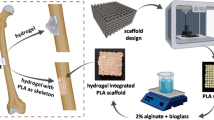
Abstract
Purpose
Synthetic polymers such as poly(lactic acid) (PLA) are well suited for preparing patient-specific bone tissue scaffolds by three-dimensional (3D) printing due to their favorable mechanical properties; however, they have limited biological activity. Natural polymers have good bioactivity and provide a better cellular microenvironment for attachment, proliferation, and differentiation, but lack the mechanical strength required as a bone substitute.
Method
In this work, porous PLA scaffolds were prepared by fused filament fabrication. For uniform cell seeding and enhanced cellular function, a silk fibroin-alginate (SF/Alg) blend hydrogel loaded with human mesenchymal cells (hMSCs) was loaded into the pores of the 3D-printed hybrid scaffolds between the struts. The physicochemical properties of the scaffold and the hMSC response were characterized.
Results
The gel-loaded 3D-printed PLA scaffolds were stable over 21 days in an aqueous buffer solution. The compressive strength of the scaffolds was ≈ 10 MPa, which is similar to that of cancellous bone. The proliferation and viability of hMSCs were significantly enhanced when loaded within the SF/Alg hydrogel in the PLA scaffolds than in the neat PLA scaffold. Furthermore, the stem cells in the gel-loaded 3D-printed PLA scaffold showed markedly higher alkaline phosphatase expression and calcium phosphate deposition, which indicates higher osteogenic differentiation with the gels. These observations were corroborated by increased expressions of osteocalcin, RUNX2, and BMP-2.
Conclusion
Thus, the combination of SF/Alg hydrogel loaded with stem cells offers a promising route for enhancing the bioactivity of 3D-printed PLA scaffolds with significant clinical potential for bone tissue engineering.
Lay Summary
Owing to their good mechanical stability, 3D-printed porous scaffolds of thermoplastics such as PLA have been used for bone tissue engineering applications. However, the presence of macro-sized pores leads to low cell attachment efficiency and distribution within the scaffolds, which results in low osteogenic activity. In this work, stem cells were encapsulated within the sonicated silk fibroin and alginate blend hydrogel and embedded within the gaps of 3D-printed PLA struts. This hybrid approach maximizes the cell density and uniform distribution and leverages the mechanical integrity of the 3D-printed PLA scaffold and osteoconductive microenvironment for proliferation and differentiation offered by the silk fibroin and alginate hydrogel. The cell-laden gels loaded within 3D-printed scaffold showed improved proliferation and osteogenic activity of stem cells, which makes the system a promising bone substitute for regeneration or healing.






Similar content being viewed by others
References
Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9(1):1–10.
Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev™ Biomed Eng. 2012;40(5):363–408.
Orciani M, Fini M, Di Primio R, Mattioli-Belmonte M. Biofabrication and bone tissue regeneration: cell source, approaches, and challenges. Front Bioeng Biotechnol. 2017;5:17.
Kinoshita Y, Maeda H. Recent developments of functional scaffolds for craniomaxillofacial bone tissue engineering applications. Sci World J. 2013;2013:863157.
Perez JR, Kouroupis D, Li DJ, Best TM, Kaplan L, Correa D. Tissue engineering and cell-based therapies for fractures and bone defects. Front Bioeng Biotechnol. 2018;6:105.
Melke J, Midha S, Ghosh S, Ito K, Hofmann S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016;31:1–16.
Tang G, Liu Z, Liu Y, Yu J, Wang X, Tan Z, Ye X. Recent trends in the development of bone regenerative biomaterials. Front Cell Dev Biol. 2021;9:665813.
Mravic M, Péault B, James AW. Current trends in bone tissue engineering. BioMed Res Int. 2014;2014:865270.
Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30(10):546–54.
Nikolova MP, Chavali MS. Recent advances in biomaterials for 3D scaffolds: a review. Bioactive materials. 2019;4:271–92.
Buyuksungur S, Hasirci V, Hasirci N. 3D printed hybrid bone constructs of PCL and dental pulp stem cells loaded GelMA. J Biomed Mater Res, Part A. 2021;109(12):2425–37.
Haleem A, Javaid M, Khan RH, Suman R. 3D printing applications in bone tissue engineering. J Clin Orthop Trauma. 2020;11:S118–24.
Su X, Wang T, Guo S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen Ther. 2021;16:63–72.
Buj-Corral I, Bagheri A, Petit-Rojo O. 3D printing of porous scaffolds with controlled porosity and pore size values. Materials. 2018;11(9):1532.
Rezania N, Asadi-Eydivand M, Abolfathi N, Bonakdar S, Mehrjoo M, Solati-Hashjin M. Three-dimensional printing of polycaprolactone/hydroxyapatite bone tissue engineering scaffolds mechanical properties and biological behavior. J Mater Sci - Mater Med. 2022;33(3):1–14.
Kumar A, Kargozar S, Baino F, Han SS. Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds: a review. Front Mater. 2019;6:313.
Chung JJ, Im H, Kim SH, Park JW, Jung Y. Toward biomimetic scaffolds for tissue engineering: 3D printing techniques in regenerative medicine. Front Bioeng Biotechnol. 2020;8:586406.
Gregor A, Filová E, Novák M, Kronek J, Chlup H, Buzgo M, Blahnová V, Lukášová V, Bartoš M, Nečas A. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J Biol Eng. 2017;11(1):1–21.
Jaidev L, Chatterjee K. Surface functionalization of 3D printed polymer scaffolds to augment stem cell response. Mater Des. 2019;161:44–54.
