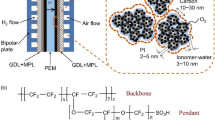
Abstract
As an important energy carrier in terms of carbon neutrality, green hydrogen produced by water electrolysis using renewable electricity has attracted worldwide attention. The polymer electrolyte water electrolyzer (PEWE) has the potential to be a mainstay in the green hydrogen market in the future because of its superior performance. However, the development of PEWE is constrained by the slow progress of the membrane electrode assembly (MEA), which is an essential component of PEWE and largely determines the cost and performance of the system. Therefore, the MEA must be optimized from the aspects of reducing cost and improving performance to promote the development of PEWEs. In this review, we first discuss the recent progress of the materials and design strategies of MEA, including the cost, activity, and stability of catalysts, distribution and thickness of ionomers, and ion transport efficiency of ion exchange membranes (IEMs). Then, the effects of all components and interlayer interfaces on the ions, electrons, and mass transfer in MEA and, consequently, the performance of PEWE are analyzed. Finally, we propose perspectives on developing MEA by optimizing the catalyst activity and stability of IEM, interface contact between adjacent components, and evaluation methods of performance.
摘要
作为碳中和的重要能源载体, 利用可再生能源电解水制取的“绿 色氢能”受到了全世界的关注. 聚合物电解质水电解槽因其优越的性能, 有望成为未来绿色制氢市场的主流. 当前, 聚合物电解质水电解槽的发 展受到膜电极发展缓慢的制约. 膜电极是聚合物电解质水电解槽的重 要组成部分, 在很大程度上决定了水电解槽系统的成本和性能. 因此, 必须从降低成本和提高性能方面对膜电极组件进行优化, 以促进其发 展. 在这篇综述中, 我们首先讨论了膜电极材料的最新进展及现有的设 计策略, 包括催化剂的成本、活性和稳定性、离聚物的分布和厚度以 及离子交换膜的离子传输效率. 然后分析了所有组分和层间界面对膜 电极中离子、电子和物质传输的影响, 以及对电解槽性能的影响. 最 后, 本论文就催化剂活性、离子交换膜稳定性、相邻组分之间的界面 以及性能评估方法等方面提出了相关建议和展望.
Similar content being viewed by others
References
Glenk G, Reichelstein S. Economics of converting renewable power to hydrogen. Nat Energy, 2019, 4: 216–222
Lu S, Zhuang Z. Electrocatalysts for hydrogen oxidation and evolution reactions. Sci China Mater, 2016, 59: 217–238
International Energy Agency. Global Hydrogen Review 2021. IEA Technical Report. 2021
Dhabi A. Green hydrogen cost reduction: Scaling up electrolysers to meet the 1.5 °C climate goal. International Renewable Energy Agency Technical Report. 2020
Abbasi R, Setzler BP, Lin S, et al. A roadmap to low-cost hydrogen with hydroxide exchange membrane electrolyzers. Adv Mater, 2019, 31: 1805876
Miller HA, Bouzek K, Hnat J, et al. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain Energy Fuels, 2020, 4: 2114–2133
Schmidt O, Gambhir A, Staffell I, et al. Future cost and performance of water electrolysis: An expert elicitation study. Int J Hydrogen Energy, 2017, 42: 30470–30492
Saba SM, Müller M, Robinius M, et al. The investment costs of electrolysis—A comparison of cost studies from the past 30 years. Int J Hydrogen Energy, 2018, 43: 1209–1223
Mayyas A, Ruth M, Pivovar B, et al. Manufacturing cost analysis for proton exchange membrane water electrolyzers. NREL/TP-6A20-72740 Technical Report. 2019
Li C, Baek JB. The promise of hydrogen production from alkaline anion exchange membrane electrolyzers. Nano Energy, 2021, 87: 106162
Li GF, Yang D, Abel Chuang PY. Defining nafion ionomer roles for enhancing alkaline oxygen evolution electrocatalysis. ACS Catal, 2018, 8: 11688–11698
Fang B, Chaudhari NK, Kim MS, et al. Homogeneous deposition of platinum nanoparticles on carbon black for proton exchange membrane fuel cell. J Am Chem Soc, 2009, 131: 15330–15338
Antolini E. Carbon supports for low-temperature fuel cell catalysts. Appl Catal B-Environ, 2009, 88: 1–24
Wu X, Scott K. RuO2 supported on Sb-doped SnO2 nanoparticles for polymer electrolyte membrane water electrolysers. Int J Hydrogen Energy, 2011, 36: 5806–5810
Mazúr P, Polonský J, Paidar M, et al. Non-conductive TiO2 as the anode catalyst support for PEM water electrolysis. Int J Hydrogen Energy, 2012, 37: 12081–12088
Kúš P, Ostroverkh A, Ševčíková K, et al. Magnetron sputtered Ir thin film on TiC-based support sublayer as low-loading anode catalyst for proton exchange membrane water electrolysis. Int J Hydrogen Energy, 2016, 41: 15124–15132
Nikiforov AV, Tomás García AL, Petrushina IM, et al. Preparation and study of IrO2/SiC-Si supported anode catalyst for high temperature PEM steam electrolysers. Int J Hydrogen Energy, 2011, 36: 5797–5805
Kondo T, Kikuchi M, Masuda H, et al. Boron-doped diamond powder as a durable support for platinum-based cathode catalysts in polymer electrolyte fuel cells. J Electrochem Soc, 2018, 165: F3072–F3077
Polonský J, Petrushina IM, Christensen E, et al. Tantalum carbide as a novel support material for anode electrocatalysts in polymer electrolyte membrane water electrolysers. Int J Hydrogen Energy, 2012, 37: 2173–2181
Schuler T, Ciccone JM, Krentscher B, et al. Hierarchically structured porous transport layers for polymer electrolyte water electrolysis. Adv Energy Mater, 2019, 10: 1903216
Faustini M, Giraud M, Jones D, et al. Hierarchically structured ultraporous Iridium-based materials: A novel catalyst architecture for proton exchange membrane water electrolyzers. Adv Energy Mater, 2019, 9: 1802136
