Abstract
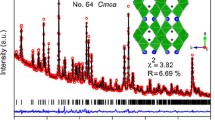
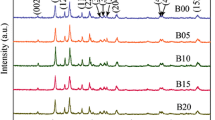
A series of BaLi2−x Na x Ti6O14(0≤x≤2) compounds as lithium storage materials were synthesized by a facile solid-state method. X-ray diffraction Rietveld refinement shows that the Bragg positions correspond to the BaLi2Ti6O14, indicating a successful preparation. The Na+ ions doped BaLi2-Ti6O14 compounds have larger unit-cell volume than the pristine one because ionic radius of Na+ ion is 55% larger than that of Li+ ion. SEM shows that the BaLi2−x Na x Ti6O14 (x=0, 0.5 and 1) powders show similar irregular shaped particles between 500 and 1000 nm. However, BaLi2−x Na x Ti6O14 (x=1.5 and 2) powders show similar rod-like shape. CV reveals that the passivating film is mainly formed during the first insertion process, and the solid electrolyte interface film on the surface of BaLi2−x Na x Ti6O14 (0≤x≤2) is formed below 0.7 V in the first cycle. Compared with other samples, BaLi0.5Na1.5Ti6O14 exhibits higher reversible capacity, better rate capability and superior cyclability. BaLi0.5Na1.5Ti6O14 delivers the delithiation capacities of 162.1 mA h g−1 at 50 mA g−1, 158.1 mA h g−1 at 100 mA g−1, 156.7 mA h g−1 at 150 mA g−1, 152.2 mA h g−1 at 200 mA g−1, 147.3 mA h g−1 at 250 mA g−1 and 142 mA h g−1 at 300 mA g−1, respectively. An interesting thing is that BaNa2Ti6O14 as anode also shows an acceptable electrochemical performance. All these improved electrochemical performances of BaLi0.5Na1.5Ti6O14 are attributed to the lowest polarization and the highest lithium ion diffusion coefficient among all samples. Hence, BaLi0.5Na1.5Ti6O14 with excellent cycling performance, simple synthesis route and wide discharge voltage range can be a possible anode candidate for lithium-ion batteries.
摘要
本文采用简单的高温固相法制备了BaLi2−x Na x Ti6O14 (0≤x≤2)系列化合物作为储锂材料. XRD Rietveld精确表明Bragg点与BaLi2Ti6O14相对应, 由于Na+的半径比Li+的半径大55%, 因此Na+掺杂的BaLi2Ti6O14化合物具有比纯BaLi2Ti6O14更大的晶胞体积. SEM测试结果表明,BaLi2−x Na x Ti6O14 (x=0, 0.5, 1)粉末呈相似的不规则的颗粒状, 粒径大约在500到1000 nm之间. 但是, BaLi2−x Na x Ti6O14 (x=1.5, 2)展示了棒状的形貌. 循环伏安结果表明, 钝化膜主要在第一次嵌锂过程时形成, BaLi2−x Na x Ti6O14 (0≤x≤2)表面的SEI膜主要在第一次循环且电位在0.7 V以下时形成. 相对于其他样品, BaLi0.5Na1.5Ti6O14具有较高的可逆容量, 较好的倍率性能和优异的循环性能. 电流密度为50、100、150、200、250和300mA g−1时, BaLi0.5Na1.5Ti6O14的脱锂容量分别为162.1、158.1、156.7、152.2、147.3和142 mA h g−1. 有趣的是, BaNa2Ti6O14作为阳极也展示了可接受的电化学性能. BaLi0.5Na1.5Ti6O14所提高的电化学性能可以归因于其最小的极化和最高的锂离子扩散系数. 因具有优异的循环性能、简单的合成路线和宽的放电区间, BaLi0.5Na1.5Ti6O14可作为锂离子电池负极候选材料.