Nilawar S, Chatterjee K. Surface decoration of redox-modulating nanoceria on 3D-printed tissue scaffolds promotes stem cell osteogenesis and attenuates bacterial colonization. Biomacromolecules. 2021;23(1):226–39.
Zhang B, Wang L, Song P, Pei X, Sun H, Wu L, Zhou C, Wang K, Fan Y, Zhang X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater Des. 2021;201:109490.
Liu Q, Li Q, Xu S, Zheng Q, Cao X. Preparation and properties of 3D printed alginate–chitosan polyion complex hydrogels for tissue engineering. Polymers. 2018;10(6):664.
Bakhtiary N, Liu C, Ghorbani F. Bioactive inks development for osteochondral tissue engineering: a mini-review. Gels. 2021;7(4):274.
Midha S, Murab S, Ghosh S. Osteogenic signaling on silk-based matrices. Biomaterials. 2016;97:133–53.
Midha S, Chameettachal S, Dey E, Ghosh S. Nonmulberry silk braids direct terminal osteocytic differentiation through activation of wnt-signaling. ACS Biomater Sci Eng. 2017;3(6):1062–74.
Wang Y, Wang X, Shi J, Zhu R, Zhang J, Zhang Z, Ma D, Hou Y, Lin F, Yang J. A biomimetic silk fibroin/sodium alginate composite scaffold for soft tissue engineering. Sci Rep. 2016;6(1):1–13.
Ming J, Zuo B. A novel silk fibroin/sodium alginate hybrid scaffolds. Polym Eng Sci. 2014;54(1):129–36.
Rajput M, Bhandaru N, Barui A, Chaudhary A, Paul RR, Mukherjee R, Chatterjee J. Nano-patterned honey incorporated silk fibroin membranes for improving cellular compatibility. RSC Adv. 2014;4(84):44674–88.
Silva R, Singh R, Sarker B, Papageorgiou DG, Juhasz JA, Roether JA, Cicha I, Kaschta J, Schubert DW, Chrissafis K. Soft-matrices based on silk fibroin and alginate for tissue engineering. Int J Biol Macromol. 2016;93:1420–31.
Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29(8):1054–64.
Kadakia P, Jain E, Hixon K, Eberlin C, Sell S. Sonication induced silk fibroin cryogels for tissue engineering applications. Mater Res Exp. 2016;3(5):055401.
Yucel T, Cebe P, Kaplan DL. Vortex-induced injectable silk fibroin hydrogels. Biophys J. 2009;97(7):2044–50.
Zhou C, Ma H. Ultrasonic degradation of polysaccharide from a red algae (Porphyra yezoensis). J Agric Food Chem. 2006;54(6):2223–8.
Prins H-J, Braat AK, Gawlitta D, Dhert WJ, Egan DA, Tijssen-Slump E, Yuan H, Coffer PJ, Rozemuller H, Martens AC. In vitro induction of alkaline phosphatase levels predicts in vivo bone forming capacity of human bone marrow stromal cells. Stem Cell Res. 2014;12(2):428–40.
BouAssaf R, Fayyad-Kazan M, Al-Nemer F, Makki R, Fayyad-Kazan H, Badran B, Berbéri A. Evaluation of the osteogenic potential of different scaffolds embedded with human stem cells originated from Schneiderian membrane: an in vitro study. BioMed Res Int. 2019;2019:1–10.
Zou L, Kidwai FK, Kopher RA, Motl J, Kellum CA, Westendorf JJ, Kaufman DS. Use of RUNX2 expression to identify osteogenic progenitor cells derived from human embryonic stem cells. Stem Cell Reports. 2015;4(2):190–8.
Wang L-T, Lee Y-W, Bai C-H, Chiang H-C, Wang H-H, Yen BL, Yen M-L. A rapid and highly predictive in vitro screening platform for osteogenic natural compounds using human runx2 transcriptional activity in mesenchymal stem cells. Front Cell Dev Biol. 2020;8:607383.
De Giglio E, Bonifacio MA, Ferreira AM, Cometa S, Ti ZY, Stanzione A, Dalgarno K, Gentile P. Multi-compartment scaffold fabricated via 3D-printing as in vitro co-culture osteogenic model. Sci Rep. 2018;8(1):1–13.
Cai H, Zou J, Wang W, Yang A. BMP2 induces hMSC osteogenesis and matrix remodeling. Mol Med Rep. 2021;23(2):1–1.
Mandal BB, Kundu S. Non-mulberry silk gland fibroin protein 3-D scaffold for enhanced differentiation of human mesenchymal stem cells into osteocytes. Acta Biomater. 2009;5(7):2579–90.
Acknowledgements
The authors acknowledge Anindo Roy for SEM imaging.
Funding
This work was supported by the Department of Science and Technology (DST), Government of India (DST/NM/NB/2018/119(G)).
Author information
Authors and Affiliations
Contributions
M.R. and K.C. designed the study. M.R. performed the experiments, analyzed the data, and prepared the first draft of the manuscript. S.N. performed XRD and FTIR experiments and assisted in 3D printing. K.C. edited the manuscript and is the senior author overseeing the work.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajput, M., Nilawar, S. & Chatterjee, K. Embedding Silk Fibroin-Alginate Hydrogel in a 3D-Printed Porous Poly(Lactic Acid) Bone Tissue Scaffold Augments Stem Cell Function. Regen. Eng. Transl. Med. 9, 384–396 (2023). https://doi.org/10.1007/s40883-022-00286-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-022-00286-7