Abstract
The increasing demand for agricultural products can be met by maximizing production potential and reducing crop losses caused by common plant-parasitic nematodes. Chemical-based nematode management is a successful technique for mitigating damage and yield losses caused by nematode pests; however, inappropriate and irresponsible application of synthetic pesticides has negative impacts on fauna, bioflora, and natural enemies such as predators and parasites. The use of biocontrol agents is the most appreciated method for nematode control among farmers because it’s safe and reduces environmental pollution. There is increasing focus on the biological control of plant-parasitic nematodes using plant growth-promoting rhizobacteria (PGPR) as a biopesticide. Moreover, PGPR strains can promote plant growth by producing various secondary metabolites of these PGPRs. This review focuses on the direct (Nitrogen fixation, phytohormone formation, phosphate solubilization, Potassium solubilization, siderophores and ammonia production) and indirect mechanisms (Hyperparasitism, antibiosis, lytic enzyme production, induced systemic resistance) of action of PGPR in plant-parasitic nematodes management, and the future prospects of PGPR-based plant-parasitic nematodes biocontrol agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are approximately 4000 plant-parasitic nematodes (PPNs) species known that may be found in all major biomes (Press and Phoenix 2005). Because of their inhabitation and parasitism behavior, it is more difficult to control plant-parasitic nematodes (Gillet et al. 2017). Plant-parasitic nematodes are polyphagous parasitic worms that cause extensive damage to important food crops, including maize, potato, and tomato (Nicol et al. 2011). Plant-parasitic nematodes control costs $173 billion per year to the global agriculture industry (Elling 2013). Species of Meloidogyne sp., Heterodera Sp., Globodera sp., and Pratylenchus sp. are among the most economically significant due to the high levels of damage and infection they inflict, their vast host range, and interaction with the host plant. Root-knot nematodes (RKNs) inhibit not only plant growth and yield but also nodulation, nitrogen fixation, and mineral nutrient absorption from the soil (Rehman et al. 2012). Root-knot nematode-infected plants exhibit symptoms both aboveground and belowground. The aboveground symptoms include poor development and fewer as well as smaller pale green leaves that wilt under high temperatures (Elnahal et al. 2022). Symptoms emerge as large galls on the roots that are two to several times the diameter of a healthy root and interfere with the plant’s ability to absorb and translocate water and dissolved nutrients. The nematode pests parasitize roots/other belowground plant parts, injuring them and allowing the entry of other soil-borne illnesses (Agrios 2005) (Fig. 1). Furthermore, there is an increasing demand for more food for the growing world population (FAO 2017). Therefore, PPN management is becoming increasingly popular throughout the world.
Above-ground and below-ground symptoms from tomato in response to Meloidogyne sp. infections (Fujimoto et al. 2021)
Several synthetic chemical nematicides such as fumigants are used to manage and control PPNs (Katooli et al. 2010). As population expansion demands an increased agricultural production and the efficacy of nematicides to protect crops against diseases and pests, the traditional method of nematode management is completely dependent on nematicides. Nevertheless, due to European Union (EU) regulations (EC No1107/2009), which state that pesticides are dangerous to human health and pollute the environment, pesticide use has considerably decreased, which has consequently increased the need for other effective nematode control methods (Zhang et al. 2017). Therefore, there exists a critical need for a nematode control method that is both ecologically acceptable and effective.
Plant-parasitic nematodes management strategies, including biocontrol methods, offer a safer and more practical option (Abd-Elgawad 2021). The term “biological control” (or biocontrol) refers to an ecologically sound and successful method for eliminating or suppressing plant-parasitic nematodes using naturally occurring species with pesticidal activity or manipulating the environment or introducing opponents (Landis and Orr 1996; Eldeeb et al. 2022). Moreover, a biological control agent is organism that adversely affects the biology and physiology (e.g., development, reproduction and respiration) and its and it is commonly referred to as a “biopesticide.” In general, biopesticides are defined as “products targeted at protecting plants and are produced from live organisms or natural compounds derived from species coevolution, rather than chemicals, and whose usage is suggested for controlling pests or bioaggressors for greater biocenosis and environmental safety” (Chandler et al. 2011). Plant growth-promoting rhizobacteria (PGPR) are host-specific biological agents that can adversely impact on the development and survival of PPNs (Alberton et al. 2020). Plant growth-promoting rhizobacteria have been defined in different ways by different authors. For instance, Kloepper and Schroth (1981) defined PGPR as a type of soil bacteria that colonize the roots of plants after being inoculated onto seeds and help them thrive. Bishnoi (2015) defined PGPR as a wide collection of soil bacteria that are important components of soil-plant systems, wherein they interact actively and effectively in the rhizosphere, regulating plant development and yield through a variety of processes. Plant growth-promoting rhizobacteria are also termed as plant health-promoting rhizobacteria or nodule-promoting rhizobacteria (Hayat et al. 2010) and can be categorized into two groups based on their habitat, viz., iPGPR (i.e., symbiotic bacteria), which reside inside plant cells, generate nodules, and are confined to specific structures, and ePGPR (i.e., free-living rhizobacteria), which live outside plant cells, do not produce nodules, but nonetheless stimulate plant development (Gray and Smith 2005). In general, the function of PGPRs can be summarized in the following three ways: first, production of certain chemicals required for plants (Backer et al. 2018); second, increasing plant resistance (Çakmakçi et al. 2006); and finally, promoting the absorption of certain minerals from the soil (Saravanakumar et al. 2008). Bacillus, Paenibacillus, Brevibacillus, Pseudomonas, Serratia, Burkholderia, and Streptomyces species are typical PGPRs used as seed treatments because of their adverse effect on disease microbes and antibiotic-producing properties (Kokalis-Burelle et al. 2006). Antibiotic synthesis, substrate competition, and induced systemic resistance in the host are some of the processes implicated in suppression of PPNs by PGPR (Nivya 2015). Moreover, numerous PGPR formulations have been reported to decrease the severity of disease caused by RKNs in experiments with muskmelon and watermelon (Kokalis-Burelle et al. 2003). Consequently, it has become more critical to explore innovative, ecologically acceptable methods, such as the use of PGPR, to control PPN populations.
Understanding the beneficial microorganism populations and their mechanisms of action in controlling PPNs at the molecular level will provide a foundation to enhance the pathogenic activity of potential biocontrol strains and develop modern biocontrol strategies in PPNs control. This review focuses on the usage of PGPR to control PPNs.
Distribution of root-knot nematode in Egypt
Meloidogyne spp. cause important diseases affecting numerous plant crops in Egypt and hence has a significant economic impact. Meloidogyne arenaria Chitwood, M. javanica Chitwood, and M. incognita Chitwood are the most economically important species in Egypt (Ibrahim et al. 2000). They are particularly damaging in tropical, subtropical, and warm regions (Ibrahim et al. 2000). Root-knot nematodes are particularly significant disease-causing agents in Egypt, affecting numerous agricultural plants and food supplies due to their widespread distribution, broad host ranges, and association with disease-causing fungi and bacteria. There have been extensive investigations in this regard, and RKNs have been documented to infect a variety of crops in all regions of Egypt (Bakr et al. 2020). Table 1 shows the distribution of Meloidogyne spp. in Egypt.
Life cycle of root-knot nematodes
Root-knot nematodes can complete their entire life cycle in 20–40 days; however, its life cycle duration is influenced by soil moisture, temperature, and host species (El-Saadony et al. 2021). The life cycle of RKNs begins with an egg from which a second-stage juvenile (J2) hatches. With the aid of their chemosensory amphids, J2s travel across the vulnerable host roots and/or through the soil by sensing chemical gradients in root tissues (Vagelas et al. 2012). Physiologically, several gradients are found near active roots, such as carbon dioxide (CO2), and it is possible that some of them operate as “long-distance attractants,” allowing the J2 to move to the root region (Banerjee and Hallem 2020). Besides CO2, other attractants, including amino acids, carbohydrates, and metabolites, are equally effective in attracting RKN J2s (Tsai et al. 2019). After finding an appropriate host, RKN J2s readily perforate below the root’s protraction zone, migrate between cells toward the root tip, and pass the Casparian strip to reach the vascular tissue (Shukla and Barberon 2021). The specific signals that govern the direction of J2s in the roots are uncertain, and only the role of CO2 as a significant attractant is known (Banerjee and Hallem 2020); however, pH can also influence the direction in which the J2s migrate (Kawanobe et al. 2020). Root-knot nematodes release a combination of enzymes that degrade the cell wall, which they use to transmit and split the soft, intermediate lamella connecting the root cells (Rai et al. 2015). A second-stage juvenile (J2) release a variety of enzymes from their subventral glands including cellulases (endoglucanases), polygalacturonases, pectate lyases, and endoxylanases (Rai et al. 2015). The J2s turns around (180 degrees).
a migrate in the opposite direction toward the vascular cylinder to settle in the differentiation zone for feeding. Then, 5–8 vascular cells are selected to form a feeding site, of which the cells undergo a series of metabolic and nutrition site (Perry and Moens 2011), metabolic and morphological alterations before being transformed into giant cells (GCs). Moreover, hypertrophied cortical cells, proliferated pericycle cells, and xylem are significantly disturbed in the region of GCs, and the GCs are surrounded by complicated xylem tissues (Bartlem et al. 2014). Protophloem forms and spreads rapidly, surrounding the GCs (Absmanner et al. 2013) and resulting in the formation of a distinct pseudo-organ termed gall that contains the GCs. Meloidogyne spp. that form tiny or no galls in their host plant exhibit reduced hyperplasia surrounding tissues (Palomares-Rius et al. 2017). The life cycle of RKNs is depicted in detail in Fig. 2.