Similar content being viewed by others
References
Massé RC, Uchaker E, Cao G. Beyond Li-ion: electrode materials for sodium- and magnesium-ion batteries. Sci China Mater, 2015, 58: 715–766
Zhou X, Chen S, Yang J, et al. Metal-organic frameworks derived okra-like SnO2 encapsulated in nitrogen-doped graphene for lithium ion battery. ACS Appl Mater Interfaces, 2017, 9: 14309–14318
Yang J, Xu Z, Sun H, et al. A three-dimensional interlayer composed of graphene and porous carbon for long-life, high capacity lithium-iron fluoride battery. Electrochim Acta, 2016, 220: 75–82
Yang J, Yan B, Ye J, et al. Carbon-coated LiCrTiO4 electrode material promoting phase transition to reduce asymmetric polarization for lithium-ion batteries. Phys Chem Chem Phys, 2014, 16: 2882–2891
Yang L, Zhang X, Li Y, et al. Graphene-encapsulated Li2MnTi3O8 nanoparticles as a high rate anode material for lithium-ion batteries. Electrochim Acta, 2015, 155: 272–278
Chen C, Ai C, Liu X, et al. High performance Li2ZnTi3O8@C anode material fabricated by a facile method without an additional carbon source. J Alloys Compd, 2017, 698: 692–698
Han JT, Huang YH, Goodenough JB. New anode framework for rechargeable lithium batteries. Chem Mater, 2011, 23: 2027–2029
Wang C, Wang S, Tang L, et al. A robust strategy for crafting monodisperse Li4Ti5O12 nanospheres as superior rate anode for lithium ion batteries. Nano Energ, 2016, 21: 133–144
Tang L, He YB, Wang C, et al. High-density microporous Li4Ti5O12 microbars with superior rate performance for lithium-ion batteries. Adv Sci, 2017, 4: 16003117 pages
Wang C, Wang S, He YB, et al. Combining fast Li-ion battery cycling with large volumetric energy density: grain boundary induced high electronic and ionic conductivity in Li4Ti5O12 spheres of densely packed nanocrystallites. Chem Mater, 2015, 27: 5647–5656
Shu J, Wu K, Wang P, et al. Lithiation and delithiation behavior of sodium lithium titanate anode. Electrochim Acta, 2015, 173: 595–606
Koseva I, Chaminade JP, Gravereau P, et al. A new family of isostructural titanates, MLi2Ti6O14 (M = Sr, Ba, Pb). J Alloys Compd, 2005, 389: 47–54
Fan SS, Zhong H, Yu HT, et al. Hollow and hierarchical Na2Li2 Ti6O14 microspheres with high electrochemical performance as anode material for lithium-ion battery. Sci China Mater, 2017, 60: 427–437
Li H, Shen L, Ding B, et al. Ultralong SrLi2Ti6O14 nanowires composed of single-crystalline nanoparticles: promising candidates for high-power lithium ions batteries. Nano Energ, 2015, 13: 18–27
Liu J, Wu B, Wang X, et al. Study of the Li+ intercalation/deintercalation behavior of SrLi2Ti6O14 by in-situ techniques. J Power Sources, 2016, 301: 362–368
Lin X, Li P, Wang P, et al. SrLi2Ti6O14: a probable host material for high performance lithium storage. Electrochim Acta, 2015, 180: 831–844
Li P, Qian S, Yu H, et al. PbLi2Ti6O14: a novel high-rate long-life anode material for rechargeable lithium-ion batteries. J Power Sources, 2016, 330: 45–54
Liu J, Li Y, Wang X, et al. Synthesis process investigation and electrochemical performance characterization of SrLi2Ti6O14 by ex situ XRD. J Alloys Compd, 2013, 581: 236–240
Cheng F, Shen J, Peng B, et al. Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts. Nat Chem, 2011, 3: 79–84
Dambournet D, Belharouak I, Amine K. MLi2Ti6O14 (M = Sr, Ba, Na) lithium insertion titanate materials: a comparative study. Inorg Chem, 2010, 49: 2822–2826
Yu H, Yan L, Qian S, et al. Fabrication of Ba0.95M0.05Li2Ti6O14 (M=Ag, Pb, Al) as high performance anode candidates for lithium secondary batteries. Electrochim Acta, 2017, 228: 453–461
Yu H, Luo M, Lan H, et al. Comparative study of Pb1−x BaxLi2Ti6O14 (0≤ x ≤1) as lithium storage materials for secondary batteries. J Power Sources, 2017, 357: 179–187
Yu H, Luo M, Qian S, et al. Ba0.9La0.1Li2Ti6O14: advanced lithium storage material for lithium-ion batteries. Electrochim Acta, 2017, 232: 132–141
Yi TF, Yang SY, Li XY, et al. Sub-micrometric Li4−x NaxTi5O12 (0≤x≤0.2) spinel as anode material exhibiting high rate capability. J Power Sources, 2014, 246: 505–511
Yin X, Huang K, Liu S, et al. Preparation and characterization of Na-doped LiFePO4/C composites as cathode materials for lithiumion batteries. J Power Sources, 2010, 195: 4308–4312
Kuang Q, Zhao Y, Liang Z. Synthesis and electrochemical properties of Na-doped Li3V2(PO4)3 cathode materials for Li-ion batteries. J Power Sources, 2011, 196: 10169–10175
Hu X, Sun J, Li Z, et al. Rechargeable room-temperature Na-CO2 batteries. Angew Chem, 2016, 128: 6592–6596
Wu MS, Chiang PCJ, Lin JC, et al. Correlation between electrochemical characteristics and thermal stability of advanced lithiumion batteries in abuse tests–short-circuit tests. Electrochim Acta, 2004, 49: 1803–1812
Luo M, Lin X, Lan H, et alx. Lithiation-delithiation kinetics of BaLi2Ti6O14 anode in high-performance secondary Li-ion batteries. J Electroanalytical Chem, 2017, 786: 86–93