Oh HS, Nong HN, Reier T, et al. Oxide-supported Ir nanodendrites with high activity and durability for the oxygen evolution reaction in acid PEM water electrolyzers. Chem Sci, 2015, 6: 3321–3328
Fang Z, Lee MS, Kim JY, et al. The effect of carbon support surface functionalization on PEM fuel cell performance, durability, and ionomer coverage in the catalyst layer. J Electrochem Soc, 2020, 167: 064506
Gostick JT, Fowler MW, Ioannidis MA, et al. Capillary pressure and hydrophilic porosity in gas diffusion layers for polymer electrolyte fuel cells. J Power Sources, 2006, 156: 375–387
Han B, Mo J, Kang Z, et al. Effects of membrane electrode assembly properties on two-phase transport and performance in proton exchange membrane electrolyzer cells. Electrochim Acta, 2016, 188: 317–326
Wang J, Wang H. Flow-field designs of bipolar plates in PEM fuel cells: Theory and applications. Fuel Cells, 2012, 12: 989–1003
Maier M, Smith K, Dodwell J, et al. Mass transport in PEM water electrolysers: A review. Int J Hydrogen Energy, 2022, 47: 30–56
Ito H, Maeda T, Nakano A, et al. Effect of flow regime of circulating water on a proton exchange membrane electrolyzer. Int J Hydrogen Energy, 2010, 35: 9550–9560
Abdin Z, Webb CJ, Gray EMA. Modelling and simulation of a proton exchange membrane (PEM) electrolyser cell. Int J Hydrogen Energy, 2015, 40: 13243–13257
Han B, Mo J, Kang Z, et al. Modeling of two-phase transport in proton exchange membrane electrolyzer cells for hydrogen energy. Int J Hydrogen Energy, 2017, 42: 4478–4489
Cai C, Rao Y, Zhou J, et al. Carbon corrosion: A novel termination mechanism of the water electrolysis plateau during voltage reversal. J Power Sources, 2020, 473: 228542
Minke C, Suermann M, Bensmann B, et al. Is iridium demand a potential bottleneck in the realization of large-scale PEM water electrolysis? Int J Hydrogen Energy, 2021, 46: 23581–23590
Bernt M, Siebel A, Gasteiger HA. Analysis of voltage losses in PEM water electrolyzers with low platinum group metal loadings. J Electrochem Soc, 2018, 165: F305–F314
Kim H, Choe S, Park H, et al. An extremely low Pt loading cathode for a highly efficient proton exchange membrane water electrolyzer. Nanoscale, 2017, 9: 19045–19049
Xie S, Choi SI, Lu N, et al. Atomic layer-by-layer deposition of Pt on Pd nanocubes for catalysts with enhanced activity and durability toward oxygen reduction. Nano Lett, 2014, 14: 3570–3576
Liu C, Wang CC, Kei CC, et al. Atomic layer deposition of platinum nanoparticles on carbon nanotubes for application in proton-exchange membrane fuel cells. Small, 2009, 5: 1535–1538
Pan Y, Zhang C, Lin Y, et al. Electrocatalyst engineering and structure-activity relationship in hydrogen evolution reaction: From nanostructures to single atoms. Sci China Mater, 2020, 63: 921–948
Shang C, Cao C, Yu D, et al. Electron correlations engineer catalytic activity of pyrochlore iridates for acidic water oxidation. Adv Mater, 2018, 31: 1805104
Li H, Tsai C, Koh AL, et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat Mater, 2016, 15: 48–53
Cheng Q, Hu C, Wang G, et al. Carbon-defect-driven electroless deposition of Pt atomic clusters for highly efficient hydrogen evolution. J Am Chem Soc, 2020, 142: 5594–5601
Liu K, Zhao X, Ren G, et al. Strong metal-support interaction promoted scalable production of thermally stable single-atom catalysts. Nat Commun, 2020, 11: 1263
Joo J, Park YJ, Kim J, et al. Mn-dopant differentiating the Ru and Ir oxidation states in catalytic oxides toward durable oxygen evolution reaction in acidic electrolyte. Small Methods, 2022, 6: 2101236
Su J, Ge R, Jiang K, et al. Assembling ultrasmall copper-doped ruthenium oxide nanocrystals into hollow porous polyhedra: Highly robust electrocatalysts for oxygen evolution in acidic media. Adv Mater, 2018, 30: 1801351
An J, Kim YB, Prinz FB. Ultra-thin platinum catalytic electrodes fabricated by atomic layer deposition. Phys Chem Chem Phys, 2013, 15: 7520–7525
Switzer JA. Atomic layer electrodeposition. Science, 2012, 338: 1300–1301
Wang JX, Inada H, Wu L, et al. Oxygen reduction on well-defined core-shell nanocatalysts: Particle size, facet, and Pt shell thickness effects. J Am Chem Soc, 2009, 131: 17298–17302
Song Z, Norouzi Banis M, Liu H, et al. Ultralow loading and high-performing Pt catalyst for a polymer electrolyte membrane fuel cell anode achieved by atomic layer deposition. ACS Catal, 2019, 9: 5365–5374
Lee WJ, Bera S, Kim CM, et al. Synthesis of highly dispersed Pt nanoparticles into carbon supports by fluidized bed reactor atomic layer deposition to boost pemfc performance. NPG Asia Mater, 2020, 12: 40
Cheng N, Stambula S, Wang D, et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat Commun, 2016, 7: 13638
Hsieh YC, Zhang Y, Su D, et al. Ordered bilayer ruthenium-platinum core-shell nanoparticles as carbon monoxide-tolerant fuel cell catalysts. Nat Commun, 2013, 4: 2466