Impact of PPNs on agriculture yield and productivity
Root-knot nematodes are tiny roundworms that belong to the phylum Nematoda and are among the most numerous animals on the planet, with more than 4100 species discovered in a range of habitats (Krif et al. 2020). Root-knot nematodes reside in the moisture layer surrounding soil particles and plant roots for the most part of their life cycle (Krif et al. 2020). To gain access into root tissues, PPNs have protruding styles or mouth spears (Palomares-Rius et al. 2017). Root-knot nematodes are divided into two types based on their feeding environments, viz., endoparasites and ectoparasites (Smant et al. 2018). Ectoparasites feed on the exterior of root surfaces rather than inside the roots (Sato et al. 2019). Endoparasitic nematodes, on the other hand, can penetrate plant roots completely or partially during the infection process (El-Ashry et al. 2021; Aioub et al. 2021). This causes physical harm as well as the possibility of secondary damage from fungal and bacterial infection concurrently with PPN infection (Zasada and Ferris 2003). Endoparasitic nematodes are estimated to cause a worldwide annual loss of 13% ($216 billion) (Singh et al. 2015). For instance, it has been reported that Meloidogyne spp. were responsible for causing an annual global loss of $157 billion (Abad et al. 2008). Moreover, the loss due to Rotylenchulus spp. was approximately 26,000 bales of cotton, equivalent to a 3% yield reduction (Lawrence et al. 2014). Therefore, establishing an effective and long-term management program to combat PPNs is becoming a top priority across the world. Nevertheless, as PPNs are tiny and primarily target plant subsurface areas, the existing integrated PPN control strategies are limited compared to those used to control other pests (Bernard et al. 2017).
Biological control
Biological control is currently a widely used approach for pest management across the world (Landis and Orr 1996). In biological control, there are four primary strategies that may be used as described in the following lines. (1) Introduction: this is a traditional strategy in which an exotic beneficial organism is introduced into a new territory and becomes completely established. This method is typically used against pests that have no natural enemies. (2) Augmentation: in vivo or in vitro reared individuals released in the natural environment to compensate for the inefficiency of existing or natural occurring microbial agents. Small numbers of native, natural enemies may be the reason for the lack of pest control. (3) Inoculation: an inoculative release is done at the start of the planting season when an indigenous microbial agent is not present or an exotic antagonist cannot persist indefinitely. This technique may have to be repeated for each subsequent growing season. (4) Inundation: this strategy involves bulk cultivation of a pathogen for application during crucial periods when rapid pest population control is required (D. L. Lee 2002). Root-knot nematode populations are regulated partially by biological control agents, and a variety of species, such as bacteria, exhibit antagonistic activity against them (Migunova and Sasanelli 2021).
Plant growth promoting rhizobacteria (PGPR) as biocontrol agents
Several bacterial species live in the “rhizosphere” of a plant’s roots and stimulate plant development by enhancing plant growth regulators. These bacteria are known as PGPR (Kumar et al. 2015). Plant growth promoting rhizobacteria are bacteria that live in the “rhizosphere” of a plant’s roots and promote plant regulators and boost nutrient availability (PGPR) (Turan et al. 2021). In the soil, diverse microorganisms such as Rhizobium sp., Xanthomonas, Bacillus sp., Arthrobacter sp., Bradyrhizobium sp., Frankia sp., Enterobacter sp., Proteus sp., Klebsiella sp., Flavobacterium sp., Pseudomonas sp., Serratia sp., Microbacterium sp., and Erwinia sp. thrive as widespread rhizosphere elements that completely colonize the rhizosphere (Teymouri et al. 2016; Abad et al. 2008). Bacillus, Azospirillum, and Pseudomonas are some of the most well-known colonized rhizobacteria genera. The use of bacteria in the rhizosphere plays a significant role as a biofertilizer and psychostimulant and in developing disease resistance and heavy metal cleanup, but it is dependent on colony formation in the rhizosphere. Rhizosphere-based microorganisms can improve plant growth through production of a set of plant growth compounds and conferring resistance to nematode infection and development (Basu et al. 2021). Moreover, PGPR have been identified as potential agents for reducing the damage caused by PPNs, resulting in for effective PPN management (Subedi et al. 2020). The use of PGPR has a significant impact on plant development and can cause the death of PPNs. Therefore, PGPR represent an excellent tool for improving agriculture practices and are a critical component of nematode control (Backer et al. 2018). Several PGPR strains are known to display a reduction in gall number of RKN species, including Bacillus firmus T11, B. cereus N10w, B. aryabhattai A08, Paenibacillus barcinonensis A10, and P. alvei T30 (Viljoen et al. 2019). A greenhouse experiment conducted on cucumber with the strains Pseudomonas fluorescens and Serratia marcescens resulted in a significant decrease in gall index and egg mass of RKN species compared with the untreated control group, and these strains are now considered biocontrol agents compared with M. incognita (Ali et al. 2021). Examples of PGPR shown in Table 2 are used as biological control agents for PPN suppression.
Mode of actions of PGPR
Direct mechanisms
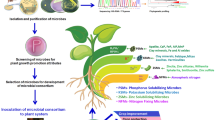
Plant growth promoting rhizobacteria help plants cope with nematode stress by producing phytohormones (cytokines, abscisic acid, auxins, and ethylene) and improving nutrient availability and uptake through phosphate and potassium solubilization, nitrogen fixation, and organic compound mineralization.
Nitrogen fixation
Nitrogen is a critical macronutrient required for plant development and growth; it is essential for protein synthesis and photosynthesis and is a substantial macronutrient of nucleic acids in the form of nitrogenous bases (Elrys et al. 2019, 2021a). Constant loss of nitrogen results in its low levels in agricultural soil. However, plants are unable to directly use atmospheric nitrogen (Cocking 2000; Elrys et al. 2021b). Plant growth promoting rhizobacteria are important for nitrogen fixation and nutrition supplementation in this situation. These nitrogen-fixing bacteria may be divided into two types as follows: symbiotic or free-living nitrogen-fixing bacteria (Raymond et al. 2004). Nitrogen-fixing PGPR strains with nematicidal activity play a significant role in sustainable agriculture as they provide both a nitrogen source and a nematode-free environment for the host plant (Vejan et al. 2016; Liao et al. 2021). In particular, Bradyrhizobium, Rhizobium, Frankia, Mesorhizobium, and Sinorhizobium are PGPR genera that can fix atmospheric nitrogen and supply it to plants, promoting plant development (Dash et al. 2017). Aggangan et al. (2013) also demonstrated that banana plants treated with nitrogen-fixing bacteria had suppressed populations of M. incognita and Radopholus similis, resulting in increased banana growth. Similarly, Paenibacillus polymyxa, a nitrogen-fixing bacterium, promotes plant development and exhibits nematicidal activity (El-Hadad et al. 2011).
Phytohormone formation
The diversity of PGPR strains may result in the production of plant growth-promoting chemicals such as phytohormones and plant growth regulators such as auxins (indole butyric acid, indole acetic acid, and phenylacetic acid), cytokines (trans-zeatin ribose, isopentenyl adenine riboside, isopentenyl adenosine, and zeatin), abscisic acid, gibberellic acid, ethylene, brassinosteroids, polyamines, jasmonates, strigolactones, salicylic acid, and other plant growth regulator compounds (Tsukanova et al. 2017) that have a direct impact on plant development and metabolism. Indole acetic acid is the most prevalent phytohormone. Phytohormones produced by PGPR are considered to promote plant development and plant–bacterial interactions (Chandra et al. 2018). The primary function of microbial phytohormones is to improve plant development by promoting elongation, cell division, tissue expansion, and other favorable effects on plant development (Khan et al. 2009). Moreover, the inclusion of bacteria that produce indole acetic acid may improve plant development and generate disease resistance (Chakraborty et al. 2006). Phytohormones produced by PGPR have been shown to protect against the negative effects of different environmental stressors (Glick 2010). Plant growth and nematode biocontrol can be improved by introducing phytohormones that produce PGPR to the field via seed application (Backer et al. 2018). For instance, Myo et al. (2019) reported that indole acetic acid production by the strain Streptomyces fradiae NKZ-259 enhanced plant growth and reduced pest population. Similarly, the strain Pseudomonas simiae MB751 produces indole acetic acid that plays a role in M. incognita control and improves plant growth (Sun et al. 2021). Therefore, any direct effect of bacteria on phytohormone synthesis may have an impact on their phytostimulating effectiveness.