Wu X, Li X, Zhu C, et al. Electrospun one-dimensional BaLi2Ti6O14 nanofibers for high rate performing lithium-ion battery. Mater Today Energ, 2016, 1–2: 17–23
Shu J. Electrochemical behavior and stability of Li4Ti5O12 in a broad voltage window. J Solid State Electrochem, 2009, 13: 1535–1539
Shu J. Study of the interface between Li4Ti5O12 electrodes and standard electrolyte solutions in 0.0–0.5 V. Electrochem Solid-State Lett, 2008, 11: A238
Yi TF, Yang SY, Xie Y. Recent advances of Li4Ti5O12 as a promising next generation anode material for high power lithium-ion batteries. J Mater Chem A, 2015, 3: 5750–5777
Yi TF, Xie Y, Zhu YR, et al. Structural and thermodynamic stability of Li4Ti5O12 anode material for lithium-ion battery. J Power Sources, 2013, 222: 448–454
Wu K, Shu J, Lin X, et al. Phase composition and electrochemical performance of sodium lithium titanates as anode materials for lithium rechargeable batteries. J Power Sources, 2015, 275: 419–428
Yi TF, Mei J, Zhu YR, et al. Li5Cr7Ti6O25 as a novel negative electrode material for lithium-ion batteries. Chem Commun, 2015, 51: 14050–14053
Lin X, Wang P, Li P, et al. Improved the lithium storage capability of BaLi2Ti6O14 by electroless silver coating. Electrochim Acta, 2015, 186: 24–33
Aravindan V, Gnanaraj J, Lee YS, et al. Insertion-type electrodes for nonaqueous Li-ion capacitors. Chem Rev, 2014, 114: 11619–11635
Borghols WJH, Wagemaker M, Lafont U, et al. Size effects in the Li4+x Ti5O12 spinel. J Am Chem Soc, 2009, 131: 17786–17792
Lin C, Deng S, Shen H, et al. Li5Cr9Ti4O24: a new anode material for lithium-ion batteries. J Alloys Compd, 2015, 650: 616–621
Fang ZK, Zhu YR, Yi TF, et al. Li4Ti5O12–LiAlO2 composite as high performance anode material for lithium-ion battery. ACS Sustain Chem Eng, 2016, 4: 1994–2003
Pan D, Wan N, Ren Y, et al. Enhanced structural and electrochemical stability of self-similar rice-shaped SnO2 nanoparticles. ACS Appl Mater Interfaces, 2017, 9: 9747–9755
Yi TF, Han X, Yang SY, et al. Enhanced electrochemical performance of Li-rich low-Co Li1.2Mn0.56Ni0.16Co0.08−x AlxO2 (0≤x≤0.08) as cathode materials. Sci China Mater, 2016, 59: 618–628
Li P, Wu K, Wang P, et al. Preparation, electrochemical characterization and in-situ kinetic observation of Na2Li2Ti6O14 as anode material for lithium ion batteries. Ceramics Int, 2015, 41: 14508–14516
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51404002), Anhui Provincial Natural Science Foundation (1508085MB25), the Natural Science Foundation of Guangdong Province (2016A030310127) and Anhui Provincial Science Fund for Excellent Young Scholars (gxyqZD2016066).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Author contributions Tao W, Xu ML performed the materials synthesis, characterization and electrochemical measurements. Zhang Q was involved in data analysis and discussion. Yi TF and Zhu YR conceived the strategy, supervised the design of experiments, and wrote the manuscript, and all authors participated in the general discussion.
Conflict of interset The authors declare that they have no conflict of interest.
Supplementary information The charge/discharge specific capacities of BaLi2−x Na x Ti6O14 (x=0.0, 0.5, 1.0, 1.5, 2.0) samples at different cycle numbers are available in the online version of the paper.
Wei Tao received his BE degree from Anhui University of Technology in 2015. He is now a second year Master student in Prof. Ting-Feng Yi's group at Anhui University of Technology. His research focuses on the application of functional materials in lithium-ion batteries.
Qianyu Zhang received his PhD degree from Fudan University, China. He was a visiting scholar at the University of California, San Diego from 2013 to 2014. He worked as an assistant researcher at Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences from 2015 to 2017. Currently, he is an associate professor at Dongguan University of Technology, China. His research involves exploring high-performance electrode materials for Li-ion battery.
Ting-Feng Yi received his BE degree in chemical engineering and technology from Liaocheng University in 2001. He then obtained his MSc degree in applied chemistry in 2004 and PhD degree in chemical engineering and technology from Harbin Institute of Technology in 2007. He joined Anhui University of Technology as an assistant professor of chemistry in 2007. He is currently a professor of Anhui University of Technology, China. His research interests include the synthesis of electrochemical functional materials and their application in lithium-ion battery, supercapacitor and lead-acid battery. For detail please see his research ID: http://www.researcherid.com/rid/F-4594-2012.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tao, W., Xu, ML., Zhu, YR. et al. Structure and electrochemical performance of BaLi2−x Na x Ti6O14 (0≤x≤2) as anode materials for lithium-ion battery. Sci. China Mater. 60, 728–738 (2017). https://doi.org/10.1007/s40843-017-9065-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-017-9065-8