Abstract
Purpose
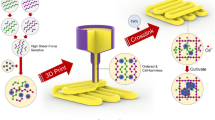
Three-dimensional (3D) bioprinting offers great potentials in rebuilding tissue mimics through engineering cell-laden constructs. Recently, the unique ability of a new type of micropore-forming bioink developed by us, containing two immiscible aqueous phases of gelatin methacryloyl (GelMA) and poly(ethylene oxide) (PEO), has become attractive since it promotes cellular behaviors. Nevertheless, this initial version of our two-phase aqueous emulsion bioink is generally unstable when experiencing prolonged storage times at room temperature, whereby it will phase-segregate and lose the micropore-forming capacity. This phase-segregation may lead to insufficient operational time window for bioprinting, especially for modalities that require a liquid-phase bioink such as digital light processing.
Methods
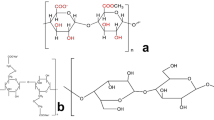
In this study, we report the development of a set of biosurfactant (rhamnolipids)-stabilized micropore-forming GelMA-based inks, with the goal of significantly improving their shelf-lives with enhanced applicability towards 3D printing.
Results
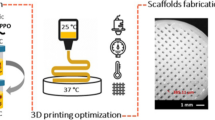
It was observed that the printed constructs using rhamnolipid-stabilized micropore-forming inks, either prepared fresh or stored for hours at room temperature, presented similar microporous structures. In contrast, the micropore-forming inks without biosurfactant-incorporation exhibited severely reduced performances after prolonged storage owing to marked phase-segregation.
Conclusion
Our study suggests that biosurfactant-incorporation enhanced stability of our micropore-forming GelMA inks and therefore present a wide range of possibilities in further development of two-phase aqueous emulsion inks and bioinks for future 3D printing and bioprinting applications.
Lay Summary
Three-dimensional (3D) bioprinting offers a collection of enabling technologies to address regenerative engineering and translational medicine problems, by allowing precisely controlled, automated fabrication of volumetric tissue constructs that are both structurally and functionally relevant to their counterparts in the human body. The biomaterials used for bioprinting are of significant importance to ensure proper tissue-production and maturation. We report a micropore-forming ink that is stabilized by biologically derived surfactant, in an effort to promote the stability of the resulting porous structures in 3D-printed architectures, for potential applications in tissue engineering and regenerative medicine.







Similar content being viewed by others
Data Availability
The datasets that support the findings of this study are available from the corresponding authors upon reasonable request. All requests for raw and analyzed data and materials will be promptly reviewed by the Brigham and Women’s Hospital and Utrecht University to verify whether the request is subject to any intellectual property or confidentiality obligations. Any data and materials that can be shared will be released via a Material Transfer Agreement.
References
Kim SS, Kaihara S, Benvenuto MS, Choi RS, Kim BS, Mooney DJ, Taylor GA, Vacanti JP. Regenerative signals for intestinal epithelial organoid units transplanted on biodegradable polymer scaffolds for tissue engineering of small intestine. Transplantation. 1999;67(2):227–33.
Atala A, Kasper FK, Mikos AG. Engineering complex tissues. Sci Transl Med. 2012;4(160):160rv12.
Khademhosseini A, Langer R. A decade of progress in tissue engineering. Nat Protoc. 2016;11(10):1775–81.
Ma X, Liu J, Zhu W, Tang M, Lawrence N, Yu C, Gou M, Chen S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv Drug Deliv Rev. 2018;132:235–51.
Moroni L, Burdick JA, Highley C, Lee SJ, Morimoto Y, Takeuchi S, Yoo JJ. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat Rev Mater. 2018;3(5):21–37.
Chen FM, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86–168.
Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8(6):457–70.
Hussey GS, Dziki JL, Badylak SF. Extracellular matrix-based materials for regenerative medicine. Nature Reviews Materials. 2018;3(7):159–73.
Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356(6337).
Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–51.
Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, Desai RM, Madl CM, Xu M, Zhao X, Chaudhuri O, Verbeke C, Kim WS, Alim K, Mammoto A, Ingber DE, Duda GN, Mooney DJ. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater. 2015;14(12):1269–77.
Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–71.
Zhou F, Hong Y, Liang R, Zhang X, Liao Y, Jiang D, Zhang J, Sheng Z, Xie C, Peng Z, Zhuang X, Bunpetch V, Zou Y, Huang W, Zhang Q, Alakpa EV, Zhang S, Ouyang H. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials. 2020;258:120287.
Tsou Y-H, Khoneisser J, Huang P-C, Xu X. Hydrogel as a bioactive material to regulate stem cell fate. Bioactive materials. 2016;1(1):39–55.
