Abstract
Yeasts have been used to manage a large number of plant diseases, but little is known about the mechanisms used by these biocontrol agents. The objectives of the present study were to evaluate the antagonistic effect of yeasts against Rhizoctonia solani and possible mechanisms of action in cowpea plants. Seventy yeast isolates were obtained from leaf, root and stem tissues of cowpea and common bean plants. Screening experiments were conducted in a greenhouse at temperatures ranging from 15 to 26 °C in the first and from 22 to 31 °C in the second experiment. Candida saopaulonensis C6A, Cryptococcus laurentii FVC10 and Bullera sinensis FVF10 (R1) reduced disease severity by 57.4%, 48.5% and 66.3%, respectively. Cowpea plants treated with FVF10 (R1) showed the highest peroxidase and catalase activities. The mechanisms of action were based on competition and induction of enzymes such as peroxidase, catalase and ascorbate peroxidase in cowpea. Candida saopaulonensis C6A, C. laurentii FVC10 and B. sinensis FVF10 (R1) are potential biocontrol agents of damping-off and stem rot caused by R. solani on cowpea plants.



Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25:3389–3402
Barbosa MAG, Michereff SJ, Mariano RLR, Maranhão E (1995) Biocontrole de Rhizoctonia solani em caupi pelo tratamento de sementes com Pseudomonas spp. fluorescentes. Summa Phytopathologica 21:151–157
Botha A (2011) The importance and ecology of yeasts in soil. Soil Biology and Biochemistry 43:1–8
Conway WS, Janisiewicz WJ, Klein JD, Sams CE (1999) Strategy for combining heat treatment, calcium infiltration, and biological control to reduce postharvest decay of ‘gala’ apples. HortScience 34:700–704
Deacon JW (1991) Significance of ecology in the development of biocontrol agent against soil-borne plant pathogens. Postharvest Biology and Technology 1:5–20
El-Mehalawy AA, Hassanein SM, Hassanein NM, Abd-Allah S (2006) Induction of resistance and biocontrol of Rhizoctonia in cotton against damping-off disease by rhizosphere yeast and fungi. Applied Ecological and Environmental Research 3:1–12
El-Mehalawy AA, Hassanim SM, Hassanim NM, Zakis SA (2007) Induction on resistence and biocontrol of Rhizoctonia in cotton against damping-off disease by rhizosphere microorganisms. New Egyptian Journal of Microbiology 17:148–168
El-Tarabily KA (2004) Supression of Rhizoctonia solani disease of sugar beet by antagonists and plant growth-promoting yeasts. Journal of Applied Microbiology 96:69–75
El-Tarabily KA, Sivasithamparam K (2006) Potential of yeasts as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Mycoscience 47:25–35
González M, Pujol M, Metraux J, González-Garcia V, Bolton MD, Borrás-Hidalgo O (2011) Tobacco leaf spot and root rot caused by Rhizoctonia solani Kühn. Molecular Plant Pathology 12:209–216
González-Garcia V, Portal OMA, Rubio SV (2006) Biology and systematics of the form genus Rhizoctonia. Spanish Journal of Agricultural Research 4:55–79
Goulart ACP (2002) Efeito do tratamento de sementes de algodão com fungicidas no controle do tombamento de plântulas causado por R. solani. Fitopatologia Brasileira 27:399–402
Havir EA, Mchale NA (1987) Biochemical and development characterization of multiple forms of catalase in tobacco leaves. Plant Physiology 84:450–455
Kar M, Mishra D (1976) Catalase, peroxidase and polyphenoloxidase activities during rice leaf senescence. Plant Physiology 57:315–319
Khalid EE (2014) Biological control of bean damping-off caused by Sclerotium rolfsii. Egyptian Journal of Phytopathology 42:1–12
Koshiba T (1993) Cytosolic ascorbate peroxidase in seedlings and leaves of maize (Zea mays). Plant & Cell Physiology 34:713–721
Kurtzman CP, Fell JW, Boekhout T (2011) The yeasts - a taxonomic study, 5th edn. Elsevier Science Publieshers, Amsterdam
McKinney RH (1923) Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. Journal of Agricultural Research 6:195–218
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7:405–410
Mohammed AS, El Hassan SM, El Balla MMA, El Sheik EAE (2008) The role of Trichoderma, VA mycorriza and dry yeasts in the control of Rhizoctonia disease of potato (Solanum tuberosum L.). The University of Khartoum. Journal of Agricultural Science 16:285–301
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-especific peroxidase en spinach chloroplasts. Plant & Cell Physiology 22:867–880
