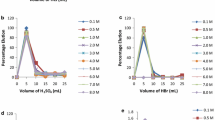
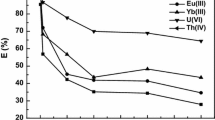
Abstract
Recently, efforts have been made to develop the extraction, recovery, and analysis of rare earth elements, especially the scandium Sc (III). This study describes the use of 15-crown-5, 12-crown-4, cryptand 2.2.2, and DC18-crown-6 as novel extractants using molecular recognition technology to effectively and selectively separate and recover Sc from aqueous media. The results show that 15-crown-5, 12-crown-4, and cryptand 2.2.2 have exhibited high selectivity for Sc, which could be of potential value in the separation and purification of Sc rare earth elements processing industry. Typically, 15-crown-5, 12-crown-4, and cryptand 2.2.2 ligands have achieved the maximum extraction efficiency > 99%, depending on the pH value of the aqueous solution. On the other hand, DC18-crown-6 only reaches 25.81%. Notably, the complex metal ions can be efficiently recovered/stripped out from the complex by HCl and HNO3. The results of the multielement extraction experiments by cryptand 2.2.2 indicate that the pH of the aqueous solution significantly impacts extraction efficiency and selectivity. Where, at pH 2 the extraction efficiency of Sc ~ 97% compared with Y ~ 18.23%, La ~ 10.21% and Ce ~ 9.2%. Moreover, the complexed Sc ions can be efficiently recovered from complexes using a precise concentration of oxalic acid, with a recovery rate of 100% at 0.1 mol/L oxalic acids, compared to 30.7% for Y, 20.3% for La, and 21.12% for Ce.
Graphical Abstract












Similar content being viewed by others
References
Haxel G (2002) Rare earth elements: critical resources for high technology, vol 87. vol 2. US Department of the Interior, US Geological Survey
Tyler G (2004) Rare earth elements in soil and plant systems-a review. Plant soil 267(1–2):191–206
Banihashemi SR, Taheri B, Razavian SM, Soltani F (2019) Selective nitric acid leaching of rare-earth elements from calcium and phosphate in fluorapatite concentrate. JOM 71(12):4578–4587. https://doi.org/10.1007/s11837-019-03605-6
Vaughan J, Tungpalan K, Parbhakar-Fox A, Fu W, Gagen EJ, Nkrumah PN, Southam G, van der Ent A, Erskine PD, Gow P, Valenta R (2021) Toward closing a loophole: recovering rare earth elements from uranium metallurgical process tailings. JOM 73(1):39–53. https://doi.org/10.1007/s11837-020-04451-7
Deng B, Li G, Luo J, Ye Q, Liu M, Rao M, Peng Z, Jiang T (2018) Selective extraction of rare earth elements over TiO2 from bauxite residues after removal of their Fe-, Si-, and Al-bearing constituents. JOM 70(12):2869–2876. https://doi.org/10.1007/s11837-018-3130-7
Zhang G, Yan P, Yang Y, McLean A (2020) Recovery of scandium from reservoir silt by electrical separation. JOM 72(7):2741–2747. https://doi.org/10.1007/s11837-020-04076-w
Avdibegovic D, Binnemans K (2020) Separation of scandium from hydrochloric acid-ethanol leachate of bauxite residue by a supported ionic liquid phase. Ind Eng Chem Res. https://doi.org/10.1021/acs.iecr.0c02943
Meng F, Li X, Wang P, Yang F, Liang D, Gao F, He C, Wei Y (2020) Recovery of scandium from bauxite residue by selective sulfation roasting with concentrated sulfuric acid and leaching. JOM 72(2):816–822. https://doi.org/10.1007/s11837-019-03931-9
Yan P, Zhang G, Yang Y, McLean A (2019) Characterization and pre-concentration of scandium in low-grade magnetite ore. JOM 71(12):4666–4673. https://doi.org/10.1007/s11837-019-03541-5
Xiao J, Zou K, Chen T, Peng Y, Ding W, Chen J, Deng B, Li H, Wang Z (2021) Extraction of Sc from Sc-bearing V-Ti magnetite tailings. JOM 73(6):1836–1844. https://doi.org/10.1007/s11837-021-04665-3
Ramasamy DL, Puhakka V, Repo E, Ben Hammouda S, Sillanpää M (2018) Two-stage selective recovery process of scandium from the group of rare earth elements in aqueous systems using activated carbon and silica composites: dual applications by tailoring the ligand grafting approach. Chem Eng J 341:351–360. https://doi.org/10.1016/j.cej.2018.02.024
Ambaye TG, Vaccari M, Castro FD, Prasad S, Rtimi S (2020) Emerging technologies for the recovery of rare earth elements (REEs) from the end-of-life electronic wastes: a review on progress, challenges, and perspectives. Environ Sci Pollut Res 27(29):36052–36074. https://doi.org/10.1007/s11356-020-09630-2