Phosphate solubilization
Phosphorus is another important element required for plant development (Malhotra et al. 2018). It is involved in various aspects of plant growth, including nucleic acid formation, protein synthesis, tissue growth, cell division, and complex energy conversion (Mahler 2004). However, phosphate compounds are available in an insoluble form in agricultural settings. Plant growth promoting rhizobacteria isolated from soil use chelation, organic acid generation, and acidification to transform inaccessible phosphorus into available forms (Alori et al. 2017), influencing host plant development and nutrient uptake. Several PGPR genera such as Arthrobacter, Bacillus, Enterobacter, Flavobacterium, Microbacterium, Pseudomonas, Rhizobium, Rhodococcus, and Serratia have been previously reported to act as phosphate solubilizers (Raj et al. 2014; Bechtaoui et al. 2019; Maldonado et al. 2020). Guang-Can et al. (2008) demonstrated that the bacterial species P. syringae, B. megaterium, B. cereus, P. cichorii, and B. caryophylli possess both phosphate solubilization and mineralization capacity, resulting in increased phosphate bioavailability. Furthermore, the phosphate-solubilizing bacterium B. firmus was found to suppress M. incognita populations in tomato roots (Terefe et al. 2009). Consistently, Seenivasan et al. (2007) reported that P. fluorescens, P. lilacinus, and T. viride caused a reduction in Globodera rostochiensis and G. pallid cyst populations in potato (Solanum tuberosum). Similarly, inoculating tomato plants with B. megaterium led to improvement in growth parameters and Nitrogen, Phosphorus and Potassium contents as well as reduction in M. incognita J2 populations (El-Hadad et al. 2011).
Siderophores and ammonia production
Living organisms require iron for biological functions such as electron transport, respiration, photosynthesis, and as a cofactor for numerous enzymes (Proença et al. 2019). Iron exists in an insoluble form in soil under aerobic conditions, making it difficult for living organisms to obtain it. Plant growth promoting rhizobacteria have developed unique methods to bind the insoluble form of iron by producing low molecular weight siderophores in environments with low iron bioavailability (Schwabe et al. 2020). Aeromonas, Azadirachta, Azotobacter, Azospirillum, Bacillus, Burkholderia, Enterobacter, Pseudomonas, Rhizobium, Serratia, and Streptomyces sp. are among the PGPR that aid in the synthesis of siderophores that carry iron into the cells of plants and promote their development (Bapiri et al. 2012; van Beelen and Fleuren‐Kemilä 1997). Similarly, Enterobacter spp., Pseudomonas spp., and Bacillus spp. possess numerous properties related to plant development, as well as nematicidal activity (El-Sayed et al. 2014). Plant growth promoting rhizobacteria strains have a direct effect on plant development by triggering cell division, physiological and biochemical metabolisms, and tissue development (Oleńska et al. 2020); however, PGPR help the host plant deal with nematode infection by providing additional assistance.
Potassium solubilization
Plants also require potassium as a macronutrient. Potassium is essential for plant development, both biochemically and physiologically (Hasanuzzaman et al. 2018). However, the majority of potassium-containing minerals exist in the soil in a permanent form that is difficult for the plant to exploit (Khanna et al. 2019a). Some rhizospheric microorganisms, such as phosphate-solubilizing bacteria, solubilize the insoluble form of potassium and release it in a form that plants may use for their own development and production (Wang et al. 2020). For potassium solubilization, PGPR use a variety of processes, including chelation, organic acid excretion, reduction, acidolysis, and exchange (Wang et al. 2020). Bacillus edaphicus, Acidithiobacillus ferrooxidans, Burkholderia spp., B. mucilaginosus spp., Pseudomonas spp., and Paenibacillus spp. are among the microbial species involved in potassium solubilization (Han and Lee 2006). In addition, the inoculation of potassium-solubilizing bacteria has a favorable effect on tomato (Solanum lycopersicum) development and nematicidal activity (El-Hadad et al. 2011).
Indirect mechanism
Hyperparasitism
Hyperparasitism is the most effective and direct type of antagonism (Pal and Gardener 2006). Hyperparasitism entails the tropic growth of the biocontrol agent toward the target organism, coiling, ultimate assault, and disintegration of the target pathogen’s cell wall or membrane through enzyme activity (Singh et al. 2017). Pasteuria penetrans exhibits excellent activity against G. rostochiensis, Aphelenchoides besseyi (Tian et al. 2007), M. graminicola, M. incognita, M. arenaria, M. hapla (Ciancio 2018), R. similis, and Pratylenchus penetrans (Abd-Elgawad and Askary 2018).
Antibiosis
Antibiotics are bacteria-produced chemical compounds with a low molecular weight. Through competition and parasitism, they play an important role in the biological control of a variety of pests (Arseneault and Filion 2017). Antibiosis is a crucial aspect of luminous pseudomonad disease control. Enzymes, metabolic by-products, and toxins are released by several PGPR to control PPNs. This prevents hatching of nematode juveniles, growth, survival, and reproduction (Subedi et al. 2020). Ammonia is generated by ammonifying bacteria through the decomposition of nitrogenous organic compounds and helps decrease root-parasitic nematode populations (Norton and Ouyang 2019). Pseudomonas fluorescens also produces secondary metabolites, such as 2,4-diacetylphloroglucinol, which has been found to suppress M. incognita populations on pepper (Capsicum annuum) (Meyer et al. 2009). Also, some rhizobacteria have been shown to produce chemicals such as hydrogen cyanide, which kills nematodes in the rhizosphere and aids in plant growth (Abd El-Rahman et al. 2019). Furthermore, P. fluorescens delays nematode development (Rizvi et al. 2012). Azospirillum, Azotobacter, and Rhizobium have also been demonstrated to significantly decrease root galling by M. javanica in root of chickpea (Cicer arietinum) (Siddiqui and Mahmood 2001).
Lytic enzyme production
Another mechanism of action of PGPR is the production of enzymes, for example, phenylalanine ammonia lyase, β-glucanase, chitinase, lipase, dehydrogenase, protease, peroxidase, and phosphatases that enhance plant growth (Won et al. 2021). Corynebacterium paurometabolous has been found to generate chitinase and hydrogen sulfide, both of which impede hatching of nematode juveniles (Mena et al. 2002). Pseudomonas fluorescens and S. marcescens were found to decrease nematode populations in roots of cucumber (Cucumis sativus) (Ali et al. 2021). On testing a strain of Lysobacter capsici newly isolated from Korean soil (Lee et al. 2015) for its biocontrol activity against root-knot nematodes in tomato (Solanum lycopersicum), this strain was found to express both chitinase and gelatinase activities. The amount of these enzymes released increased significantly following the addition of eggs and J2 of M. incognita to the culture medium. The lower numbers of galls and egg masses found in tomato roots inoculated with this PGPR, compared to those occurring in untreated plants, was found to be a consequence of the synthesis of these enzymes. Moreover, Li et al. (2019) revealed that inoculation of tomato plants with strain B. cereus (BCM2) increased the release from the bacterium of nematode-inhibitory molecules, especially 2,4-di-tert-butylphenol and 3,3 dimethyloctane. Subsequently, it has been demonstrated that a crude protein extract of BCM2, also contained chitosanase, alkaline serine protease, and neutral protease, and induced 100% mortality in second-stage juveniles of M. incognita.
