Abstract
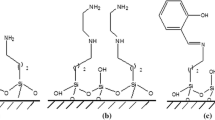
A very effective adsorbent for thorium has been obtained by modification of Santa Barbara Amorphous (SBA-15) using the chelating agent thenoyltrifluoroacetone (TTA). The prepared adsorbent (SBA-15-TTA) was characterized using scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR), surface area, porosity, and zeta potential analyses. We investigated the factors affecting Th(IV) adsorption on TTA-SBA-15, such as initial pH, contact time, temperature, and initial metal concentration. The effective initial pH for adsorption was found to be 4. The binding sites on TTA-SBA-15 adsorbent were saturated using an initial Th(IV) concentration of 100 mg L−1. The Dubinin–Radushkevich isotherm suggested a strong chemical interaction between adsorbent and adsorbate. Contact time and temperature had no significant effect on the adsorption. Therefore, the studies show that TTA-SBA-15 is a promising adsorbent to treat Th(IV)-contaminated effluents.






Similar content being viewed by others
References
M.E. Nasab, S.A. Milani, A. Sam, Extractive separation of Th(IV), U(VI), Ti(IV), La(III) and Fe(III) from Zarigan ore. J. Radioanal. Nucl. Chem. 288, 677–683 (2011). https://doi.org/10.1007/s10967-011-1008-z
M.O.A. El-Magied, A.A. Tolba, H.S. El-Gendy et al., Studies on the recovery of Th(IV) ions from nitric acid solutions using amino-magnetic glycidyl methacrylate resins and application to granite leach liquors. Hydrometallurgy 169, 89–98 (2017). https://doi.org/10.1016/j.hydromet.2016.12.011
S.V. Bhat, J.S. Melo, B.B. Chaugule et al., Biosorption characteristics of uranium(VI) from aqueous medium onto Catenella repens, a red alga. J. Hazard. Mater. 158, 628–635 (2008). https://doi.org/10.1016/j.jhazmat.2008.02.042
W. Liu, X. Dai, Z. Bai et al., Highly sensitive and selective uranium detection in natural water systems using a luminescent mesoporous metal–organic framework equipped with abundant Lewis basic sites: a combined batch, X-ray absorption spectroscopy, and first principles simulation investigation. Environ. Sci. Technol. 51, 3911–3921 (2017). https://doi.org/10.1021/acs.est.6b06305
D. Sheng, L. Zhu, C. Xu et al., Efficient and selective uptake of TcO4 − by a cationic metal–organic framework material with open Ag+ sites. Environ. Sci. Technol. 51, 3471–3479 (2017). https://doi.org/10.1021/acs.est.7b00339
T. Zheng, Z. Yang, D. Gui et al., Overcoming the crystallization and designability issues in the ultrastable zirconium phosphonate framework system. Nat. Commun. 8, 15369 (2017). https://doi.org/10.1038/ncomms15369
Y. Wang, Z. Liu, Y. Li et al., Umbellate distortions of the uranyl coordination environment result in a stable and porous polycatenated framework that can effectively remove cesium from aqueous solutions. J. Am. Chem. Soc. 137, 6144–6147 (2015). https://doi.org/10.1021/jacs.5b02480
D. Alipour, A.R. Keshtkar, M.A. Moosavian, Adsorption of thorium(IV) from simulated radioactive solutions using a novel electrospun PVA/TiO2/ZnO nanofiber adsorbent functionalized with mercapto groups: study in single and multi-component systems. Appl. Surf. Sci. 366, 19–29 (2016). https://doi.org/10.1016/j.apsusc.2016.01.049
L. Weijuan, T. Zuyi, Comparative study on Th(IV) sorption on alumina and silica from aqueous solutions. J. Radioanal. Nucl. Chem. 254(1), 187–192 (2002). https://doi.org/10.1023/A:1020874405480
L. Yan, F. Qiaohui, W. Wangsuo, Sorption of Th(IV) on goethite: effects of pH, ionic strength, FA and phosphate. J. Radioanal. Nucl. Chem. 289, 865–871 (2011). https://doi.org/10.1007/s10967-011-1166-z
G. Zhijun, N. Lijun, T. Zuyi, Sorption of Th(IV) ions onto TiO2: effects of contact time, ionic strength, thorium concentration and phosphate. J. Radioanal. Nucl. Chem. 266(2), 333–338 (2005). https://doi.org/10.1007/s10967-005-0912-5