Wang W, Pranolo Y, Cheng CY (2011) Metallurgical processes for scandium recovery from various resources: a review. Hydrometallurgy 108(1):100–108. https://doi.org/10.1016/j.hydromet.2011.03.001
Zhang N, Li H-X, Liu X-M (2016) Recovery of scandium from bauxite residue—red mud: a review. Rare Met 35(12):887–900
Wang W, Cheng CY (2011) Separation and purification of scandium by solvent extraction and related technologies: a review. J Chem Technol Biotechnol 86(10):1237–1246
Liu Z, Li H (2015) Metallurgical process for valuable elements recovery from red mud—a review. Hydrometallurgy 155:29–43
Shyam Sunder GS, Adhikari S, Rohanifar A, Poudel A, Kirchhoff JR (2020) Evolution of environmentally friendly strategies for metal extraction. Separations 7(1):4
Salman AD, Juzsakova T, Ákos R, Ibrahim RI, Al-Mayyahi MA, Mohsen S, Abdullah TA, Domokos E (2021) Synthesis and surface modification of magnetic Fe3O4@SiO2 core-shell nanoparticles and its application in uptake of scandium (III) ions from aqueous media. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-12170-4
Ashour RM, El-sayed R, Abdel-Magied AF, Abdel-khalek AA, Ali MM, Forsberg K, Uheida A, Muhammed M, Dutta J (2017) Selective separation of rare earth ions from aqueous solution using functionalized magnetite nanoparticles: kinetic and thermodynamic studies. Chem Eng J 327:286–296. https://doi.org/10.1016/j.cej.2017.06.101
Mohammedi H, Miloudi H, Boos A, Bertagnolli C (2020) Lanthanide recovery by silica-Cyanex 272 material immobilized in alginate matrix. Environ Sci Pollut Res 27(21):26943–26953. https://doi.org/10.1007/s11356-020-08484-y
Bradshaw J, Izatt R, Savage P, Bruening R, Krakowiak K (2000) The design of ion selective macrocycles and the solid-phase extraction of ions using molecular recognition technology: a synopsis. Supramol Chem 12:23–26. https://doi.org/10.1080/10610270008029802
Makanyire T, Sanchez-Segado S, Jha A (2016) Separation and recovery of critical metal ions using ionic liquids. Adv Manuf 4(1):33–46. https://doi.org/10.1007/s40436-015-0132-3
Liu Y, Gao Y, Wei Z, Zhou Y, Zhang M, Hou H, Tian G, He H (2018) Extraction behavior and third phase formation of neodymium(III) from nitric acid medium in N,N′-dimethyl-N,N′-dioctyl-3-oxadiglcolamide. J Radioanal Nucl Chem 318(3):2087–2096. https://doi.org/10.1007/s10967-018-6261-y
Ruepert A, Keizer K, Steg L, Maricchiolo F, Carrus G, Dumitru A, García Mira R, Stancu A, Moza D (2016) Environmental considerations in the organizational context: a pathway to pro-environmental behaviour at work. Energy Res Soc Sci 17:59–70. https://doi.org/10.1016/j.erss.2016.04.004
Hu J, Zou D, Chen J, Li D (2020) A novel synergistic extraction system for the recovery of scandium (III) by Cyanex272 and Cyanex923 in sulfuric acid medium. Sep Purif Technol 233:115977. https://doi.org/10.1016/j.seppur.2019.115977
Yang Y, Arora G, Fernandez FA, Crawford JD, Surowiec K, Lee EK, Bartsch RA (2011) Lower-rim versus upper-rim functionalization in di-ionizable calix[4]arene-crown-5 isomers. Synthesis and divalent metal ion extraction. Tetrahedron 67(7):1389–1397. https://doi.org/10.1016/j.tet.2010.12.006
Lakshmanan V, Vijayan S (2018) A review on application of crown ethers in separation of rare earths and precious metals: proceedings of the first global conference on extractive metallurgy, pp 1913–1930. https://doi.org/10.1007/978-3-319-95022-8_159
Rounaghi GH, Mohajeri M, Doaei M, Ghaemi A (2010) Solvent influence upon complex formation between benzo-15-crown-5 and Mg2+, Ca2+ and Sr2+ cations in some pure and binary mixed solvents using conductometric method. J Incl Phenom Macrocycl Chem 67(3):443–450. https://doi.org/10.1007/s10847-009-9727-2
Abdurrahmanoglu S, Gündüz C, Çakır Ü, Çiçek B, Bulut M (2005) The synthesis and complexation study of some coumestan and coumestan analog derivatives of crown ethers using conductometry. Dyes Pigm 65(3):197–204. https://doi.org/10.1016/j.dyepig.2004.07.011
Kimura K, Shono T (1992) Chapter 4 - applications of crown compounds to analytical and separation chemistry: ion sensor and liquid chromatography. In: Hiraoka M (ed) Studies in organic chemistry, vol 45. Elsevier, Amsterdam, pp 198–264
