Abstract
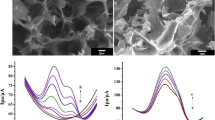
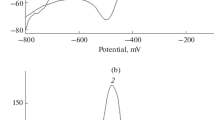
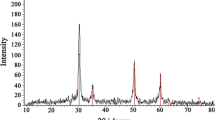
Palladium-copper nanoparticles were placed on activated carbon to give a nanocomposite for electrochemical sensing of riboflavin (vitamin B2). The activated carbon was produced by pyrolysis of natural waste of pistachio nutshells after KOH activation and under a nitrogen atmosphere. The carbons possess a large surface area and micro/meso-porosity. The nanocomposite was characterized by a variety of techniques to confirm structures and morphology. A screen-printed electrode modified with the composite was examined by EIS, CV, DPV, and amperometry. The effects of pH value, scan rate, and stability of the modified electrode were studied. Under optimized conditions, vitamin B2 displays a well-expressed oxidation peak at −0.15 V (vs. Ag/AgCl) in solutions with a pH value of 7.0. The voltammetric signal increases linearly in the 0.02 to 9 μM concentrations range and a lower detection limit of 7.6 pM. The sensor was successfully applied to the determination of vitamin B2 even in the presence of other common vitamins and in (spiked) raw milk samples.

A highly porous carbon was modified with palladium-copper alloy nanoparticles and used to coat an electrode for sensing of riboflavin (vitamin B2) by voltammetry.








Similar content being viewed by others
References
Hassan M, Zhang S, Lind J, Wang T, Bo X, Lin H, Li WH, Zhou M (2018) The biomass of ground cherry husks derived carbon nanoplates for electrochemical sensing. Sensors Actuators B Chem 255(3):3248–3256. https://doi.org/10.1016/j.snb.2017.09.151
Lam E, Luong JHT (2014) Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal 4(10):3393–3410. https://doi.org/10.1021/cs5008393
Veerakumar P, Rajkumar C, Chen SM, Thirumalraj B, Lin KC (2018) Activated porous carbon supported rhenium composites as electrode materials for electrocatalytic and supercapacitor applications. Electrochim Acta 271(1):433–447. https://doi.org/10.1016/j.electacta.2018.03.165
Işıtan S, Ceylan S, Topcu Y, Hintz C, Tefft J, Chellappa T, Guo J, Goldfarb JL (2016) Product quality optimization in an integrated biorefinery: conversion of pistachio nutshell biomass to biofuels and activated biochars via pyrolysis. Energy Convers Manag 127(1):576–588. https://doi.org/10.1016/j.enconman.2016.09.031
Veerakumar P, Maiyalagan T, Raj BGS, Guruprasad K, Jiang Z, Lin KC (2018) Paper flower-derived porous carbons with high-capacitance by chemical and physical activation for sustainable applications. Arab J Chem. https://doi.org/10.1016/j.arabjc.2018.08.009
Rufford T, Hulicova-Jurcakova D, Khosla K, Zhu Z, Lu GQ (2010) Microstructure and electrochemical double-layer capacitance of carbon electrodes prepared by zinc chloride activation of sugar cane bagasse. J Power Sources 195(3):912–918. https://doi.org/10.1016/j.jpowsour.2009.08.048
Lou BS, Veerakumar P, Chen SM, Veeramani V, Madhu R, Liu SB (2016) Ruthenium nanoparticles decorated curl-like porous carbons for high performance supercapacitors. Sci Rep 6:19949. https://doi.org/10.1038/srep19949
Okutucu C, Duman G, Ucar S, Yasa I, Yanik J (2011) Production of fungicidal oil and activated carbon from pistachio shell. J Anal Appl Pyrolysis 91(1):140–146. https://doi.org/10.1016/j.jaap.2011.02.002
Hassan A, Youssef A, Priecel P (2013) Removal of deltamethrin insecticide over highly porous activated carbon prepared from pistachio nutshells. Carbon Lett 14(4):234–242. https://doi.org/10.5714/CL.2013.14.4.234
