Abstract
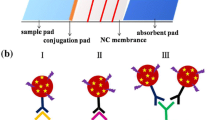
A novel lateral flow immunoassay biosensor, combining the receptor-ligand reaction and antigen-antibody reaction was developed for the detection of β-lactam antibiotics in milk. The receptor protein, the carboxy-terminal domain of β-lactam sensor-transducer mutant from Bacillus. licheniformis, served as the recognition element. Colloidal gold (CG)-labelled receptor antibody-receptor ternary complex was applied as a signal transducer probe. The presence of β-lactams was identified through a color change in the test zone, where the reaction between the complex of CG/anti-receptor antibody/receptor and β-lactam antigen on the test line could be inhibited by β-lactam residues in the sample, thus leading to a reduction in color signal. Based on the indirect labeling of the receptor with an antibody as a linker, the ability to detect 33 β-lactams was below or near the corresponding maximum residue limit. The proposed lateral flow immunoassay biosensor appears to be an excellent field-based screening tool for the qualitative screening of β-lactams in milk.

摘要
的侧流免疫传感器, 用于牛奶中β-内酰胺类抗生素的残留检测. 该传感器以地衣芽孢杆菌来源的突变型β-内酰胺类抗生素传感受体蛋白(BlaR-CTD-M)为识别元件, 以胶体金(CG)标记的受体抗体-受体复合物为信号传递探针. 基于样品中游离的β-内酰胺类抗生素竞争抑制CG/受体抗体-受体复合物与检测线处固定的β-内酰胺类抗生素抗原的结合, 根据检测线的颜色变化可实现β-内酰胺类抗生素的快速定性检测. 通过受体抗体作为连接臂, 以CG间接标记受体, 使该传感器能检测牛奶中33种β-内酰胺类抗生素, 检测限符合最大残留限量. 本文所建的传感器检测方法是一种现场快速定性筛查β-内酰胺类抗生素残留的有效工具.
Similar content being viewed by others
References
World Health Organization. Global action plan on antimicrobial resistance. Geneva: WHO Press, 2015. https://www.eahp.eu/sites/defauh7files/global_action_plan_eng.pdf
Qiao M, Ying GG, Singer AC, et al. Review of antibiotic resistance in China and its environment. Environ Int, 2018, 110: 160–172
Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol Infec, 2016, 22: S9–S14
Ma L, Li B, Zhang T. New insights into antibiotic resistome in drinking water and management perspectives: A metagenomic based study of small-sized microbes. Water Res, 2019, 152: 191–201
Holmes AH, Moore LSP, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet, 2016, 387: 176–187
Godziszewska J, Guzek D, Głąbski K, et al. Mobile antibiotic resistance: The spread of genes determining the resistance of bacteria through food products. Postepy Hig Med Dosw, 2016, 70: 803–810
Piddock LJ. The crisis of no new antibiotics—What is the way forward? Lancet Infect Dis, 2012, 12: 249–253
Huang KHG, Cluzet V, Hamilton K, et al. The impact of reported beta-lactam allergy in hospitalized patients with hematologic malignancies requiring antibiotics. Clin Infect Dis, 2018, 67: 27–33
Moreno E, Laffond E, Muñoz-Bellido FJ, et al. Using beta-lactam antibiotics in patients with a history of beta-lactam allergy: Current concepts. Polish Archives Internal Med, 2017, 127: 540–549
Tyers M, Wright GD. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat Rev Microbiol, 2019, 17: 141–155
Wright GD. Antibiotic adjuvants: Rescuing antibiotics from resistance. Trends Microbiol, 2016, 24: 862–871
Tängdén T, Giske CG. Global dissemination of extensively drug-resistant carbapenemase-producing enterobacteriaceae: Clinical perspectives on detection, treatment and infection control. J Intern Med, 2015, 277: 501–512
Legrand T, Vodovar D, Tournier N, et al. Simultaneous determination of eight β-lactam antibiotics, amoxicillin, cefazolin, cefepime, cefotaxime, ceftazidime, cloxacillin, oxacillin, and piperacillin, in human plasma by using ultra-high-performance liquid chromatography with ultraviolet detection. Antimicrob Agents Chemother, 2016, 60: 4734–4742
Carlier M, Stove V, De Waele JJ, et al. Ultrafast quantification of β-lactam antibiotics in human plasma using UPLC-MS/MS. J Chromatogr B, 2015, 978–979: 89–94
Šestáková N, Theurillat R, Sendi P, et al. Monitoring of cefepime in human serum and plasma by micellar electrokinetic capillary chromatography: Improvement of sample preparation and validation by liquid chromatography coupled to mass spectrometry. J Sep Sci, 2017, 40: 1805–1814
Li YF, Sun YM, Beier RC, et al. Immunochemical techniques for multianalyte analysis of chemical residues in food and the environment: A review. TrAC Trends Anal Chem, 2017, 88: 25–40
Liu J, Zhang HC, Duan CF, et al. Production of anti-amoxicillin ScFv antibody and simulation studying its molecular recognition mechanism for penicillins. J Environ Sci Health Part B, 2016, 51: 742–750
Tooke CL, Hinchliffe P, Bragginton EC, et al. β-Lactamases and β-lactamase inhibitors in the 21st century. J Mol Biol, 2019, 431: 3472–3500
Brem J, Cain R, Cahill S, et al. Structural basis of metallo-β-lactamase, serine-β-lactamase and penicillin-binding protein inhibition by cyclic boronates. Nat Commun, 2016, 7: 12406