Rounaghi GH, Mohajeri M, Tarahomi S, Rahmanian R (2011) Study of complex formation of dibenzo-18-crown-6 with Ce3+, Y3+, $\mathrm{UO}_{2}^{2 +}$ and Sr2+ cations in acetonitrile–dioxane binary solvent mixtures. J Solution Chem 40(3):377–389. https://doi.org/10.1007/s10953-011-9651-0
Khayatian G, Salehi F (2006) Conductance and thermodynamic study of the complexation of ammonium ion with different crown ethers in binary nonaqueous solvents. J Chin Chem Soc. https://doi.org/10.1002/jccs.200800055
Alivertis D, Paraskevopoulos G, Theodorou V, Skobridis K (2009) Dendritic effects of crown ether-functionalized dendrimers on the solvent extraction of metal ions. Tetrahedron Lett 50(44):6019–6021. https://doi.org/10.1016/j.tetlet.2009.08.053
Pozzi G, Quici S, Fish RH (2008) Perfluorocarbon soluble crown ethers as phase transfer catalysts. Adv Synth Catal 350:2425–2436. https://doi.org/10.1002/adsc.200800393
Rounaghi GH, Tarahomi S, Mohajeri M (2009) A conductometric study of complexation reaction between dibenzo-24-crown-8 with yttrium cation in some binary mixed non-aqueous solvents. J Incl Phenom Macrocycl Chem 63:319–325. https://doi.org/10.1007/s10847-008-9525-2
Çiçek B, Yıldız A (2011) Synthesis, metal ion complexation and computational studies of thio oxocrown ethers. Molecules. https://doi.org/10.3390/molecules16108670
Yongzhu J, Hirose K, Nakamura T, Nishioka R, Ueshige T, Tobe Y (2006) Preparation and evaluation of a chiral stationary phase covalently bound with a chiral pseudo-18-crown-6 ether having a phenolic hydroxy group for enantiomer separation of amino compounds. J Chromatogr A 1129(2):201–207. https://doi.org/10.1016/j.chroma.2006.07.003
Salman AD, Juzsakova T, Jalhoom MG, Le Phuoc C, Mohsen S, Adnan Abdullah T, Zsirka B, Cretescu I, Domokos E, Stan CD (2020) Novel hybrid nanoparticles: synthesis, functionalization, characterization, and their application in the uptake of scandium (III) ions from aqueous media. Materials. https://doi.org/10.3390/ma13245727
Shirikova A, Pasechnik L, Yatsenko S (2014) Prospects of application of microencapsulated extractants for extraction of scandium and rare-earth elements. Non-Ferrous Metals 1:41–44
Kostikova GV, Krasnova OG, Tsivadze AY, Zhilov VI (2018) Scandium extraction with benzo-15-crown-5 from neutral nitrate–trichloroacetate solutions. Russ J Inorg Chem 63(4):555–560. https://doi.org/10.1134/S0036023618040125
Pyrzyńska K, Kilian K, Pęgier M (2019) Separation and purification of scandium: from industry to medicine. Sep Purif Rev 48(1):65–77. https://doi.org/10.1080/15422119.2018.1430589
Marczenko Z, Balcerzak M (2000) Chapter 39 - rare-earth elements. In: Marczenko Z, Balcerzak M (eds) Analytical spectroscopy library, vol 10. Elsevier, Amsterdam, pp 341–349
Su J, Burnette R (2008) First principles investigation of noncovalent complexation: a [2.2.2]-cryptand ion-binding selectivity study. ChemPhysChem 9:1989–1996. https://doi.org/10.1002/cphc.200800376
Hancock R (1986) Macrocycles and their selectivity for metal ions on the basis of size. Pure Appl Chem 58:1445–1452. https://doi.org/10.1351/pac198658111445
Chekhlov AN (2003) Crystal structure of (2.2.2-cryptand)lithium perchlorate. Russ J Coord Chem 29(12):828–832. https://doi.org/10.1023/B:RUCO.0000008393.57920.7d
Moeller T, Martin DF, Thompson LC, Ferrús R, Feistel GR, Randall WJ (1965) The coordination chemistry of yttrium and the rare earth metal ions. Chem Rev 65(1):1–50. https://doi.org/10.1021/cr60233a001
Henderson P (2013) Rare earth element geochemistry. Elsevier, Amsterdam
Gongyi G, Yuli C, Yu L (1988) Solvent extraction off scandium from wolframite residue. JOM 40(7):28–31. https://doi.org/10.1007/BF03258146
Acknowledgements
The authors would like to thank the Hungarian GINOP-2.2.1-15-2017-00106 project: “Complex utilization of red mud and recovery of rare earth metals from red mud” and project of The University of Danang B2019-DN03-40 for the generous support of the research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Additional information
The contributing editor for this article was Hongmin Zhu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salman, A.D., Juzsakova, T., Jalhoom, M.G. et al. Potential Application of Macrocyclic Compounds for Selective Recovery of Rare Earth Scandium Elements from Aqueous Media. J. Sustain. Metall. 8, 135–147 (2022). https://doi.org/10.1007/s40831-021-00484-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00484-7