Induced systemic resistance (ISR)
Induced resistance refers to a plant’s improved capacity to defend itself against a diverse variety of pests after being appropriately stimulated (Kamal et al. 2014). systemic acquired resistance (SAR) refers to the enhanced defense response caused by an inducing substance following infection by a pathogen or pest (Wendehenne et al. 2014). Induced systemic resistance can be induced by PGPR, whereas SAR refers to the resistance generated by other microorganisms. Both generated resistances protect against a wide spectrum of diseases, including those caused by fungi, nematodes, bacteria, insects, and viruses (Beneduzi et al. 2012). Numerous defense enzymes are linked to systemic resistance, such as superoxide dismutase, polyphenol oxidase, peroxidase, catalase, lipoxygenase, chitinase, phenylalanine ammonia lyase, β-1,3-glucanase ascorbate, peroxidase, and proteinase (Pokhare et al. 2012). These enzymes initiate the resistance-inducing process by generating phenolic chemicals and phytoalexins (Mohammadi et al. 2020). Extensive research has demonstrated that PGPR reduce the population of plant-parasitic nematodes by increasing the plant’s systemic resistance (Khanna et al. 2019b). This induced tolerance is achieved through cell wall intensification, callose sedimentation, phenolic compound accumulation, and upregulation of biochemical compounds such as jasmonic acid, pathogenesis-related (PR) proteins, lipopolysaccharides, phytoalexin, siderophores, chitinase, and salicylic acid (Khanna et al. 2019b). Another study showed that B. cereus significantly decreased M. incognita and M. javanica populations in roots of Arabidopsis through the development of systemic resistance (Jiang et al. 2020). Hackenberg et al. (1999) found that Agrobacterium radiobacter (G12) plays an essential role in preventing Globodera spp. juveniles from penetrating potato (S. tuberosum) roots. Interestingly, tomato roots (S. lycopersicum) treated with P. fluorescens, Pf128 plus Bacillus subtilis, Bbv57, showed increased activity of enzymes and decreased M. incognita populations (Meena et al. 2012). This demonstrates the possible function of PGPR in establishing systemic resistance to nematodes by animating and compiling defense enzymes such as polyphenol oxidase, peroxidase, and phenylalanine ammonia lyase. The overall mechanisms of action of PGPR as nematode biocontrol agents are presented in Fig. 3.
Challenges in PGPR application
The capacity of microbial inoculations to compete with native microbial populations and environmental conditions is critical for their application in phytoremediation, bioremediation, as biofertilizers, and as biocontrols. Major problems with microbial survival arise under naturally occurring, field conditions circumstances. The physical and chemical qualities of the soil, the biological activity of indigenous microbes, and agricultural management strategies such as crop rotation all influence the microbial inoculation of crop plants. Most inoculants are successfully based on the basis of first come, first served. So, what are the reasons, and which are the critical areas where new inputs are required in order to increase commercialization of PGPR products? The selectivity of PGPRs is one of the key factors preventing them from becoming more widely used. The majority of conventional agrochemicals are broad-spectrum compounds that affect a wide range of organisms. Plant growth promoting rhizobacteria, on the other hand, tend to be highly targeted. This can result in variable quality and efficacy under field conditions.
Applications of PGPR as multifunctional agents
The other advantages of inoculating plants with PGPR include drought resistance, as shown for the strains P. fluorescens DR11 and Enterobacter hormaechei DR16 (Ilyas et al. 2020), tolerance to salinity stress, as shown for Bacillus pumilus and Achromobacter piechaudii (Sagar et al. 2020), tolerance to biotic stress as demonstrated for Paenibacillus xylanexedens and Bacillus amyloliquefaciens (Reshma et al. 2018), increased nutrient absorption, as shown for P. polymyxa (Chen and Liu 2019), seed germination enhancement, shown for S. marcescens and P. fluorescens (Almaghrabi et al. 2014), biostimulation by phytohormone production, as demonstrated for Azospirillum lipoferum and B. subtilis (Kalam et al. 2017), soil fertility enhancement, described for B. subtilis and B. cereus (Jang et al. 2017), bioremediation of pollutants, as shown by P. fluorescens and S. marcescens (Romeh and Hendawi 2017), and amendment of plant secondary metabolites, demonstrated for B. subtilis and Azotobacter chroococcum (Ordookhani et al. 2011).
Commercialization of PGPR
The use of PGPR as nematode biocontrol agents and for plant growth promotion, soil fertility improvement, and phytopathogen biocontrol supports sustainable agriculture by providing environmentally benign alternatives to synthetic agrochemicals such as chemical fertilizers and pesticides. A protocol for the development and commercialization of PGPR-based biocontrol agents is shown in Fig. 4. Although various strains of PGPR are currently available on the market as biological nematicides, a simple question (i.e., repeatability) remains to be answered before PGPR may be commercialized (Table 3). However, the efficacy of these products must be evaluated further. PGPR products must have a wide range of applications, a long shelf life, be safe to use, have a viable market, be readily available, be consistent in terms of their efficacy and have a low investment cost to be economically successful.
Steps of producing for commercializing plant growth-promoting rhizobacteria-based biocontrol (Basu et al. 2021)
Future prospects
Future research into improving the growing conditions, safety, broad-spectrum activity, shelf life, and stability of PGPR products is critical to their commercialization success. The agricultural revolution has increased worldwide agriculture productivity and prompted the introduction of synthetic chemical fertilizers to enhance yield and preserve crops. Excessive agrochemical usage is becoming a serious concern to animals, humans and the environment, and consequently, numerous chemicals have been prohibited across the world. Meeting the growing food demands of the world’s population is a huge challenge that may be addressed by enhancing agricultural output through adequate safe and productive strategies. These conditions may be achieved using a single yet complex method, i.e., the application of PGPR, which helps increase crop safety, soil fertility, and plant development and lead to sustainable agriculture. This technique is well recognized as a safe method of insect management and plant growth enhancement across the world. The application of genetic engineering to improve PGPR technology, and other innovations, would ensure agroecosystem productivity and stability, resulting in an optimal and sustainable agricultural system.
Conclusion
Increased yield with improved crop protection and higher soil fertility using an environmentally feasible technique is critically needed. For the optimal use of efficient PGPR strains for sustainable agriculture it is necessary to gain in-depth knowledge of the PPNs population and their colonization. Moreover, genetic engineering technologies may be used to enhance the biocontrol efficiency of PGPR by synergistically overexpressing multiple anti-phytopathogen characteristics for effective pest management. The complete potential of the consortium of efficient bacterial strains for reducing the detrimental effects of different biotic stressors on plant growth can be exploited. Commercialization of PGPR-based biopesticides should receive further attention.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PGPR:
-
Plant growth promoting rhizobacteria
- PPNs:
-
Plant-parasitic nematodes
- BCA:
-
Biological Control Agent
- RKN:
-
Root-knot nematodes
- J2s:
-
Second-stage juveniles
- GCs:
-
Giant cells
- HCN:
-
Hydrogen cyanamide
- ISR:
-
Induced Systemic Resistance
- SOD:
-
Superoxide dismutase
- PPO:
-
Polyphenol oxidase
- PO:
-
Peroxidase
- CAT:
-
Catalase
- LOX:
-
Lipoxygenase
- PAL:
-
Phenylalanine ammonia lyase
- APX:
-
Peroxidase
- JA:
-
Jasmonic acid
- LPS's:
-
Lipopolysaccharides
- SA:
-
Salicylic acid
References
Abad P, Gouzy J, Aury J-M, Castagnone-Sereno P, Danchin EG, Deleury E et al (2008) Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol 26(8):909–915
Abd El-Aal EM, Shahen M, Sayed S, Kesba H, Ansari MJ, El-Ashry RM et al (2021) In vivo and in vitro management of Meloidogyne incognita (Tylenchida: Heteroderidae) using rhizosphere bacteria, Pseudomonas spp. and Serratia spp. compared with oxamyl. Saudi J Biol Sci 28(9):4876–4883
Abd El-Rahman A, Shaheen HA, Abd El-Aziz RM, Ibrahim DS (2019) Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egypt J Biol Pest Control 29(1):1–11
Abd-Elgawad MM (2021) Optimizing safe approaches to manage plant-parasitic nematodes. Plants 10(9):1911