Wang JX, Zhang Y, Capuano CB, et al. Ultralow charge-transfer resistance with ultralow Pt loading for hydrogen evolution and oxidation using Ru@Pt core-shell nanocatalysts. Sci Rep, 2015, 5: 12220
Yarlagadda V, Carpenter MK, Moylan TE, et al. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Lett, 2018, 3: 618–621
Castanheira L, Silva WO, Lima FHB, et al. Carbon corrosion in proton-exchange membrane fuel cells: Effect of the carbon structure, the degradation protocol, and the gas atmosphere. ACS Catal, 2015, 5: 2184–2194
Forouzandeh F, Li X, Banham DW, et al. Understanding the corrosion resistance of meso- and micro-porous carbons for application in PEM fuel cells. J Electrochem Soc, 2018, 165: F3230–F3240
Kang S, Ham K, Lee J. Moderate oxophilic CoFe in carbon nanofiber for the oxygen evolution reaction in anion exchange membrane water electrolysis. Electrochim Acta, 2020, 353: 136521
Paunović P, Gogovska DS, Popovski O, et al. Preparation and characterization of Co-Ru/TiO2/MWCNTs electrocatalysts in PEM hydrogen electrolyzer. Int J Hydrogen Energy, 2011, 36: 9405–9414
Shiva Kumar S, Ramakrishna SUB, Rama Devi B, et al. Phosphorus-doped graphene supported palladium (Pd/PG) electrocatalyst for the hydrogen evolution reaction in PEM water electrolysis. Int J Green Energy, 2018, 15: 558–567
Song J, Wei C, Huang ZF, et al. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem Soc Rev, 2020, 49: 2196–2214
Hu S, Ge S, Liu H, et al. Low-dimensional electrocatalysts for acidic oxygen evolution: Intrinsic activity, high current density operation, and long-term stability. Adv Funct Mater, 2022, 32: 2201726
Cherevko S, Zeradjanin AR, Topalov AA, et al. Dissolution of noble metals during oxygen evolution in acidic media. ChemCatChem, 2014, 6: 2219–2223
Danilovic N, Subbaraman R, Chang KC, et al. Activity-stability trends for the oxygen evolution reaction on monometallic oxides in acidic environments. J Phys Chem Lett, 2014, 5: 2474–2478
Li G, Yu H, Wang X, et al. Highly effective IrxSn1−xO2 electrocatalysts for oxygen evolution reaction in the solid polymer electrolyte water electrolyser. Phys Chem Chem Phys, 2013, 15: 2858–2866
Pham CV, Bühler M, Knöppel J, et al. IrO2 coated TiO2 core-shell microparticles advance performance of low loading proton exchange membrane water electrolyzers. Appl Catal B-Environ, 2020, 269: 118762
Marshall A, Sunde S, Tsypkin M, et al. Performance of a PEM water electrolysis cell using IrxRuyTazO2 electrocatalysts for the oxygen evolution electrode. Int J Hydrogen Energy, 2007, 32: 2320–2324
Kadakia K, Datta MK, Velikokhatnyi OI, et al. Novel (Ir,Sn,Nb)O2 anode electrocatalysts with reduced noble metal content for PEM based water electrolysis. Int J Hydrogen Energy, 2012, 37: 3001–3013
Sun W, Liu JY, Gong XQ, et al. OER activity manipulated by IrO6 coordination geometry: An insight from pyrochlore iridates. Sci Rep, 2016, 6: 38429
Lin Y, Tian Z, Zhang L, et al. Chromium-ruthenium oxide solid solution electrocatalyst for highly efficient oxygen evolution reaction in acidic media. Nat Commun, 2019, 10: 162
Kumari S, Ajayi BP, Kumar B, et al. A low-noble-metal W1−xIrxO3−δ water oxidation electrocatalyst for acidic media via rapid plasma synthesis. Energy Environ Sci, 2017, 10: 2432–2440
Zhou Z, Zaman WQ, Sun W, et al. Cultivating crystal lattice distortion in IrO2via coupling with MnO2 to boost the oxygen evolution reaction with high intrinsic activity. Chem Commun, 2018, 54: 4959–4962
Chen S, Huang H, Jiang P, et al. Mn-doped RuO2 nanocrystals as highly active electrocatalysts for enhanced oxygen evolution in acidic media. ACS Catal, 2019, 10: 1152–1160
Lee H, Kim JY, Lee SY, et al. Comparative study of catalytic activities among transition metal-doped IrO2 nanoparticles. Sci Rep, 2018, 8: 16777
Yang L, Chen H, Shi L, et al. Enhanced iridium mass activity of 6H-phase, Ir-based perovskite with nonprecious incorporation for acidic oxygen evolution electrocatalysis. ACS Appl Mater Interfaces, 2019, 11: 42006–42013
Wen Y, Chen P, Wang L, et al. Stabilizing highly active Ru sites by suppressing lattice oxygen participation in acidic water oxidation. J Am Chem Soc, 2021, 143: 6482–6490
Pi Y, Shao Q, Wang P, et al. General formation of monodisperse IrM (M = Ni, Co, Fe) bimetallic nanoclusters as bifunctional electrocatalysts for acidic overall water splitting. Adv Funct Mater, 2017, 27: 1700886
Park DH, Kim MH, Lee HJ, et al. Development of Ni-Ir oxide composites as oxygen catalysts for an anion-exchange membrane water electrolyzer. Adv Mater Inter, 2022, 9: 2102063
Luo Y, Chiang SW, Tang L, et al. Manipulating electrocatalysis using mosaic catalysts. Small Sci, 2021, 1: 2000059
Lv H, Zhang G, Hao C, et al. Activity of IrO2 supported on tantalum-doped TiO2 electrocatalyst for solid polymer electrolyte water electrolyzer. RSC Adv, 2017, 7: 40427–40436
Hu W, Chen S, Xia Q. IrO2/Nb-TiO2 electrocatalyst for oxygen evolution reaction in acidic medium. Int J Hydrogen Energy, 2014, 39: 6967–6976
Gou W, Zhang M, Zou Y, et al. Iridium-chromium oxide nanowires as highly performed OER catalysts in acidic media. ChemCatChem, 2019, 11: 6008–6014