Zheng Shu X, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25(7–8):1339–48.
Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26(18):3919–28.
Miri AK, Nieto D, Iglesias L, Goodarzi Hosseinabadi H, Maharjan S, Ruiz-Esparza GU, Khoshakhlagh P, Manbachi A, Dokmeci MR, Chen S, Shin SR, Zhang YS, Khademhosseini A. Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. Adv Mater. 2018;30(27):e1800242.
Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–80.
Zhang YS, Yue K, Aleman J, Moghaddam KM, Bakht SM, Yang J, Jia W, Dell'Erba V, Assawes P, Shin SR, Dokmeci MR, Oklu R, Khademhosseini A. 3D bioprinting for tissue and organ fabrication. Ann Biomed Eng. 2017;45(1):148–63.
Murphy SV, De Coppi P, Atala A. Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng. 2020;4(4):370–80.
Levato R, Jungst T, Scheuring RG, Blunk T, Groll J, Malda J. From shape to function: the next step in bioprinting. Adv Mater. 2020;32(12):e1906423.
Moroni L, Boland T, Burdick JA, De Maria C, Derby B, Forgacs G, Groll J, Li Q, Malda J, Mironov VA, Mota C, Nakamura M, Shu W, Takeuchi S, Woodfield TBF, Xu T, Yoo JJ, Vozzi G. Biofabrication: a guide to technology and terminology. Trends Biotechnol. 2018;36(4):384–402.
Lee JM, Sing SL, Zhou M, Yeong WY. 3D bioprinting processes: a perspective on classification and terminology. International journal of bioprinting. 2018;4(2):151.
Li W, Mille LS, Robledo JA, Uribe T, Huerta V, Zhang YS. Recent advances in formulating and processing biomaterial inks for vat polymerization-based 3D printing. Adv Healthc Mater. 2020;9(15):e2000156.
Cui X, Li J, Hartanto Y, Durham M, Tang J, Zhang H, Hooper G, Lim K, Woodfield T. Advances in extrusion 3D bioprinting: a focus on multicomponent hydrogel-based bioinks. Adv Healthc Mater. 2020;9(15):e1901648.
Vanaei S, Parizi MS, Vanaei S, Salemizadehparizi F, Vanaei HR. An overview on materials and techniques in 3d bioprinting toward biomedical application. Engineered Regeneration. 2021;2:1–18.
Zhang YS, Haghiashtiani G, Hübscher T, Kelly DJ, Lee JM, Lutolf M, McAlpine MC, Yeong WY, Zenobi-Wong M, Malda J. 3D extrusion bioprinting methods. Nature Reviews Methods Primers. 2021;1(1):75.
Pedde RD, Mirani B, Navaei A, Styan T, Wong S, Mehrali M, Thakur A, Mohtaram NK, Bayati A, Dolatshahi-Pirouz A, Nikkhah M, Willerth SM, Akbari M. Emerging biofabrication strategies for engineering complex tissue constructs. Adv Mater. 2017;29(19).
Bao G, Jiang T, Ravanbakhsh H, Reyes A, Ma Z, Strong M, Wang H, Kinsella JM, Li J, Mongeau L. Triggered micropore-forming bioprinting of porous viscoelastic hydrogels. Mater Horiz. 2020;7(9):2336–47.
Ying G, Manriquez J, Wu D, Zhang J, Jiang N, Maharjan S, Hernandez Medina DH, Zhang YS. An open-source handheld extruder loaded with pore-forming bioink for in situ wound dressing. Mater Today Bio. 2020;8:100074.
Ying G, Jiang N, Parra C, Tang G, Zhang J, Wang H, Chen S, Huang NP, Xie J, Zhang YS. Bioprinted injectable hierarchically porous gelatin methacryloyl hydrogel constructs with shape-memory properties. Adv Funct Mater. 2020;30(46).
Ying GL, Jiang N, Maharjan S, Yin YX, Chai RR, Cao X, Yang JZ, Miri AK, Hassan S, Zhang YS. Aqueous two-phase emulsion bioink-enabled 3D bioprinting of porous hydrogels. Adv Mater. 2018;30(50):e1805460.
Levato R, Lim KS, Li W, Asua AU, Peña LB, Wang M, Falandt M, Bernal PN, Gawlitta D, Zhang YS, Woodfield TBF, Malda J. High-resolution lithographic biofabrication of hydrogels with complex microchannels from low-temperature-soluble gelatin bioresins. Materials Today Bio. 2021;12:100162.
Ying G, Jiang N, Yu C, Zhang YS. Three-dimensional bioprinting of gelatin methacryloyl (GelMA). Bio-Design and Manufacturing. 2018;1(4):215–24.