Abd-Elgawad MM, Askary TH (2018) Fungal and bacterial nematicides in integrated nematode management strategies. Egypt J Biol Pest Control 28(1):1–24
Absmanner B, Stadler R, Hammes UZ (2013) Phloem development in nematode-induced feeding sites: the implications of auxin and cytokinin. Front Plant Sci 4:241
Aggangan NS, Tamayao PJS, Aguilar EA, Anarna JA, Dizon TO (2013) Arbuscular mycorrhizal fungi and nitrogen fixing bacteria as growth promoters and as biological control agents against nematodes in tissue-cultured banana var. Lakatan Phil J Sci 142(2):153–165
Agrios G (2005) Sclerotinia diseases. Plant pathology, 5th edn. Elsevier Academic Press, New York, pp 546–550
Aioub AA, El-Ashry RM, Hashem AS, Elesawy AE, Elsobki AE (2021) Compatibility of entomopathogenic nematodes with insecticides against the cabbage white butterfly, Pieris rapae L. (Lepidoptera: Pieridae). Egypt J Biol Pest Control 31(1):1–12
Alberton D, Valdameri G, Moure VR, Monteiro RA, Pedrosa FdO, Müller-Santos M et al (2020) What Did We Learn From Plant Growth-Promoting Rhizobacteria (PGPR)-Grass Associations Studies Through Proteomic and Metabolomic Approaches? Front Sustain Food Syst. https://doi.org/10.3389/fsufs.2020.607343
Ali AA, El-Ashry RM, Aioub AA (2021) Animal manure rhizobacteria co-fertilization suppresses phytonematodes and enhances plant production: evidence from field and greenhouse. J Plant Dis Protect 1:15
Almaghrabi OA, Massoud SI, Abdelmoneim TS (2013) Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi J Biol Sci 20(1):57–61
Almaghrabi OA, Abdelmoneim T, Albishri HM, Moussa T (2014) Enhancement of maize growth using some plant growth promoting rhizobacteria (PGPR) under laboratory conditions. Life Sci J 11(11):764–772
Alori ET, Glick BR, Babalola OO (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol 8:971. https://doi.org/10.3389/fmicb.2017.00971
Arseneault T, Filion M (2017) Biocontrol through antibiosis: exploring the role played by subinhibitory concentrations of antibiotics in soil and their impact on plant pathogens. Can J Plant Path 39(3):267–274
Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E et al (2018) Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front Plant Sci 9:1473. https://doi.org/10.3389/fpls.2018.01473
Bakr R, Mahdy M, Mousa E (2011) A survey of root-knot and citrus nematodes in some new reclaimed lands in Egypt. Pak J Nematol 29(2):165–170
Bakr RA, Mahdy MES, Mousa ESM (2020) Survey of root-knot nematodes Meloidogyne spp. associated with different economic crops and weeds in Egypt. Egypt J Crop Protect 15(2):1–14
Banerjee N, Hallem EA (2020) The role of carbon dioxide in nematode behaviour and physiology. Parasitology 147(8):841–854. https://doi.org/10.1017/S0031182019001422
Bapiri A, Asgharzadeh A, Mujallali H, Khavazi K, Pazira E (2012) Evaluation of zinc solubilization potential by different strains of fluorescent pseudomonads. J Appl Sci Environ Manag 16:3
Bartlem DG, Jones MG, Hammes UZ (2014) Vascularization and nutrient delivery at root-knot nematode feeding sites in host roots. J Exp Bot 65(7):1789–1798
Basu A, Prasad P, Das SN, Kalam S, Sayyed R, Reddy M et al (2021) Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: recent developments, constraints, and prospects. Sustainability 13(3):1140
Basyony AG, Abo-Zaid GA (2018) Biocontrol of the root-knot nematode, Meloidogyne incognita, using an eco-friendly formulation from Bacillus subtilis, lab. and greenhouse studies. Egypt J Biol Pest Control 28:1
Basyony A, Ibrahim IK, Zeyadah S, Kawanna MA (2020) Survey of plant parasitic nematode associated with spinach, Swiss chard and table beet in North Egypt. Alex Sci Exch J 41:471–477
Bechtaoui N, Raklami A, Tahiri AI, Benidire L, El Alaoui A, Meddich A et al (2019) Characterization of plant growth promoting rhizobacteria and their benefits on growth and phosphate nutrition of faba bean and wheat. Biol Open 8(7):bio043968
Beneduzi A, Ambrosini A, Passaglia LM (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35:1044–1051
Bernard GC, Egnin M, Bonsi C (2017) The impact of plant-parasitic nematodes on agriculture and methods of control. Nematol-Concepts, Diagnos Control 10(121):151
Bharali A, Bhagawati B, Uday K (2019) Bio-efficacy of native bioagents and biofertilizers for the management of root-knot nematode Meloidogyne incognita infecting black gram Vigna mungo. Int J Curr Microbiol App Sci 8(2):1484–1501
Bhuiyan S, Garlick K, Anderson J, Wickramasinghe P, Stirling G (2018) Biological control of root-knot nematode on sugarcane in soil naturally or artificially infested with Pasteuria penetrans. Australas Plant Pathol 47(1):45–52
Bishnoi U (2015) PGPR interaction: an ecofriendly approach promoting the sustainable agriculture system. Adv Bot Res 75:81–113
Burkett-Cadena M, Kokalis-Burelle N, Lawrence KS, Van Santen E, Kloepper JW (2008) Suppressiveness of root-knot nematodes mediated by rhizobacteria. Biol Control 47(1):55–59
Çakmakçi R, Dönmez F, Aydın A, Şahin F (2006) Growth promotion of plants by plant growth-promoting rhizobacteria under greenhouse and two different field soil conditions. Soil Biol Biochem 38(6):1482–1487
Castillo JD, Lawrence KS, Kloepper JW (2013) Biocontrol of the reniform nematode by Bacillus firmus GB-126 and Paecilomyces lilacinus 251 on cotton. Plant Dis 97(7):967–976
Chakraborty U, Chakraborty B, Basnet M (2006) Plant growth promotion and induction of resistance in Camellia sinensis by Bacillus megaterium. J Basic Microbiol 46(3):186–195
Chandler D, Bailey AS, Tatchell GM, Davidson G, Greaves J, Grant WP (2011) The development, regulation and use of biopesticides for integrated pest management. Philos Trans Royal Soc B: Biol Sci 366(1573):1987–1998
Chandra S, Askari K, Kumari M (2018) Optimization of indole acetic acid production by isolated bacteria from Stevia rebaudiana rhizosphere and its effects on plant growth. J Genet Eng Biotechnol 16(2):581–586. https://doi.org/10.1016/j.jgeb.2018.09.001
Chen Q, Liu S (2019) Identification and characterization of the phosphate-solubilizing bacterium Pantoea sp. S32 in reclamation soil in Shanxi China. Front Microbiol 10:2171
Ciancio A (2018) Biocontrol potential of Pasteuria spp. for the management of plant parasitic nematodes. CAB Rev 13:1–13
Cocking EC (2000) Helping plants get more nitrogen from the air. Eur Rev 8(2):193–200
Dash NP, Kumar A, Kaushik MS, Abraham G, Singh PK (2017) Agrochemicals influencing nitrogenase, biomass of N2-fixing cyanobacteria and yield of rice in wetland cultivation. Biocatal Agric Biotechnol 9:28–34
El-Ashry R, Ali MA, Elsobki AE, Aioub AA (2021) Integrated management of Meloidogyne incognita on tomato using combinations of abamectin, Purpureocillium lilacinum, rhizobacteria, and botanicals compared with nematicide. Egypt J Biol Pest Control 31(1):1–10
Eldeeb AM, Farag AAG, Al-Harbi MS, Kesba H, Sayed S, Elesawy AE et al (2022) Controlling of MELOIDGYNE incognita (Tylenchida: Heteroderidae) using nematicides, Linum usitatissimum extract and certain organic acids on four peppers cultivars under greenhouse conditions. Saudi J Biol Sci 29(5):3107–3113
El-Hadad M, Mustafa M, Selim SM, El-Tayeb T, Mahgoob A, Aziz NHA (2011) The nematicidal effect of some bacterial biofertilizers on Meloidogyne incognita in sandy soil. Braz J Microbiol 42:105–113
Elling AA (2013) Major emerging problems with minor Meloidogyne species. Phytopathology 103(11):1092–1102
El-Nagdi W, Abd-El-Khair H, Soliman G, Ameen H, El-Sayed GM (2019) Application of protoplast fusants of Bacillus licheniformis and Pseudomonas aeruginosa on Meloidogyne incognita in tomato and eggplant. Middle East J Appl Sci 9:622–629
Elnahal AS, El-Saadony MT, Saad AM, Desoky ESM, El-Tahan AM, Rady MM et al (2022) The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: a review. Eur J Plant Pathol 1:34
Elrys AS, Raza S, Abdo AI, Liu Z, Chen Z, Zhou J (2019) Budgeting nitrogen flows and the food nitrogen footprint of Egypt during the past half century: challenges and opportunities. Environ Int 130:104895
Elrys AS, Ali A, Zhang H, Cheng Y, Zhang J, Cai ZC et al (2021a) Patterns and drivers of global gross nitrogen mineralization in soils. Glob Change Biol 27(22):5950–5962
Elrys AS, Wang J, Metwally MA, Cheng Y, Zhang JB, Cai ZC et al (2021b) Global gross nitrification rates are dominantly driven by soil carbon-to-nitrogen stoichiometry and total nitrogen. Glob Change Biol 27(24):6512–6524
El-Saadony MT, Abuljadayel DA, Shafi ME, Albaqami NM, Desoky E-SM, El-Tahan AM et al (2021) Control of foliar phytoparasitic nematodes through sustainable natural materials: current progress and challenges. Saudi J Biol Sci 28(12):7314–7326. https://doi.org/10.1016/j.sjbs.2021.08.035
El-Sayed WS, Akhkha A, El-Naggar MY, Elbadry M (2014) In vitro antagonistic activity, plant growth promoting traits and phylogenetic affiliation of rhizobacteria associated with wild plants grown in arid soil. Front Microbiol 5:651
FAO, F. (2017). The future of food and agriculture–Trends and challenges. Annual Report, 296.