Xu J, Gao Q, Zhang Y, Tan Y, Tian W, Zhu L, Jiang L (2014) Preparing two-dimensional microporous carbon from pistachio nutshell with high areal capacitance as supercapacitor materials. Sci Rep 4(5545). https://doi.org/10.1038/srep05545
Wu F, Tseng R, Hu C, Wang C (2005) Effects of pore structure and electrolyte on the capacitive characteristics of steam-and KOH activated carbons for supercapacitors. J Power Sources 144(1):302–309. https://doi.org/10.1016/j.jpowsour.2004.12.020
Veerakumar P, Veeramani V, Chen SM, Madhu R, Liu SB (2016) Palladium nanoparticle incorporated porous activated carbon: electrochemical detection of toxic metal ions. ACS Appl Mater Interfaces 8(2):1319–1326. https://doi.org/10.1021/acsami.5b10050
Zhang L, Ye C, Li X, Ding Y, Liang H, Zhao G, Wang YA (2018) CuNi/C nanosheet array based on a metal–organic framework derivate as a supersensitive non-enzymatic glucose sensor. Nano-Micro Lett 10(1):28. https://doi.org/10.1007/s40820-017-0178-9
Wu J, Shan S, Luo J, Joseph P, Petkov V, Zhong CJ (2015) PdCu nanoalloy electrocatalysts in oxygen reduction reaction: role of composition and phase state in catalytic synergy. ACS Appl Mater Interfaces 7(46):25906–25913. https://doi.org/10.1021/acsami.5b08478
Xu C, Liu A, Qiu H, Liu Y (2011) Nanoporous PdCu alloy with enhanced electrocatalytic performance. Electrochem Commun 13(8):766–769. https://doi.org/10.1016/j.elecom.2011.04.007
Liu A, Geng H, Xu C, Qiu H (2011) A three-dimensional hierarchical nanoporous PdCu alloy for enhanced electrocatalysis and biosensing. Anal Chim Acta 703(2):172–178. https://doi.org/10.1016/j.aca.2011.07.039
Yang H, Wang Z, Li C, Xu C (2017) Nanoporous PdCu alloy as an excellent electrochemical sensor for H2O2 and glucose detection. J Colloid Interface Sci 491(1):321–328. https://doi.org/10.1016/j.jcis.2016.12.041
Mehmeti E, Stanković DM, Chaiyo S, Švorc Ľ, Kalcher K (2016) Manganese dioxide-modified carbon paste electrode for voltammetric determination of riboflavin. Microchim Acta 183(5):1619–1624. https://doi.org/10.1007/s00604-016-1789-4
Sriramprabha R, Divagar M, Ponpandian N, Viswanathan C (2018) Tin oxide/reduced graphene oxide nanocomposite-modified electrode for selective and sensitive detection of riboflavin. J Electrochem Soc 165(11):B498–B507. https://doi.org/10.1149/2.0761811jes
Parvin MH, Azizi E, Arjomandi J, Lee JY (2018) Highly sensitive and selective electrochemical sensor for detection of vitamin B12 using an Au/PPy/FMNPs@TD-modified electrode. Sensors Actuators B Chem 261:335–344. https://doi.org/10.1016/j.snb.2018.01.168
Roushani M, Shahdost-fard F (2015) A novel ultrasensitive aptasensor based on silver nanoparticles measured via enhanced voltammetric response of electrochemical reduction of riboflavin as redox probe for cocaine detection. Sensors Actuators B Chem 207:764–771. https://doi.org/10.1016/j.snb.2014.10.131
Chen M, Andrenyak DM, Moody DE, Foltz RL (2005) Determination of riboflavin by high-performance liquid chromatography with riboflavin-depleted urine as calibration and control matrix. J Chromatogr B 820(1):147–150. https://doi.org/10.1016/j.jchromb.2005.03.018
Ashraf M, Javed S, Abbas Q, Khokhar MY, Nangyal H, Sherwani SK, Kausar R (2014) Spectrophotmetric determination of riboflavin with spermine-copper chloride complexes in pharmaceutical preparations. Am Euras J Agric Environ Sci 14:1397–1401. https://doi.org/10.5829/idosi.aejaes.2014.14.12.9189