Zhang J, Wang Z, Wen K, et al. Penicillin-binding protein 3 of Streptococcus pneumoniae and its application in screening of β-lactams in milk. Anal Biochem, 2013, 442: 158–165
Li Y, Xu X, Liu L, et al. Rapid detection of 21 β-lactams using an immunochromatographic assay based on the mutant BlaR-CTD protein from Bacillus licheniformis. Analyst, 2020, 145: 3257–3265
Li Y, Wang Z, Sun L, et al. Nanoparticle-based sensors for food contaminants. TrAC Trends Anal Chem, 2019, 113: 74–83
Duan J, Zhan J. Recent developments on nanomaterials-based optical sensors for Hg2+ detection. Sci China Mater, 2015, 58: 223–240
Li Y, Liu L, Song S, et al. A rapid and semi-quantitative gold nanoparticles based strip sensor for polymyxin B sulfate residues. Nanomaterials, 2018, 8: 144–155
Ma L, Wang Z, Liu H, et al. Monoclonal antibody production and the development of a quantitative time-resolved fluoroimmunoassay for rifaximin in milk. Food Agric Immunol, 2019, 30: 1135–1147
Zhang S, Wu ZY, Zhou K, et al. Development of a competitive indirect ELISA for high-throughput screening of hydrocortisone in cosmetic sample. Food Agric Immunol, 2019, 30: 594–605
Chen Y, Liu L, Xu L, et al. Gold immunochromatographic sensor for the rapid detection of twenty-six sulfonamides in foods. Nano Res, 2017, 10: 2833–2844
Meng X, Ji D, Zhang W, et al. Determination of 3.5-dinitro-N′-(5-nitrofurfurylidene) salicylic acid hydrazide in fish using immunochromatographic strip tests. Food Agric Immunol, 2019, 30: 475–486
Hwang J, Lee S, Choo J. Application of a SERS-based lateral flow immunoassay strip for the rapid and sensitive detection of staphylococcal enterotoxin B. Nanoscale, 2016, 8: 11418–11425
Song S, Suryoprabowo S, Liu L, et al. Development of an immunochromatographic strip test for rapid detection of sodium nifurstyrenate in fish. Food Agric Immunol, 2019, 30: 236–247
Wang Z, Guo L, Liu L, et al. Colloidal gold-based immunochromatographic strip assay for the rapid detection of three natural estrogens in milk. Food Chem, 2018, 259: 122–129
Wang W, Liu L, Song S, et al. Gold nanoparticle-based strip sensor for multiple detection of twelve Salmonella strains with a genus-specific lipopolysaccharide antibody. Sci China Mater, 2016, 59: 665–674
Suryoprabowo S, Liu L, Kuang H, et al. Gold immunochromatographic assay for simultaneous detection of sibutramine and sildenafil in slimming tea and coffee. Sci China Mater, 2020, 63: 654–659
Li Y, Liu L, Song S, et al. Development of a gold nanoparticle immunochromatographic assay for the on-site analysis of 6-benzylaminopurine residues in bean sprouts. Food Agric Immunol, 2018, 29: 14–26
Huang CR, Lin CH, Hsiao SC, et al. Drug interaction between valproic acid and carbapenems in patients with epileptic seizures. Kaohsiung J Med Sci, 2017, 33: 130–136
Acknowledgements
This work was financially supported by the National Key R&D Program of China (2019YFC1606603).
Author information
Authors and Affiliations
Contributions
Author contributions Kuang H and Xu C conceived and designed the experiments; Li Y performed the experiments; Sun L, Liu L and Kuang H performed the data analysis. All the authors drafted the manuscript, and read and approved the manuscript prior to submission.
Corresponding authors
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Yue Li is a PhD candidate under the supervision of Prof. Chuanlai Xu at Jiangnan University. Her research interests focus on developing rapid and sensitive methods to determine analytes using immunochromatography strip test.
Chuanlai Xu is the Director of the International Lab of Biointerface & Biodetection, Jiangnan University, leader of the Food Safety Innovation Team in Priority Areas Accredited by the Ministry of Science and Technology and fellow of the Royal Society of Chemistry. His research interests include: (1) fabrication of nano-devices and their applications in biotechnology; (2) preparation of antibodies against hazards (such as heavy metals, pesticides, and veterinary drug) and their applications in rapid detection.
Supporting information
40843_2020_1578_MOESM1_ESM.pdf
Integration of Antibody-Antigen and Receptor-Ligand Reactions to Establish a Gold Strip Biosensor for Detection of 33 β-Lactam Antibiotics
Rights and permissions
About this article
Cite this article
Li, Y., Liu, L., Xu, C. et al. Integration of antibody-antigen and receptor-ligand reactions to establish a gold strip biosensor for detection of 33 β-lactam antibiotics. Sci. China Mater. 64, 2056–2066 (2021). https://doi.org/10.1007/s40843-020-1578-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-020-1578-0