Fatima S, Anjum T (2017) Identification of a potential ISR determinant from pseudomonas aeruginosa PM12 against fusarium wilt in tomato. Front Plant Sci 8:848
Fujimoto T, Abe H, Mizukubo T, Seo S (2021) Phytol, a constituent of chlorophyll, induces root-knot nematode resistance in Arabidopsis via the ethylene signaling pathway. Mol Plant Microbe Interact 34(3):279–285
Geng C, Nie X, Tang Z, Zhang Y, Lin J, Sun M et al (2016) A novel serine protease, Sep1, from Bacillus firmus DS-1 has nematicidal activity and degrades multiple intestinal-associated nematode proteins. Sci Rep 6(1):1–12
Ghahremani Z, Escudero N, Beltrán-Anadón D, Saus E, Cunquero M, Andilla J et al (2020) Bacillus firmus strain I-1582, a nematode antagonist by itself and through the plant. Front Plant Sci 11:796
Gillet F-X, Bournaud C, de Souza A, Júnior JD, Grossi-de-Sa MF (2017) Plant-parasitic nematodes: towards understanding molecular players in stress responses. Ann Bot 119(5):775–789. https://doi.org/10.1093/aob/mcw260
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28(3):367–374
Gray E, Smith D (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol Biochem 37(3):395–412
Guang-Can T, Shu-Jun T, Miao-Ying C, Guang-Hui X (2008) Phosphate-solubilizing and-mineralizing abilities of bacteria isolated from soils. Pedosphere 18(4):515–523
Hackenberg C, Vrain TC, Sikora RA (1999) Rhizosphere colonization pattern of Agrobacterium radiobacter strain G12A, an antagonistic rhizobacterium to the potato cyst nematode Globodera pallida. Microbiol Res 154(1):57–61
Han H-S, Lee K (2006) Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ 52(3):130
Hasanuzzaman M, Bhuyan M, Nahar K, Hossain M, Mahmud JA, Hossen M et al (2018) Potassium: a vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 8(3):31
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Annals Microbiol 60(4):579–598
Ibrahim IK, Mokbel AA (2009) Occurrence and distribution of the root-knot nematodes Meloidogyne spp. and their host plants in Northern Egypt. Egypt J Exp Biol (botany) 5:1–7
Ibrahim I, Handoo Z, El-Sherbiny A (2000) A survey of phytoparasitic nematodes on cultivated and non-cultivated plants in Northwestern Egypt. J Nematol 32(4S):478
Ibrahim, I, & Handoo, Z.(2015) Survey of phytoparasitic nematodes associated with some crop plants in Northern Egypt. In J Nemat, (Vol. 47, pp. 247–247, Vol. 3): SOC Nematologists PO BOX 311, Marceline, MO 64658 USA
Ibrahim, I. (1994) Potentially important phytoparasitic nematodes in agroforestry plantations and the associated host plants. In: 1 International Symposium on Silviculture of Protection Foresty in Arid Regions and the Agroforestry Potential, Alexandria (Egypt), 21–24 Mar 1994
Ilyas, N, Mumtaz, K, Akhtar, N, Yasmin, H, Sayyed, R, Khan, W, et al. (2020) Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability, 12(21) 8876
Ismail A, Mahfouz S, Mohamed M, Hassan RA, Abou-Setta LM (2014) Environmentally safe substances for controlling the root-knot nematode, Meloidogyne arenaria infested potato and their influence on yield and alkaloidal content in Egypt. Arch of Phytopathol Plant Prot 47(5):600–609
Jang JH, Woo SY, Kim SH, Khaine I, Kwak MJ, Lee HK et al (2017) Effects of increased soil fertility and plant growth-promoting rhizobacteria inoculation on biomass yield, energy value, and physiological response of poplar in short-rotation coppices in a reclaimed tideland: A case study in the Saemangeum area of Korea. Biomass Bioenergy 107:29–38
Jiang C, Fan Z, Li Z, Niu D, Li Y, Zheng M et al (2020) Bacillus cereus AR156 triggers induced systemic resistance against Pseudomonas syringae pv. tomato DC3000 by suppressing miR472 and activating CNLs-mediated basal immunity in Arabidopsis. Mol Plant Pathol 21(6):854–870
Kalam, S, Basu, A, & Ankati, S. (2017). Plant root-associated biofilms in bioremediation. Biofilms in Plant and Soil Health; Ahmad, I, Husain, FM (eds) 337 355
Kamal R, Gusain YS, Kumar V (2014) Interaction and symbiosis of AM fungi, actinomycetes and plant growth promoting rhizobacteria with plants: strategies for the improvement of plants health and defense system. Int J Curr Microbial Appl Sci 3(7):564–585
Katooli N, Moghadam E, Taheri A, Nasrollahnejad S (2010) Management of root-knot nematode (Meloidogyne incognita) on cucumber with the extract and oil of nematicidal plants. Int J Agric Res 5(8):582–586
Kawanobe M, Sugihara S, Miyamaru N, Yoshida K, Nonomura E, Oshiro H et al (2020) Distribution of root-lesion and stunt nematodes, and their relationship with soil properties and nematode fauna in sugarcane fields in Okinawa. Japan Agron 10(6):762
Khan MS, Zaidi A, Wani P, Ahemad M, Oves M (2009) Functional diversity among plant growth-promoting rhizobacteria: current status. Microbial strategies for crop improvement. Springer, Newyork, pp 105–132
Khan MR, Mohidin FA, Khan U, Ahamad F (2016) Native Pseudomonas spp. suppressed the root-knot nematode in in vitro and in vivo, and promoted the nodulation and grain yield in the field grown mungbean. Biol Control 101:159–168
Khanna K, Jamwal VL, Sharma A, Gandhi SG, Ohri P, Bhardwaj R et al (2019a) Evaluation of the role of rhizobacteria in controlling root-knot nematode infection in Lycopersicon esculentum plants by modulation in the secondary metabolite profiles. AoB Plants 11(6):plz069
Khanna K, Sharma A, Ohri P, Bhardwaj R, Abd-Allah EF, Hashem A et al (2019b) Impact of plant growth promoting rhizobacteria in the orchestration of Lycopersicon esculentum mill resistance to plant parasitic nematodes: a metabolomic approach to evaluate defense responses under field conditions. Biomolecules 9(11):676
Kloepper J, Schroth M (1981) Plant growth-promoting rhizobacteria and plant growth under gnotobiotic conditions. Phytopathology 71(6):642–644
Köhl J, Kolnaar R, Ravensberg WJ (2019) Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front Plant Sci 10:845
Kokalis-Burelle N (2015) Pasteuria penetrans for control of Meloidogyne incognita on tomato and cucumber, and M. arenaria on snapdragon. J Nematol 47(3):207
Kokalis-Burelle N, Vavrina C, Reddy M, Kloepper J (2003) Amendment of muskmelon and watermelon transplant media with plant growth-promoting rhizobacteria: effects on seedling quality, disease, and nematode resistance. HortTechnology 13(3):476–482
Kokalis-Burelle N, Kloepper J, Reddy M (2006) Plant growth-promoting rhizobacteria as transplant amendments and their effects on indigenous rhizosphere microorganisms. Appl Soil Ecol 31(1–2):91–100
Korayem A, Youssef M, Mohamed M, Lashein A (2014) A survey of plant parasitic nematodes associated with different plants in North Sinai. Mid East J Agric Res 3(3):522–529
Krif G, Mokrini F, Aissami AE, Laasli S-E, Imren M, Özer G et al (2020) Diversity and management strategies of plant parasitic nematodes in Moroccan organic farming and their relationship with soil physico-chemical properties. Agriculture 10(10):447
Kumar A, Maurya BR, Raghuwanshi R (2015) Characterization of bacterial strains and their impact on plant growth promotion and yield of wheat and microbial populations of soil. Afr J Agric Res 10(12):1367–1375
Landis, DA, & Orr, DB. (1996). Biological control: approaches and applications. Electronic IPM Textbook
Lastochkina O, Pusenkova L, Yuldashev R, Babaev M, Garipova S, Blagova D et al (2017) Effects of Bacillus subtilis on some physiological and biochemical parameters of Triticum aestivum L. (wheat) under salinity. Plant Physiol Biochem 121:80–88
Lawrence, K, Hagan, A, Norton, R, Hu, J, Faske, T, Hutmacher, R, et al. Cotton disease loss estimate committee report, 2014. In Proceedings of the 2015 Beltwide Cotton Conferences, San Antonio, TX. Cordova: National Cotton Council, 2015 (pp. 188–190)
Lee DL (2002) The biology of nematodes. CRC Press, United States