Shumyantseva VV, Bulko TV, Petushkova NA, Samenkova NF, Kuznetsova GP, Archakov AI (2004) Fluorescent assay for riboflavin binding to cytochrome P450 2B4. J Inorg Biochem 98(2):365–370. https://doi.org/10.1016/j.jinorgbio.2003.10.024
Arnold A, Lipsius ST, Greene DJ (1941) Riboflavin determination by the microbiological method. J Food Sci 6(1):39–43. https://doi.org/10.1111/j.1365-2621.1941.tb16266.x
Muthusankar G, Rajkumar C, Chen SM, Karkuzhali R, Gopu G, Sangili A, Sengottuvelan N, Sankar R (2019) Sonochemical driven simple preparation of nitrogen-doped carbon quantum dots/SnO2 nanocomposite: a novel electrocatalyst for sensitive voltammetric determination of riboflavin. Sensors Actuators B 281(15):602–612. https://doi.org/10.1016/j.snb.2018.10.145
Kariuki NN, Wang X, Mawdsley JR, Ferrandon MS, Niyogi SG, Vaughey JT, Myers DJ (2010) Colloidal synthesis and characterization of carbon-supported Pd-Cu nanoparticle oxygen reduction electrocatalysts. Chem Mater 22(14):4144–4152. https://doi.org/10.1021/cm100155z
Veerakumar P, Salamalai K, Thanasekaran P, Lin KC (2018) Simple preparation of porous carbon-supported ruthenium: propitious catalytic activity in the reduction of ferrocyanate(III) and a cationic dye. ACS Omega 3(10):12609–12621. https://doi.org/10.1021/acsomega.8b01680
Yang ZR, Wang SQ, Wang J, Zhou AJ, Xu CW (2017) Pd supported on carbon containing nickel, nitrogen and sulfur for ethanol electrooxidation. Sci Rep 7:15479. https://doi.org/10.1038/s41598-017-15060-x
Karnan M, Subramani K, Srividhya PK, Sathish M (2017) Electrochemical studies on corncob derived activated porous carbon for supercapacitors application in aqueous and non-aqueous electrolytes. Electrochim Acta 228(20):586–596. https://doi.org/10.1016/j.electacta.2017.01.095
Kim D, Kim JM, Jeon Y, Lee J, Oh J, Antink WH, Kim D, Piao Y (2018) Novel two-step activation of biomass-derived carbon for highly sensitive electrochemical determination of acetaminophen. Sensors Actuators B Chem 259(15):50–58. https://doi.org/10.1016/j.snb.2017.12.066
Apaydin-Varol E, Putun E, Putun AE (2007) Slow pyrolysis of pistachio shell. Fuel 86(12–13):1892–1899. https://doi.org/10.1016/j.fuel.2006.11.041
Chen D, Sun P, Liu H, Yang J (2017) Bimetallic Cu–Pd alloy multipods and their highly electrocatalytic performance for formic acid oxidation and oxygen reduction. J Mater Chem A 5(9):4421–4429. https://doi.org/10.1039/C6TA10476B
Han SH, Bai J, Liu HM, Zeng JH, Jiang JX, Chen Y, Lee JM (2016) One-pot fabrication of hollow and porous Pd-Cu alloy nanospheres and their remarkably improved catalytic performance for hexavalent chromium reduction. ACS Appl Mater Interfaces 8(45):30948–30955. https://doi.org/10.1021/acsami.6b10343
Laviron E (1974) Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J Electroanal Chem 52(3):355–393. https://doi.org/10.1016/S0022-0728(74)80448-1
Acknowledgments
The authors are grateful for the financial support (MOST 106- 2113-M-027-003-MY3 to S.-M.C. and MOST-102-2113-M- 002-009-MY3 to K.-C.L.) from the Ministry of Science and Technology (MOST), Taiwan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 6188 kb)
Rights and permissions
About this article
Cite this article
Sangili, A., Veerakumar, P., Chen, SM. et al. Voltammetric determination of vitamin B2 by using a highly porous carbon electrode modified with palladium-copper nanoparticles. Microchim Acta 186, 299 (2019). https://doi.org/10.1007/s00604-019-3396-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3396-7