Ghadge SD, Patel PP, Datta MK, et al. Fluorine substituted (Mn,Ir)O2: F high performance solid solution oxygen evolution reaction electro-catalysts for PEM water electrolysis. RSC Adv, 2017, 7: 17311–17324
Aizaz Ud Din M, Irfan S, Dar SU, et al. Synthesis of 3D IrRuMn sphere as a superior oxygen evolution electrocatalyst in acidic environment. Chem Eur J, 2020, 26: 5662–5666
Kim J, Shih PC, Qin Y, et al. A porous pyrochlore Y2[Ru1.6Y0.4]O7−δ electrocatalyst for enhanced performance towards the oxygen evolution reaction in acidic media. Angew Chem Int Ed, 2018, 57: 13877–13881
Wang Y, Hou S, Ma R, et al. Modulating crystallinity and surface electronic structure of IrO2via gadolinium doping to promote acidic oxygen evolution. ACS Sustain Chem Eng, 2021, 9: 10710–10716
Tong J, Liu Y, Peng Q, et al. An efficient Sb-SnO2-supported IrO2 electrocatalyst for the oxygen evolution reaction in acidic medium. J Mater Sci, 2017, 52: 13427–13443
Yang L, Yu G, Ai X, et al. Efficient oxygen evolution electrocatalysis in acid by a perovskite with face-sharing IrO6 octahedral dimers. Nat Commun, 2018, 9: 5236
Datta MK, Kadakia K, Velikokhatnyi OI, et al. High performance robust F-doped tin oxide based oxygen evolution electro-catalysts for PEM based water electrolysis. J Mater Chem A, 2013, 1: 4026–4037
Oh HS, Nong HN, Reier T, et al. Electrochemical catalyst-support effects and their stabilizing role for IrOx nanoparticle catalysts during the oxygen evolution reaction. J Am Chem Soc, 2016, 138: 12552–12563
Yun TG, Heo Y, Bin Bae H, et al. Elucidating intrinsic contribution of d-orbital states to oxygen evolution electrocatalysis in oxides. Nat Commun, 2021, 12: 824
Wang H, Lu Z, Xu S, et al. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proc Natl Acad Sci USA, 2013, 110: 19701–19706
Luo Y, Li X, Cai X, et al. Two-dimensional MoS2 confined Co(OH)2 electrocatalysts for hydrogen evolution in alkaline electrolytes. ACS Nano, 2018, 12: 4565–4573
Wu MY, Da PF, Zhang T, et al. Designing hybrid NiP2/NiO nanorod arrays for efficient alkaline hydrogen evolution. ACS Appl Mater Interfaces, 2018, 10: 17896–17902
Kim J, Kim J, Kim H, et al. Nanoporous nickel phosphide cathode for a high-performance proton exchange membrane water electrolyzer. ACS Appl Mater Interfaces, 2019, 11: 30774–30785
Yao RQ, Zhou YT, Shi H, et al. Nanoporous surface high-entropy alloys as highly efficient multisite electrocatalysts for nonacidic hydrogen evolution reaction. Adv Funct Mater, 2021, 31: 2009613
Luo Y, Zhang Z, Yang F, et al. Stabilized hydroxide-mediated nickelbased electrocatalysts for high-current-density hydrogen evolution in alkaline media. Energy Environ Sci, 2021, 14: 4610–4619
Seetharaman S, Balaji R, Ramya K, et al. Graphene oxide modified non-noble metal electrode for alkaline anion exchange membrane water electrolyzers. Int J Hydrogen Energy, 2013, 38: 14934–14942
Yu Q, Luo Y, Mahmood A, et al. Engineering two-dimensional materials and their heterostructures as high-performance electrocatalysts. Electrochem Energ Rev, 2019, 2: 373–394
Hinnemann B, Moses PG, Bonde J, et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J Am Chem Soc, 2005, 127: 5308–5309
Zhang C, Luo Y, Tan J, et al. High-throughput production of cheap mineral-based two-dimensional electrocatalysts for high-current-density hydrogen evolution. Nat Commun, 2020, 11: 3724
Yang L, Wang D, Liu M, et al. Glue-assisted grinding exfoliation of large-size 2D materials for insulating thermal conduction and large-current-density hydrogen evolution. Mater Today, 2021, 51: 145–154
Zhang C, Tan J, Pan Y, et al. Mass production of 2D materials by intermediate-assisted grinding exfoliation. Natl Sci Rev, 2020, 7: 324–332
Holzapfel PKR, Bühler M, Escalera-López D, et al. Fabrication of a robust PEM water electrolyzer based on non-noble metal cathode catalyst: [Mo3S13]2− clusters anchored to N-doped carbon nanotubes. Small, 2020, 16: 2003161
King LA, Hubert MKA, Capuano C, et al. A non-precious metal hydrogen catalyst in a commercial polymer electrolyte membrane electrolyser. Nat Nanotechnol, 2019, 14: 1071–1074
Tajuddin AAH, Elumalai G, Xi Z, et al. Corrosion-resistant non-noble metal electrodes for PEM-type water electrolyzer. Int J Hydrogen Energy, 2021, 46: 38603–38611
Huynh M, Bediako DK, Nocera DG. A functionally stable manganese oxide oxygen evolution catalyst in acid. J Am Chem Soc, 2014, 136: 6002–6010
Li A, Ooka H, Bonnet N, et al. Stable potential windows for long-term electrocatalysis by manganese oxides under acidic conditions. Angew Chem Int Ed, 2019, 58: 5054–5058
Li A, Kong S, Guo C, et al. Enhancing the stability of cobalt spinel oxide towards sustainable oxygen evolution in acid. Nat Catal, 2022, 5: 109–118
Henkensmeier D, Najibah M, Harms C, et al. Overview: State-of-the art commercial membranes for anion exchange membrane water electrolysis. J Electrochem Energy Convers Storage, 2021, 18: 024001
Xiao L, Zhang S, Pan J, et al. First implementation of alkaline polymer electrolyte water electrolysis working only with pure water. Energy Environ Sci, 2012, 5: 7869–7871