Lee YS, Nguyen XH, Naing KW, Park YS, Kim KY (2015) Role of lytic enzymes secreted by Lysobacter capsici YS1215 in the control of root-knot nematode of tomato plants. Indian J Microbiol 55(1):74–80
Li J, Zou C, Xu J, Ji X, Niu X, Yang J et al (2015) Molecular mechanisms of nematode-nematophagous microbe interactions: basis for biological control of plant-parasitic nematodes. Annu Rev Phytopathol 53:67–95
Li X, Hu H-J, Li J-Y, Wang C, Chen S-L, Yan S-Z (2019) Effects of the endophytic bacteria Bacillus cereus BCM2 on tomato root exudates and Meloidogyne incognita infection. Plant Dis 103(7):1551–1558
Liang L-M, Zou C-G, Xu J, Zhang K-Q (2019) Signal pathways involved in microbe–nematode interactions provide new insights into the biocontrol of plant-parasitic nematodes. Philos Trans R Soc B 374(1767):20180317
Liao C, Tian Q, Liu F (2021) Nitrogen availability regulates deep soil priming effect by changing microbial metabolic efficiency in a subtropical forest. J for Res 32(2):713–723
Lopes EP, Ribeiro RCF, Xavier AA, Alves RM, de Castro MT, Martins MJ et al (2019) Effect of Bacillus subtilis on Meloidogyne javanica and on tomato growth promotion. J Exp Agric Int 1:8
Mahler, R. L. (2004). Nutrients plants require for growth, university of Idaho, college of agricultural and life science
Maldonado S, Rodríguez A, Ávila B, Morales P, González MP, Araya Angel JPA et al (2020) Enhanced crop productivity and sustainability by using native phosphate solubilizing rhizobacteria in the agriculture of arid zones. Front Sustain Food Syst 4:263. https://doi.org/10.3389/fsufs.2020.607355
Malhotra H, Sharma S, Pandey R (2018) Phosphorus nutrition: plant growth in response to deficiency and excess. Plant nutrients and abiotic stress tolerance. Springer, Singapore, pp 171–190
Meena KS, Jonathan E, Devrajan K, Raguchander T (2012) Pseudomonas fluorescens induced systemic resistance in tomato against Meloidogyne incognita. Indian J Nematol 42(1):5–10
Mena J, Pimentel E, Hernández A, Veloz L, Vázquez R, León L et al (2002) Mechanism of action of Corynebacterium pauronetabolum strain C-924 on nematodes. Nematology 4(2):287
Meyer SL, Halbrendt JM, Carta LK, Skantar AM, Liu T, Abdelnabby HM et al (2009) Toxicity of 2, 4-diacetylphloroglucinol (DAPG) to plant-parasitic and bacterial-feeding nematodes. J Nematol 41(4):274
Migunova VD, Sasanelli N (2021) Bacteria as biocontrol tool against phytoparasitic nematodes. Plants 10(2):389. https://doi.org/10.3390/plants10020389
Mohammadi MA, Han X, Zhang Z, Xi Y, Boorboori M, Wang-Pruski G (2020) Phosphite application alleviates Pythophthora infestans by modulation of photosynthetic and physio-biochemical metabolites in potato leaves. Pathogens 9(3):170
Mohammed S, El Saedy MA, Enan MR, Ibrahim NE, Ghareeb A, Moustafa SA (2008) Biocontrol efficiency of Bacillus thuringiensis toxins against root-knot nematode, Meloidogyne incognita. J Cell Mol Biol 7(1):57–66
Myo EM, Ge B, Ma J, Cui H, Liu B, Shi L et al (2019) Indole-3-acetic acid production by Streptomyces fradiae NKZ-259 and its formulation to enhance plant growth. BMC Microbiol 19(1):1–14
Nascimento FX, Vicente CS, Barbosa P, Espada M, Glick BR, Mota M et al (2013) Evidence for the involvement of ACC deaminase from Pseudomonas putida UW4 in the biocontrol of pine wilt disease caused by Bursaphelenchus xylophilus. Biocontrol 58(3):427–433
Nicol J, Turner S, Coyne D, den Nijs L, Hockland S, Maafi ZT (2011) Current nematode threats to world agriculture. Genomics and molecular genetics of plant-nematode interactions. Springer, Dordrecht, pp 21–43
Nikoo, FS, Sahebani, N, Aminian, H, Mokhtarnejad, L, and Ghaderi, R. (2014). Induction of systemic resistance and defense-related enzymes in tomato plants using Pseudomonas fluorescens CHAO and salicylic acid against root-knot nematode Meloidogyne javanica. J Plant Prot Res, 15(4)
Nivya R (2015) A study on plant growth promoting activity of the endophytic bacteria isolated from the root nodules of Mimosa pudica plant. Int J Innov Res Sci Eng Technol 4:6959–6968
Norton J, Ouyang Y (2019) Controls and Adaptive Management of Nitrification in Agricultural Soils. Front Microbiol 10:1931. https://doi.org/10.3389/fmicb.2019.01931
Oleńska E, Małek W, Wójcik M, Swiecicka I, Thijs S, Vangronsveld J (2020) Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: a methodical review. Sci Total Environ 743:140682. https://doi.org/10.1016/j.scitotenv.2020.140682
Ordookhani K, Sharafzadeh S, Zare M (2011) Influence of PGPR on growth, essential oil and nutrients uptake of sweet basil. Adv Environ Biol 5(4):672–677
Pal, KK, & Gardener, BM. (2006). Biological control of plant pathogens
Palomares-Rius JE, Escobar C, Cabrera J, Vovlas A, Castillo P (2017) Anatomical alterations in plant tissues induced by plant-parasitic nematodes. Front Plant Sci 8:1987–1987. https://doi.org/10.3389/fpls.2017.01987
Perry R, Moens M (2011) Introduction to Plant-Parasitic Nematodes; Modes of Parasitism. Springer, Dordrecht, pp 3–20
Pokhare, S, Shakil, N, Kumar, J, & Singh, K. (2012). Foliar application of chemical elicitors induces biochemical changes in wheat against the cereal cyst nematode, Heterodera Avenae. Nematologia Mediterranea
Press M, Phoenix G (2005) Impacts of parasitic plants on natural communities: Tansley review. New Phytol 166:737–751. https://doi.org/10.1111/j.1469-8137.2005.01358.x
Proença DN, Heine T, Senges CHR, Bandow JE, Morais PV, Tischler D (2019) Bacterial metabolites produced under iron limitation kill pinewood nematode and attract caenorhabditis elegans. Front Microbiol 10:2166. https://doi.org/10.3389/fmicb.2019.02166
Raddy H, Fouad A, Montasser S, Abdel-Lateef M, El-Samadisy A (2013) Efficacy of six nematicides and six commercial bioproducts against root-knot nematode, Meloidogyne incognita on tomato. J Appl Sci Res 9(7):4410–4417
Radhakrishnan R, Hashem A, Abd-Allah EF (2017) Bacillus: a biological tool for crop improvement through bio-molecular changes in adverse environments. Front Physiol 8:667
Rahul S, Chandrashekhar P, Hemant B, Chandrakant N, Laxmikant S, Satish P (2014) Nematicidal activity of microbial pigment from Serratia marcescens. Nat Prod Res 28(17):1399–1404
Rai KM, Balasubramanian VK, Welker CM, Pang M, Hii MM, Mendu V (2015) Genome wide comprehensive analysis and web resource development on cell wall degrading enzymes from phyto-parasitic nematodes. BMC Plant Biol 15:187–187. https://doi.org/10.1186/s12870-015-0576-4
Raj D, Linda R, Babyson RS (2014) Molecular characterization of phosphate solubilizing bacteria (PSB) and plant growth promoting rhizobacteria (PGPR) from pristine soils. Int J Innov Sci Eng Technol 1:317–324
Raymond J, Siefert JL, Staples CR, Blankenship RE (2004) The natural history of nitrogen fixation. Mol Biol Evol 21(3):541–554
Rehman B, Ganai MA, Parihar K, Siddiqui MA, Usman A (2012) Management of root knot nematode, meloidogyne incognita affecting chickpea, Cicer arietinum for sustainable production. Biosci Int 1(1):1–5
Reitz M, Rudolph K, Schroder I, Hoffmann-Hergarten S, Hallmann J, Sikora R (2000) Lipopolysaccharides of Rhizobium etli strain G12 act in potato roots as an inducing agent of systemic resistance to infection by the cyst nematode Globodera pallida. Appl Environ Microbiol 66(8):3515–3518
Reshma, P, Naik, M, Aiyaz, M, Niranjana, S, Chennappa, G, Shaikh, S, et al. (2018). Induced systemic resistance by 2, 4-diacetylphloroglucinol positive fluorescent Pseudomonas strains against rice sheath blight
Rizvi R, Mahmood I, Tiyagi SA, Khan Z (2012) Conjoint effect of oil-seed cakes and Pseudomonas fluorescens on the growth of chickpea in relation to the management of plant-parasitic nematodes. Braz Arch Biol Technol 55(6):801–808