Yu C, Schimelman J, Wang P, Miller KL, Ma X, You S, Guan J, Sun B, Zhu W, Chen S. Photopolymerizable biomaterials and light-based 3D printing strategies for biomedical applications. Chem Rev. 2020;120(19):10695–743.
Lee M, Rizzo R, Surman F, Zenobi-Wong M. Guiding lights: tissue bioprinting using photoactivated materials. Chem Rev. 2020;120(19):10950–1027.
Farias CBB, Almeida FC, Silva IA, Souza TC, Meira HM, Rita de Cássia F, Luna JM, Santos VA, Converti A, Banat IM. Production of green surfactants: market prospects. Electron J Biotechnol. 2021.
Turos E, Garay-Jimenez JC, Sona AJ. An evaluation of non-ionic surfactants on the cytotoxicity and activity of poly (butyl acrylate/styrene) nanoparticle emulsions. J Nanopart Res. 2019;21(7):1–8.
Abdel-Mawgoud AM, Lepine F, Deziel E. Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol. 2010;86(5):1323–36.
Schmid A, Kollmer A, Witholt B. Effects of biosurfactant and emulsification on two-liquid phase Pseudomonas oleovorans cultures and cell-free emulsions containing n-decane. Enzym Microb Technol. 1998;22(6):487–93.
Maharjan S, Alva J, Cámara C, Rubio AG, Hernández D, Delavaux C, Correa E, Romo MD, Bonilla D, Santiago ML. Symbiotic photosynthetic oxygenation within 3D-bioprinted vascularized tissues. Matter. 2021;4(1):217–40.
Gong J, Schuurmans CCL, Genderen AMV, Cao X, Li W, Cheng F, He JJ, Lopez A, Huerta V, Manriquez J, Li R, Li H, Delavaux C, Sebastian S, Capendale PE, Wang H, Xie J, Yu M, Masereeuw R, et al. Complexation-induced resolution enhancement of 3D-printed hydrogel constructs. Nat Commun. 2020;11(1):1267.
Li W, Wang M, Mille LS, Robledo JA, Huerta V, Uribe T, Cheng F, Li H, Gong J, Ching T, Murphy CA, Lesha A, Hassan S, Woodfield TBF, Lim KS, Zhang YS. A smartphone-enabled portable digital light processing 3D printer. Adv Mater. 2021;33(35):2102153.
M. Wang, W. Li, L.S. Mille, T. Ching, Z. Luo, G. Tang, C.E. Garciamendez, A. Lesha, M. Hashimoto, Y.S. Zhang, Digital light processing based bioprinting with composable gradients, Adv. Mater. 2022;34(1):2107038.
Li H, Cheng F, Li W, Cao X, Wang Z, Wang M, Robledo-Lara JA, Liao J, Chavez-Madero C, Hassan S, Xie J, Trujillo-de Santiago G, Alvarez MM, He J, Zhang YS. Expanding sacrificially printed microfluidic channel-embedded paper devices for construction of volumetric tissue models in vitro. Biofabrication. 2020;12(4):045027.
Cheng F, Cao X, Li H, Liu T, Xie X, Huang D, Maharjan S, Bei HP, Gómez A, Li J. Generation of cost-effective paper-based tissue models through matrix-assisted sacrificial 3d printing. Nano Lett. 2019;19(6):3603–11.
Liang Y, Zhao X, Hu T, Chen B, Yin Z, Ma PX, Guo B. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small. 2019;15(12):e1900046.
Fu F, Shang L, Chen Z, Yu Y, Zhao Y. Bioinspired living structural color hydrogels, science. Robotics. 2018;3(16):eaar8580.
Fu F, Chen Z, Zhao Z, Wang H, Shang L, Gu Z, Zhao Y. Bio-inspired self-healing structural color hydrogel. Proct Natl Acad Sci USA. 2017;114(23):5900.
Liu W, Heinrich MA, Zhou Y, Akpek A, Hu N, Liu X, Guan X, Zhong Z, Jin X, Khademhosseini A, Zhang YS. Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks. Adv Healthc Mater. 2017;6(12).
Code Availability
Not applicable.
Funding
The authors gratefully acknowledge funding from the National Institutes of Health (R21EB025270, R21EB026175), the National Science Foundation (NSF-CBET-1936105), and the Brigham Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
All authors consent to participate.
Consent for Publication
All authors consent for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 3755 kb)
Rights and permissions
About this article
Cite this article
Qin, XS., Wang, M., Li, W. et al. Biosurfactant-Stabilized Micropore-Forming GelMA Inks Enable Improved Usability for 3D Printing Applications. Regen. Eng. Transl. Med. 8, 471–481 (2022). https://doi.org/10.1007/s40883-022-00250-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-022-00250-5