Ahn SH, Lee BS, Choi I, et al. Development of a membrane electrode assembly for alkaline water electrolysis by direct electrodeposition of nickel on carbon papers. Appl Catal B-Environ, 2014, 154–155: 197–205
Chen P, Hu X. High-efficiency anion exchange membrane water electrolysis employing non-noble metal catalysts. Adv Energy Mater, 2020, 10: 2002285
Razmjooei F, Morawietz T, Taghizadeh E, et al. Increasing the performance of an anion-exchange membrane electrolyzer operating in pure water with a nickel-based microporous layer. Joule, 2021, 5: 1776–1799
Krivina RA, Ou Y, Xu Q, et al. Oxygen electrocatalysis on mixed-metal oxides/oxyhydroxides: From fundamentals to membrane electrolyzer technology. Acc Mater Res, 2021, 2: 548–558
Xu D, Stevens MB, Cosby MR, et al. Earth-abundant oxygen electrocatalysts for alkaline anion-exchange-membrane water electrolysis: Effects of catalyst conductivity and comparison with performance in three-electrode cells. ACS Catal, 2018, 9: 7–15
Luo Y, Zhang S, Pan H, et al. Unsaturated single atoms on monolayer transition metal dichalcogenides for ultrafast hydrogen evolution. ACS Nano, 2020, 14: 767–776
Yu Q, Luo Y, Qiu S, et al. Tuning the hydrogen evolution performance of metallic 2D tantalum disulfide by interfacial engineering. ACS Nano, 2019, 13: 11874–11881
Tuaev X, Paraknowitsch JP, Illgen R, et al. Nitrogen-doped coatings on carbon nanotubes and their stabilizing effect on Pt nanoparticles. Phys Chem Chem Phys, 2012, 14: 6444–6447
Yang SH, Ferreira P, la O’ GJ, et al. Coarsening of Pt nanoparticles in proton exchange membrane fuel cells upon potential cycling. ECS Trans, 2006, 1: 185–195
Pfeifer V, Jones TE, Velasco Vélez JJ, et al. In situ observation of reactive oxygen species forming on oxygen-evolving iridium surfaces. Chem Sci, 2017, 8: 2143–2149
Kötz R, Neff H, Stucki S. Anodic iridium oxide films: XPS-studies of oxidation state changes and O2 evolution. J Electrochem Soc, 1984, 131: 72–77
Cherevko S, Geiger S, Kasian O, et al. Oxygen evolution activity and stability of iridium in acidic media. Part 2.—Electrochemically grown hydrous iridium oxide. J Electroanal Chem, 2016, 774: 102–110
Fierro S, Kapałka A, Comninellis C. Electrochemical comparison between IrO2 prepared by thermal treatment of iridium metal and IrO2 prepared by thermal decomposition of H2IrCl6 solution. Electrochem Commun, 2010, 12: 172–174
Selamet OF, Pasaogullari U, Spernjak D, et al. Two-phase flow in a proton exchange membrane electrolyzer visualized in situ by simultaneous neutron radiography and optical imaging. Int J Hydrogen Energy, 2013, 38: 5823–5835
Martelli GN, Ornelas R, Faita G. Deactivation mechanisms of oxygen evolving anodes at high current densities. Electrochim Acta, 1994, 39: 1551–1558
Yu H, Bonville L, Jankovic J, et al. Microscopic insights on the degradation of a PEM water electrolyzer with ultra-low catalyst loading. Appl Catal B-Environ, 2020, 260: 118194
Yu S, Li K, Wang W, et al. Tuning catalyst activation and utilization via controlled electrode patterning for low-loading and high-efficiency water electrolyzers. Small, 2022, 18: 2107745
Yu Q, Zhang Z, Qiu S, et al. A Ta-TaS2 monolith catalyst with robust and metallic interface for superior hydrogen evolution. Nat Commun, 2021, 12: 6051
Moysiadou A, Hu X. Stability profiles of transition metal oxides in the oxygen evolution reaction in alkaline medium. J Mater Chem A, 2019, 7: 25865–25877
Li N, Bediako DK, Hadt RG, et al. Influence of iron doping on tetravalent nickel content in catalytic oxygen evolving films. Proc Natl Acad Sci USA, 2017, 114: 1486–1491
Hung SF, Hsu YY, Chang CJ, et al. Unraveling geometrical site confinement in highly efficient iron-doped electrocatalysts toward oxygen evolution reaction. Adv Energy Mater, 2018, 8: 1701686
Anantharaj S, Kundu S, Noda S. “The Fe effect”: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts. Nano Energy, 2021, 80: 105514
Cao X, Novitski D, Holdcroft S. Visualization of hydroxide ion formation upon electrolytic water splitting in an anion exchange membrane. ACS Mater Lett, 2019, 1: 362–366
Xu J, Liu G, Li J, et al. The electrocatalytic properties of an IrO2/SnO2 catalyst using SnO2 as a support and an assisting reagent for the oxygen evolution reaction. Electrochim Acta, 2012, 59: 105–112
Cheng J, Zhang H, Chen G, et al. Study of IrxRu1−xO2 oxides as anodic electrocatalysts for solid polymer electrolyte water electrolysis. Electrochim Acta, 2009, 54: 6250–6256
Antonucci V, Di Blasi A, Baglio V, et al. High temperature operation of a composite membrane-based solid polymer electrolyte water electrolyser. Electrochim Acta, 2008, 53: 7350–7356
Millet P, Dragoe D, Grigoriev S, et al. Genhypem: A research program on PEM water electrolysis supported by the european commission. Int J Hydrogen Energy, 2009, 34: 4974–4982
Cheng J, Zhang H, Ma H, et al. Preparation of Ir0.4Ru0.6MoxOy for oxygen evolution by modified adams’ fusion method. Int J Hydrogen Energy, 2009, 34: 6609–6613