Romeh A, Hendawi M (2017) Biochemical interactions between Glycine max L silicon dioxide (SiO2) and plant growth-promoting bacteria (PGPR) for improving phytoremediation of soil contaminated with fenamiphos and its degradation products. Pestic Biochem Physiol 142(32):43. https://doi.org/10.1016/j.pestbp.2017.01.001
Sagar A, Sayyed R, Ramteke P, Sharma S, Marraiki N, Elgorban AM et al (2020) ACC deaminase and antioxidant enzymes producing halophilic Enterobacter sp PR14 promotes the growth of rice and millets under salinity stress. Physiol Mol Biol Plants 26(9):1847–1854
Saravanakumar D, Lavanya N, Muthumeena B, Raguchander T, Suresh S, Samiyappan R (2008) Pseudomonas fluorescens enhances resistance and natural enemy population in rice plants against leaffolder pest. J Appl Entomol 132(6):469–479
Sato K, Kadota Y, Shirasu K (2019) Plant immune responses to parasitic nematodes. Front Plant Sci 10:1165–1165. https://doi.org/10.3389/fpls.2019.01165
Schwabe R, Senges CHR, Bandow JE, Heine T, Lehmann H, Wiche O et al (2020) Cultivation dependent formation of siderophores by Gordonia rubripertincta CWB2. Microbiol Res 238:126481
Seenivasan N, Devrajan K, Selvaraj N (2007) Management of potato cyst nematodes, Globodera spp. through biological control. Indian J Nematol 37(1):27–29
Shukla V, Barberon M (2021) Building and breaking of a barrier: suberin plasticity and function in the endodermis. Curr Opin Plant Biol 64:102153. https://doi.org/10.1016/j.pbi.2021.102153
Siddiqui ZA, Mahmood I (2001) Effects of rhizobacteria and root symbionts on the reproduction of Meloidogyne javanica and growth of chickpea. Biores Technol 79(1):41–45
Siddiqui I, Shaukat S (2004) Systemic resistance in tomato induced by biocontrol bacteria against the root-knot nematode, Meloidogyne javanica is independent of salicylic acid production. J Phytopathol 152(1):48–54
Singh P, Siddiqui ZA (2010) Biocontrol of root-knot nematode Meloidogyne incognita by the isolates of Pseudomonas on tomato. Arch Phytopathol Plant Protect 43(14):1423–1434
Singh S, Singh B, Singh A (2015) Nematodes: a threat to sustainability of agriculture. Procedia Environ Sci 29:215–216
Singh HB, Sarma BK, Keswani C (2017) Advances in PGPR research. CABI, UK
Smant G, Helder J, Goverse A (2018) Parallel adaptations and common host cell responses enabling feeding of obligate and facultative plant parasitic nematodes. Plant J 93(4):686–702
Subedi P, Gattoni K, Liu W, Lawrence KS, Park S-W (2020) Current utility of plant growth-promoting rhizobacteria as biological control agents towards plant-parasitic nematodes. Plants 9(9):1167. https://doi.org/10.3390/plants9091167
Sun X, Zhang R, Ding M, Liu Y, Li L (2021) Biocontrol of the root-knot nematode Meloidogyne incognita by a nematicidal bacterium Pseudomonas simiae MB751 with cyclic dipeptide. Pest Manag Sci 77(10):4365–4374
Terefe M, Tefera T, Sakhuja P (2009) Effect of a formulation of Bacillus firmus on root-knot nematode Meloidogyne incognita infestation and the growth of tomato plants in the greenhouse and nursery. J Invertebr Pathol 100(2):94–99
Teymouri M, Akhtari J, Karkhane M, Marzban A (2016) Assessment of phosphate solubilization activity of Rhizobacteria in mangrove forest. Biocatal Agric Biotechnol 5:168–172
Tian B, Yang J, Zhang K-Q (2007) Bacteria used in the biological control of plant-parasitic nematodes: populations, mechanisms of action, and future prospects. FEMS Microbiol Ecol 61(2):197–213
Timper P, Kone D, Yin J, Ji P, Gardener BBM (2009) Evaluation of an antibiotic-producing strain of Pseudomonas fluorescens for suppression of plant-parasitic nematodes. J Nematol 41(3):234
Tranier MS, Pognant-Gros J, Quiroz RDLC, González CNA, Mateille T, Roussos S (2014) Commercial biological control agents targeted against plant-parasitic root-knot nematodes. Braz Arch Biol Technol 57:831–841
Tsai AY-L, Higaki T, Nguyen C-N, Perfus-Barbeoch L, Favery B, Sawa S (2019) Regulation of root-knot nematode behavior by seed-coat mucilage-derived attractants. Mol Plant 12(1):99–112
Tsukanova K, Meyer J, Bibikova T (2017) Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S Afr J Bot 113:91–102
Tülek A, Kepenekçi İI, Oksal E, Hazir S (2018) Comparative effects of entomopathogenic fungi and nematodes and bacterial supernatants against rice white tip nematode. Egypt J Biol Pest Control 28(1):1–6
Turan, M, Arjumend, T, Argın, S, Yıldırım, E, Katırcıoğlu, H, Gürkan, B, et al. (2021). Plant Root Enhancement by Plant Growth Promoting Rhizobacteria. Plant Roots, vol. 2021
Turatto MF, Dourado FdS, Zilli JE, Botelho GR (2018) Control potential of Meloidogyne javanica and Ditylenchus spp. using fluorescent Pseudomonas and Bacillus spp. Braz J Microbiol 49:54–59
Vagelas IK, Dennett MD, Pembroke B, Gowen SR (2012) Adhering Pasteuria penetrans endospores affect movements of root-knot nematode juveniles. Phytopathol Mediterr 51(3):618–624
van Beelen P, Fleuren-Kemilä AK (1997) Influence of pH on the toxic effects of zinc, cadmium, and pentachlorophenol on pure cultures of soil microorganisms. Environ Toxicol Chem: an Int J 16(2):146–153
Vejan P, Abdullah R, Khadiran T, Ismail S, Nasrulhaq Boyce A (2016) Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Molecules 21(5):573
Viljoen JJ, Labuschagne N, Fourie H, Sikora RA (2019) Biological control of the root-knot nematode Meloidogyne incognita on tomatoes and carrots by plant growth-promoting rhizobacteria. Trop Plant Pathol 44(3):284–291
Wang J, Li R, Zhang H, Wei G, Li Z (2020) Beneficial bacteria activate nutrients and promote wheat growth under conditions of reduced fertilizer application. BMC Microbiol 20(1):1–12
Wendehenne D, Gao Q-M, Kachroo A, Kachroo P (2014) Free radical-mediated systemic immunity in plants. Curr Opin Plant Biol 20:127–134
Won S-J, Moon J-H, Ajuna HB, Choi S-I, Maung CEH, Lee S et al (2021) Biological control of leaf blight disease caused by Pestalotiopsis maculans and growth promotion of Quercus acutissima Carruth container seedlings using Bacillus velezensis CE 100. Int J Mol Sci 22(20):11296
Xiang N, Lawrence KS, Kloepper JW, Donald PA, McInroy JA, Lawrence GW (2017) Biological control of Meloidogyne incognita by spore-forming plant growth-promoting rhizobacteria on cotton. Plant Dis 101(5):774–784
Xiang N, Lawrence KS, Donald PA (2018) Biological control potential of plant growth-promoting rhizobacteria suppression of Meloidogyne incognita on cotton and Heterodera glycines on soybean: a review. J Phytopathol 166(7–8):449–458
Xie S, Jiang H, Ding T, Xu Q, Chai W, Cheng B (2018) Bacillus amyloliquefaciens FZB42 represses plant miR846 to induce systemic resistance via a jasmonic acid-dependent signalling pathway. Mol Plant Pathol 19(7):1612–1623
Youssef M, Abd Abd-El-Khair H, El-Nagdi WM (2017) Management of root knot nematode, Meloidogyne incognita infecting sugar beet as affected by certain bacterial and fungal suspensions. Agric Eng Int: CIGR J 293:301
Zasada I, Ferris H (2003) Sensitivity of Meloidogyne javanica and Tylenchulus semipenetrans to isothiocyanates in laboratory assays. Phytopathology 93(6):747–750
Zhang S, Gan Y, Ji W, Xu B, Hou B, Liu J (2017) Mechanisms and characterization of Trichoderma longibrachiatum T6 in suppressing nematodes (Heterodera avenae) in wheat. Front Plant Sci 8:1491
Acknowledgements
Not applicable
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A. Aioub collected and summarized the results. A. Elesawy has conducted the systematic literature review. E. Ammar has participated in writing the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aioub, A.A.A., Elesawy, A.E. & Ammar, E.E. Plant growth promoting rhizobacteria (PGPR) and their role in plant-parasitic nematodes control: a fresh look at an old issue. J Plant Dis Prot 129, 1305–1321 (2022). https://doi.org/10.1007/s41348-022-00642-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-022-00642-3