M. Kruk, M. Jaroniec, A. Sayari, New insights into pore-size expansion of mesoporous silicates using long-chain amines. Microporous Mesoporous Mater. 35–36, 545–553 (2000). https://doi.org/10.1016/S1387-1811(99)00249-8
H. Yoshitake, Highly-controlled synthesis of organic layers on mesoporous silica: their structure and application to toxic ion adsorptions. New J. Chem. 29, 1107–1117 (2005). https://doi.org/10.1039/b504957a
D. Zhao, J. Feng, Q. Huo et al., Triblock copolymer syntheses of mesoporous silica with periodic 50–300 angstrom pores. Science 279, 548–552 (1998). https://doi.org/10.1126/science.279.5350.548
E. Da’na, Adsorption of heavy metals on functionalized-mesoporous silica: a review. Microporous Mesoporous Mater. 247, 145–157 (2017). https://doi.org/10.1016/j.micromeso.2017.03.050
L. Dolatyari, M.R. Yaftian, S. Rostamnia, Removal of uranium(VI) ions from aqueous solutions using Schiff base functionalized SBA-15 mesoporous silica materials. J. Environ. Manag. 169, 8–17 (2016). https://doi.org/10.1016/j.jenvman.2015.12.005
S. Wang, K. Wang, C. Dai et al., Adsorption of Pb2+ on amino-functionalized core–shell magnetic mesoporous SBA-15 silica composite. Chem. Eng. J. 262, 897–903 (2015). https://doi.org/10.1016/j.cej.2014.10.035
L. Giraldoa, J.C.M. Piraján, Study on the adsorption of heavy metal ions from aqueous solution on modified SBA-15. Mater. Res. 16(4), 745–754 (2013). https://doi.org/10.1590/S1516-14392013005000051
A. Shahbazi, H. Younesi, A. Badiei, Batch and fixed-bed column adsorption of Cu(II), Pb(II) and Cd(II) from aqueous solution onto functionalised SBA-15 mesoporous silica. Can. J. Chem. Eng. 91, 739–750 (2013). https://doi.org/10.1002/cjce.21691
G.R. Harvianto, S.H. Kim, C.S. Ju, Solvent extraction and stripping of lithium ion from aqueous solution and its application to seawater. Rare Met. 35(12), 948–953 (2016). https://doi.org/10.1007/s12598-015-0453-1
D.R. Raut, P.K. Mohapatra, Extraction of uranyl ion using 2-thenoyltrifluoro acetone (HTTA) in room temperature ionic liquids. Sep. Sci. Technol. 50, 380–386 (2015). https://doi.org/10.1080/01496395.2014.973523
Y. Li, B. Yan, Y. Li, Hybrid materials of SBA-16 functionalized by rare earth (Eu3+, Tb3+) complexes of modified β-diketone (TTA and DBM): covalently bonding assembly and photophysical properties. J. Solid State Chem. 183(4), 871–877 (2010). https://doi.org/10.1016/j.jssc.2010.02.006
B. Yan, Y. Li, B. Zhou, Covalently bonding assembly and photophysical properties of luminescent hybrids Eu–TTA–Si and Eu–TTA–Si–MCM-41 by modified thenoyltrifluoroacetone. Microporous Mesoporous Mater. 120, 317–324 (2009). https://doi.org/10.1016/j.micromeso.2008.11.021
J. Bassil, A. Al-Barazi, P. Da Costa et al., Catalytic combustion of methane over mesoporous silica supported palladium. Catal. Today 176(1), 36–40 (2011). https://doi.org/10.1016/j.cattod.2011.05.026
J. Du, H. Xu, J. Shen et al., Catalytic dehydrogenation and cracking of industrial dipentene over M/SBA-15 (M = Al, Zn) catalysts. Appl. Catal. A Gener. 296, 186–193 (2005). https://doi.org/10.1016/j.apcata.2005.08.030
E. Ghedini, F. Menegazzo, M. Signoretto et al., Mesoporous silica as support for Pd-catalyzed H2O2 direct synthesis: effect of textural properties of the support on the activity and selectivity. J. Catal. 273(2), 266–273 (2010). https://doi.org/10.1016/j.jcat.2010.06.003
P. Wang, Z. Wang, J. Li et al., Preparation, characterization and catalytic characteristics of Pd nanoparticles encapsulated in mesoporous silica. Microporous Mesoporous Mater. 116(1), 400–405 (2008). https://doi.org/10.1016/j.micromeso.2008.04.029