Song S, Zhang H, Ma X, et al. Electrochemical investigation of electrocatalysts for the oxygen evolution reaction in PEM water electrolyzers. Int J Hydrogen Energy, 2008, 33: 4955–4961
Song S, Zhang H, Liu B, et al. An improved catalyst-coated membrane structure for PEM water electrolyzer. Electrochem Solid-State Lett, 2007, 10: B122
Marshall A, Børresen B, Hagen G, et al. Electrochemical characterisation of IxSn1−xO2 powders as oxygen evolution electrocatalysts. Electrochim Acta, 2006, 51: 3161–3167
Millet P, Ngameni R, Grigoriev SA, et al. PEM water electrolyzers: From electrocatalysis to stack development. Int J Hydrogen Energy, 2010, 35: 5043–5052
Leng Y, Chen G, Mendoza AJ, et al. Solid-state water electrolysis with an alkaline membrane. J Am Chem Soc, 2012, 134: 9054–9057
Su X, Gao L, Hu L, et al. Novel piperidinium functionalized anionic membrane for alkaline polymer electrolysis with excellent electrochemical properties. J Membrane Sci, 2019, 581: 283–292
Parrondo J, Arges CG, Niedzwiecki M, et al. Degradation of anion exchange membranes used for hydrogen production by ultrapure water electrolysis. RSC Adv, 2014, 4: 9875–9879
Lindquist GA, Oener SZ, Krivina R, et al. Performance and durability of pure-water-fed anion exchange membrane electrolyzers using baseline materials and operation. ACS Appl Mater Interfaces, 2021, 13: 51917–51924
Koshikawa H, Murase H, Hayashi T, et al. Single nanometer-sized NiFe-layered double hydroxides as anode catalyst in anion exchange membrane water electrolysis cell with energy conversion efficiency of 74.7% at 1.0 A cm−2. ACS Catal, 2020, 10: 1886–1893
Chen HY, Chen GC, Liao KW, et al. Island-type hybrid catalysts applied for anion exchange membrane water electrolysis. Catalysts, 2022, 12: 102
Faid AY, Barnett AO, Seland F, et al. Tuning Ni-MoO2 catalyst-ionomer and electrolyte interaction for water electrolyzers with anion exchange membranes. ACS Appl Energy Mater, 2021, 4: 3327–3340
Jang MJ, Yang J, Jeong J, et al. Promotion effect of modified Ni/C by La-Ce oxide for durable hydrogen evolution reaction. ACS Sustain Chem Eng, 2021, 9: 12508–12513
Pandiarajan T, John Berchmans L, Ravichandran S. Fabrication of spinel ferrite based alkaline anion exchange membrane water electrolysers for hydrogen production. RSC Adv, 2015, 5: 34100–34108
Park YS, Jeong J, Noh Y, et al. Commercial anion exchange membrane water electrolyzer stack through non-precious metal electrocatalysts. Appl Catal B-Environ, 2021, 292: 120170
Busacca C, Zignani SC, Di Blasi A, et al. Electrospun NiMn2O4 and NiCo2O4 spinel oxides supported on carbon nanofibers as electrocatalysts for the oxygen evolution reaction in an anion exchange membrane-based electrolysis cell. Int J Hydrogen Energy, 2019, 44: 20987–20996
Li D, Park EJ, Zhu W, et al. Highly quaternized polystyrene ionomers for high performance anion exchange membrane water electrolysers. Nat Energy, 2020, 5: 378–385
Morawietz T, Handl M, Oldani C, et al. High-resolution analysis of ionomer loss in catalytic layers after operation. J Electrochem Soc, 2018, 165: F3139–F3147
Orfanidi A, Rheinländer PJ, Schulte N, et al. Ink solvent dependence of the ionomer distribution in the catalyst layer of a PEMFC. J Electrochem Soc, 2018, 165: F1254–F1263
Lee JH, Doo G, Kwon SH, et al. Dispersion-solvent control of ionomer aggregation in a polymer electrolyte membrane fuel cell. Sci Rep, 2018, 8: 10739
Kim TH, Yoo JH, Maiyalagan T, et al. Influence of the nafion agglomerate morphology on the water-uptake behavior and fuel cell performance in the proton exchange membrane fuel cells. Appl Surf Sci, 2019, 481: 777–784
Sharma R, Andersen SM. Zoom in catalyst/ionomer interface in polymer electrolyte membrane fuel cell electrodes: Impact of catalyst/ ionomer dispersion media/solvent. ACS Appl Mater Interfaces, 2018, 10: 38125–38133
Trinke P, Keeley GP, Carmo M, et al. Elucidating the effect of mass transport resistances on hydrogen crossover and cell performance in pem water electrolyzers by varying the cathode ionomer content. J Electrochem Soc, 2019, 166: F465–F471
Salvatore DA, Gabardo CM, Reyes A, et al. Designing anion exchange membranes for CO2 electrolysers. Nat Energy, 2021, 6: 339–348
Varcoe JR, Atanassov P, Dekel DR, et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ Sci, 2014, 7: 3135–3191
Díaz JC, Kamcev J. Ionic conductivity of ion-exchange membranes: Measurement techniques and salt concentration dependence. J Membrane Sci, 2021, 618: 118718
Fuel Cells and Hydrogen Joint Undertaking. 2019 Annual Work Plan and Budget. FCHJU Technical Report. 2019
Li N, Zhang Q, Wang C, et al. Phenyltrimethylammonium functionalized polysulfone anion exchange membranes. Macromolecules, 2012, 45: 2411–2419
Li N, Yan T, Li Z, et al. Comb-shaped polymers to enhance hydroxide transport in anion exchange membranes. Energy Environ Sci, 2012, 5: 7888–7892
Li L, Lin CX, Wang XQ, et al. Highly conductive anion exchange membranes with long flexible multication spacer. J Membrane Sci, 2018, 553: 209–217