Negri CE, Gonçalves SS, Xafranski H, Bergamasco MD, Aquino VR, Castro PTO, Colombo AL (2014) Cryptic and rare Aspergillus species in Brazil: prevalence in clinical samples and in vitro susceptibility to triazoles. Journal of Clinical Microbiology 52:3633–3640
Noronha MA, Michereff SJ, Mariano RLR (1995) Efeito do tratamento de sementes de caupi com Bacillus subtilis no controle de Rhizoctonia solani. Fitopatologia Brasileira 20:174–178
Pal KK, Gardener BM (2006) Biological control of plant pathogens. Plant Health Instructor 1:1–25
Santos FS, Souza PE, Oliveira CA, Magalhães FHL, Laurenti MA (2005) Ajuste do inóculo de R. solani AG-4 em substrato para estudo de rhizoctoniose em algodoeiro e feijoeiro. Summa Phytopathologica 31:374–376
Sartorato A, Nechet KL, Halfeld-Vieira BA (2006) Diversidade genética de isolados de Rhizoctonia solani coletados em feijão-caupi no estado de Roraima. Fitopatologia Brasileira 31:297–301
Sikora EJ (2004) Rhizoctonia root rot on garden beans. Alabama cooperative extension system. Available at: www.aces.edu/pubs/docs/A/ANR-1006/. Accessed on June 12, 2014
Silva JAT, Medeiros EV, Silva JM, Tenório DA, Moreira KA, Nascimento TCES, Souza-Motta C (2016) Trichoderma aureoviride URM 5158 and Trichoderma hamatum URM 6656 are biocontrol agents that act against cassava root rot through different mechanisms. Journal of Phytopathology 164:1003–1011
Silva JAT, Medeiros EV, Silva JM, Tenório DA, Moreira KA, Nascimento TCES, Souza-Motta C (2017) Antagonistic activity of Trichoderma spp. against Scytalidium lignicola CMM 1098 and antioxidant enzymatic activity in cassava. Phytoparasitica 45:219–225
Sneh B, Jabaji-Hare S, Neate S, Dijst G (1996) Rhizoctonia species: taxonomy, molecular biology, ecology, pathology and disease control. 1st Ed. Kluwer Academic Publishers, Dordrecht
Sui Y, Wisniewski M, Droby S, Liu J, Müller V (2015) Responses of yeast biocontrol agents to environmental stress. Applied and Environmental Microbiology 81:2968–2975
Tanaka M (1994) Patógeno causadores de tombamento do algodoeiro e seus efeitos sobre a germinação das sementes em diferentes temperaturas. Fitopatologia Brasileira 19:29–33
Teixidó N, Vinas I, Usall J, Sanchis V, Magan N (1998) Ecophysiological responses of the biocontrol yeast Candida sake to water, temperature and pH stress. Journal of Applied Microbiology 84:192–200
Tománková K, Luhová L, Petrivalský M, Pec P, Lebeda A (2006) Biochemical aspects of reactive oxygen species formation in the interaction between Lycopersicon spp. and Oidium neolycopersici. Physiological and Molecular Plant Pathology 68:22–32
Tuzun S, Kloepper JW (1995) Potential applications of plant growth-promotig rhizobacteria to induced systemic disease resistence. In: Romeiro RS (ed) Controle biológico de enfermidades de plantas. Editora UFV, Viçosa. pp. 39–56
Urbanek H, Kuzniak-Gebarowska E, Herka K (1991) Elicitation of defense responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiologiae Plantarum 13:43–50
White TJ, Bruns T, Lee S, Taylor RJ (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Shinsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, London, pp 315–322
Zhao Y, TU K, Shao X, Jing W, Su Z (2008) Effects of the yeast Pichia guilliermondii against Rhizopus nigricans on tomato fruit. Postharvest Biology and Technology 49:113–120
Acknowledgements
We thank CAPES for granting a scholarship to the first author, as well as CNPq for the research scholarship granted to DL, CSL, and EVM (306401/2015-0). We thank Dr. Antônio Félix da Costa (IPA) for his contribution concerning the plant material used in the present study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Jorge T. de Souza
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
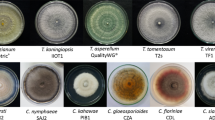
Supplementary Figure S1
The antagonist effect of yeast isolates against Rhizoctonia solani mycelial growth in vitro, calculated in relation to the control treatment without any yeast isolate. (JPG 95 kb)
Rights and permissions
About this article
Cite this article
de Tenório, D.A., de Medeiros, E.V., Lima, C.S. et al. Biological control of Rhizoctonia solani in cowpea plants using yeast. Trop. plant pathol. 44, 113–119 (2019). https://doi.org/10.1007/s40858-019-00275-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-019-00275-2