A.M. Venezia, G. Di Carlo, L.F. Liotta et al., Effect of Ti(IV) loading CH4 oxidation activity and SO2 tolerance of Pd catalysts supported on silica SBA-15 and HMS. Appl. Catal. B Environ. 106, 529–539 (2011). https://doi.org/10.1016/j.apcatb.2011.06.013
F. Yin, S. Ji, P. Wu et al., Deactivation behavior of Pd-based SBA-15 mesoporous silica for the catalytic combustion of methane. J. Catal. 257(1), 108–116 (2008). https://doi.org/10.1016/j.jcat.2008.04.010
S.B. Sarrin, Analytical use of arsenazo III: determination of thorium, zirconium, uranium and rare earth elements. Talanta 8, 673–685 (1961). https://doi.org/10.1016/0039-9140(61)80164-1
L. Cromières, V. Moulin, B. Fourest et al., Sorption of thorium onto hematite colloids. Radiochim. Acta 82, 249–256 (1998). https://doi.org/10.1524/ract.1998.82.special-issue.249
P. Sar, S.F. D’Souza, Biosorption of thorium (IV) by a Pseudomonas biomass. Biotechnol. Lett. 24, 239–243 (2002). https://doi.org/10.1023/A:1014153913287
Ş. Sert, C. Kütahyali, S. İnan et al., Biosorption of lanthanum and cerium from aqueous solutions by Platanus orientalis leaf powder. Hydrometallurgy 90, 13–18 (2008). https://doi.org/10.1016/j.hydromet.2007.09.006
S. Sert, M. Eral, Uranium adsorption studies on aminopropyl modified mesoporous sorbent (NH2–MCM-41) using statistical design method. J. Nucl. Mater. 406, 285–292 (2010). https://doi.org/10.1016/j.jnucmat.2010.08.024
S. Yusan, S. Akyil, Sorption of uranium(VI) from aqueous solutions by akaganeite. J. Hazard. Mater. 160, 388–395 (2008). https://doi.org/10.1016/j.jhazmat.2008.03.009
H.K. Boparai, M. Joseph, D.M. O’Carroll, Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 186, 458–465 (2011). https://doi.org/10.1016/j.jhazmat.2010.11.029
D.L. Guerraa, R.R. Viana, C. Airoldi, Adsorption of thorium cation on modified clays MTTZ derivative. J. Hazard. Mater. 168, 1504–1511 (2009). https://doi.org/10.1016/j.jhazmat.2009.03.034
S.R. Yousefi, S.J. Ahmadi, F. Shemirania et al., Simultaneous extraction and preconcentration of uranium and thorium in aqueous samples by new modified mesoporous silica prior to inductively coupled plasma optical emission spectrometry determination. Talanta 80, 212–217 (2009). https://doi.org/10.1016/j.talanta.2009.06.058
L. Dolatyari, M.R. Yaftian, S. Rostamnia, Adsorption characteristics of Eu(III) and Th(IV) ions onto modified mesoporous silica SBA-15 materials. J. Taiwan Inst. Chem. Eng. 60, 174–184 (2016). https://doi.org/10.1016/j.jtice.2015.11.004
L. Zuo, S. Yu, H. Zhou et al., Th(IV) adsorption on mesoporous molecular sieves: effects of contact time, solid content, pH, ionic strength, foreign ions and temperature. J. Radioanal. Nucl. Chem. 288, 379–387 (2011). https://doi.org/10.1007/s10967-010-0930-9
L.Y. Yuan, Z.Q. Bai, R. Zhao et al., Introduction of bifunctional groups into mesoporous silica for enhancing uptake of thorium(IV) from aqueous solution. Appl. Mater. Interfaces 6, 4786–4796 (2014). https://doi.org/10.1021/am405584h
S. Yusan, C. Gok, S. Erenturk et al., Adsorptive removal of thorium (IV) using calcined and flux calcined diatomite from Turkey: evaluation of equilibrium, kinetic and thermodynamic data. Appl. Clay Sci. 67–68, 106–116 (2012). https://doi.org/10.1016/j.clay.2012.05.012
C. Kütahyalı, M. Eral, Sorption studies of uranium and thorium on activated carbon prepared from olive stones: kinetic and thermodynamic aspects. J. Nucl. Mater. 396, 251–256 (2010). https://doi.org/10.1016/j.jnucmat.2009.11.018
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Scientific Technological Research Council of Turkey (No. 113Y557).
Rights and permissions
About this article
Cite this article
Gök, M., Sert, Ş., Özevci, G. et al. Efficient adsorption of Th(IV) from aqueous solution by modified SBA-15 mesoporous silica. NUCL SCI TECH 29, 95 (2018). https://doi.org/10.1007/s41365-018-0432-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-018-0432-y