Hao J, Gao X, Jiang Y, et al. Crosslinked high-performance anion exchange membranes based on poly(styrene-b-(ethylene-co-butylene)-b-styrene). J Membrane Sci, 2018, 551: 66–75
Abouzari-Lotf E, Jacob MV, Ghassemi H, et al. Highly conductive anion exchange membranes based on polymer networks containing imidazolium functionalised side chains. Sci Rep, 2021, 11: 3764
Chandesris M, Médeau V, Guillet N, et al. Membrane degradation in PEM water electrolyzer: Numerical modeling and experimental evidence of the influence of temperature and current density. Int J Hydrogen Energy, 2015, 40: 1353–1366
Salarizadeh P, Javanbakht M, Askari MB, et al. Novel proton conducting core-shell PAMPS-PVBS@Fe2TiO5 nanoparticles as a reinforcement for SPEEK based membranes. Sci Rep, 2021, 11: 4926
Wang HH, Hu C, Park JH, et al. Reinforced poly(fluorenyl-co-terphenyl piperidinium) anion exchange membranes for fuel cells. J Membrane Sci, 2022, 644: 120160
Huang Z, Lv B, Zhou L, et al. Ultra-thin h-BN doped high sulfonation sulfonated poly(ether-ether-ketone) of PTFE-reinforced proton exchange membrane. J Membrane Sci, 2022, 644: 120099
Siracusano S, Baglio V, Stassi A, et al. Performance analysis of short-side-chain aquivion perfluorosulfonic acid polymer for proton exchange membrane water electrolysis. J Membrane Sci, 2014, 466: 1–7
Yu TH, Sha Y, Liu WG, et al. Mechanism for degradation of nafion in PEM fuel cells from quantum mechanics calculations. J Am Chem Soc, 2011, 133: 19857–19863
Escobedo G, Raiford K, Nagarajan GS, et al. Strategies for mitigation of PFSA polymer degradation in PEM fuel cells. ECS Trans, 2006, 1: 303–311
Dekel DR, Willdorf S, Ash U, et al. The critical relation between chemical stability of cations and water in anion exchange membrane fuel cells environment. J Power Sources, 2018, 375: 351–360
Curtin DE, Lousenberg RD, Henry TJ, et al. Advanced materials for improved PEMFC performance and life. J Power Sources, 2004, 131: 41–48
Yu J, Yi B, Xing D, et al. Degradation mechanism of polystyrene sulfonic acid membrane and application of its composite membranes in fuel cells. Phys Chem Chem Phys, 2003, 5: 611–615
Haugen GM, Meng F, Aieta NV, et al. The Effect of heteropoly acids on stability of PFSA PEMs under fuel cell operation. Electrochem Solid-State Lett, 2007, 10: B51
Ramani V, Kunz HR, Fenton JM. Stabilized composite membranes and membrane electrode assemblies for elevated temperature/low relative humidity PEFC operation. J Power Sources, 2005, 152: 182–188
Edson JB, Macomber CS, Pivovar BS, et al. Hydroxide based decomposition pathways of alkyltrimethylammonium cations. J Membrane Sci, 2012, 399–400: 49–59
Mohanty AD, Bae C. Mechanistic analysis of ammonium cation stability for alkaline exchange membrane fuel cells. J Mater Chem A, 2014, 2: 17314–17320
Noh S, Jeon JY, Adhikari S, et al. Molecular engineering of hydroxide conducting polymers for anion exchange membranes in electrochemical energy conversion technology. Acc Chem Res, 2019, 52: 2745–2755
Lin B, Dong H, Li Y, et al. Alkaline stable C2-substituted imidazolium-based anion-exchange membranes. Chem Mater, 2013, 25: 1858–1867
Gu S, Cai R, Luo T, et al. Quaternary phosphonium-based polymers as hydroxide exchange membranes. ChemSusChem, 2010, 3: 555–558
Gu S, Wang J, Kaspar RB, et al. Permethyl cobaltocenium (Cp*2Co+) as an ultra-stable cation for polymer hydroxide-exchange membranes. Sci Rep, 2015, 5: 11668
Romulus J, Henssler JT, Weck M. Postpolymerization modification of block copolymers. Macromolecules, 2014, 47: 5437–5449
Lee WH, Kim YS, Bae C. Robust hydroxide ion conducting poly(biphenyl alkylene)s for alkaline fuel cell membranes. ACS Macro Lett, 2015, 4: 814–818
Karim MR, Hatakeyama K, Matsui T, et al. Graphene oxide nanosheet with high proton conductivity. J Am Chem Soc, 2013, 135: 8097–8100
Sun P, Ma R, Bai X, et al. Single-layer nanosheets with exceptionally high and anisotropic hydroxyl ion conductivity. Sci Adv, 2017, 3: e1602629
Liu J, Yu L, Cai X, et al. Sandwiching h-BN monolayer films between sulfonated poly(ether ether ketone) and nafion for proton exchange membranes with improved ion selectivity. ACS Nano, 2019, 13: 2094
Shao JJ, Raidongia K, Koltonow AR, et al. Self-assembled two-dimensional nanofluidic proton channels with high thermal stability. Nat Commun, 2015, 6: 7602
Ding L, Wei Y, Wang Y, et al. A two-dimensional lamellar membrane: MXene nanosheet stacks. Angew Chem Int Ed, 2017, 56: 1825–1829
Qian X, Chen L, Yin L, et al. CdPS3 nanosheets-based membrane with high proton conductivity enabled by Cd vacancies. Science, 2020, 370: 596–600
Xie Z, Navessin T, Shi K, et al. Functionally graded cathode catalyst layers for polymer electrolyte fuel cells. J Electrochem Soc, 2005, 152: A1171
Kim K, Kim H, Lee K, et al. Effect of nafion® gradient in dual catalyst layer on proton exchange membrane fuel cell performance. Int J Hydrogen Energy, 2008, 33: 2783–2789
Klingele M, Breitwieser M, Zengerle R, et al. Direct deposition of proton exchange membranes enabling high performance hydrogen fuel cells. J Mater Chem A, 2015, 3: 11239–11245
Bühler M, Klose C, Hegge F, et al. A novel fabrication technique for electrodes of PEM water electrolyzers. ECS Trans, 2017, 80: 1069–1075
Choi WC, Kim JD, Woo SI. Modification of proton conducting membrane for reducing methanol crossover in a direct-methanol fuel cell. J Power Sources, 2001, 96: 411–414
Jang S, Kim M, Kang YS, et al. Facile multiscale patterning by creep-assisted sequential imprinting and fuel cell application. ACS Appl Mater Interfaces, 2016, 8: 11459–11465
Bae JW, Cho YH, Sung YE, et al. Performance enhancement of polymer electrolyte membrane fuel cell by employing line-patterned nafion membrane. J Industrial Eng Chem, 2012, 18: 876–879
Lee DH, Yun GT, Doo G, et al. Hierarchical wrinkle-structured catalyst layer/membrane interface for ultralow Pt-loading polymer electrolyte membrane fuel cells (PEMFCs). Nano Lett, 2022, 22: 1174–1182
Joseph D, Büsselmann J, Harms C, et al. Porous nafion membranes. J Membrane Sci, 2016, 520: 723–730
Hizir FE, Ural SO, Kumbur EC, et al. Characterization of interfacial morphology in polymer electrolyte fuel cells: Micro-porous layer and catalyst layer surfaces. J Power Sources, 2010, 195: 3463–3471
Aoyama Y, Suzuki K, Tabe Y, et al. Water transport and PEFC performance with different interface structure between micro-porous layer and catalyst layer. J Electrochem Soc, 2016, 163: F359–F366
Zhang X, Shao J, Huang W, et al. Three dimensional carbon substrate materials for electrolysis of water. Sci China Mater, 2018, 61: 1143–1153
Debe MK. Tutorial on the fundamental characteristics and practical properties of nanostructured thin film (NSTF) catalysts. J Electrochem Soc, 2013, 160: F522–F534
Xie Z, Yu S, Yang G, et al. Ultrathin platinum nanowire based electrodes for high-efficiency hydrogen generation in practical electrolyzer cells. Chem Eng J, 2021, 410: 128333
Park YS, Lee JH, Jang MJ, et al. Co3S4 nanosheets on Ni foam via electrodeposition with sulfurization as highly active electrocatalysts for anion exchange membrane electrolyzer. Int J Hydrogen Energy, 2020, 45: 36–45
Luo Y, Tang L, Khan U, et al. Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density. Nat Commun, 2019, 10: 269
Yang F, Luo Y, Yu Q, et al. A durable and efficient electrocatalyst for saline water splitting with current density exceeding 2000 mA cm−2. Adv Funct Mater, 2021, 31: 2010367
Siemens Energy. Overview of the PEM Silyzer Family. Siemens Technical Report. 2020
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52188101), the National Science Fund for Distinguished Young Scholars (52125309), Guangdong Basic and Applied Basic Research Foundation (2021A1515110829), Guangdong Innovative and Entrepreneurial Research Team Program (2017ZT07C341), and Shenzhen Basic Research Project (JCYJ20200109144620815).
Author information
Authors and Affiliations
Contributions
Liu H, Yu Q, and Liu B conceived the original idea. The manuscript was drafted by Liu H, Yu Q and Liu B. Other authors include Kang X, Zhao T, Zhang Z, Ge S, Hu S, Luo Y, Yang F, Li S, Sun C and Cheng H discussed and commented on the manuscript.
Corresponding authors
Additional information
Conflict of interest
The authors declare that they have no conflict of interest.
Heming Liu is a PhD student at Tsinghua-Berkeley Shenzhen Institute, Tsinghua University. His current research mainly focuses on water electrolysis at high current density and electrochemical carbon dioxide reduction.
Qiangmin Yu is a research scientist of Tsinghua Shenzhen International Graduate School, Tsinghua University, China. He received his PhD degree in inorganic chemistry from Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences (FJIRSM, CAS) in 2017. He worked at Tsinghua-Berkeley Shenzhen Institute, Tsinghua University as a postdoctoral researcher in collaboration with Profs. Hui-Ming Cheng and Bilu Liu from 2017 to 2019. His research interests focus on the controllable preparation of low-dimensional materials and their applications in the production of sustainable fuels and industrial chemicals by electrolysis.
Bilu Liu is an associate professor and PI at Tsinghua Shenzhen International Graduate School, Tsinghua University, China. He received his bachelor’s degree in materials chemistry from the University of Science and Technology of China (USTC) in 2006, and PhD degree in materials science from the Institute of Metal Research (IMR), CAS in 2012. His research interests cover the chemistry and materials science of low-dimensional materials with emphasis on carbon nanostructures, two-dimensional materials, and heterostructures. His work relates to the controlled mass preparation of these materials and their applications in electronics, optoelectronics, and catalysis.
Rights and permissions
About this article
Cite this article
Liu, H., Kang, X., Zhao, T. et al. Engineering membrane electrode assembly for advanced polymer electrolyte water electrolyzer. Sci. China Mater. 65, 3243–3272 (2022). https://doi.org/10.1007/s40843-022-2128-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-022-2128-4