Abstract
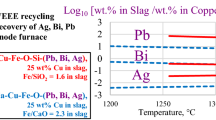
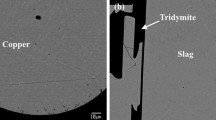
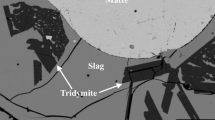
The present article continues the series reviewing the thermochemistry of complex multicomponent pyrometallurgical systems. The recovery of multiple metals through the Pb-based primary and recycling processes is an economic driving force for the circular economy of the future. In this study, equilibrium distributions of Ag, Au, Bi, and Zn between PbO–FeO–Fe2O3–SiO2 silica saturated slag and Pb metal phases were investigated experimentally, using high-temperature equilibration followed by rapid quenching. The measurement of phase compositions was done using microanalytical methods. Electron Probe X-ray Microanalysis (EPMA) was applied for those elements which present at relatively high concentrations. The Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) was used to measure very low concentrations of Ag and Au in slag. The starting mixtures of materials were planned using FactSage software and preliminary thermodynamic database to target specific proportions of phases and concentrations of Pb in slag after the achievement of equilibrium. Measured slag/metal distribution coefficients follow the sequence Zn ≫ Ag > Bi ≫ Au. Newly obtained results were critically assessed and used to improve the thermodynamic database. Integration between experiments and thermodynamic database development permits cross-analysis of distribution coefficients obtained in the present study with the parallel measurements of distribution in copper-based systems. The liquid slag phase was described using a two-sublattice Modified Quasichemical Model (MQM). Single sublattice MQM was applied for the liquid metal phase.




Similar content being viewed by others
References
Khaliq A, Rhamdhani MA, Brooks G, Masood S (2014) Metal extraction processes for electronic waste and existing industrial routes: a review Australian perspective. Resources 3:152–179. https://doi.org/10.3390/resources3010152
Tesfaye F, Lindberg D, Hamuyuni J, Taskinen P, Hupa L (2017) Improving urban mining practices for optimal recovery of resources from e-waste. Miner Eng 111:209–221. https://doi.org/10.1016/j.mineng.2017.06.018
Shishin D, Hayes PC, Jak E (2018) Multicomponent thermodynamic databases for complex non-ferrous pyrometallurgical processes. In: Davis B, Moats M, Wang S (eds) Extraction 2018: Proceedings of the first global conference on extractive metallurgy. Springer, New York, pp 853-868. https://doi.org/10.1007/978-3-319-95022-8_68
Jak E (2018) Modelling metallurgical furnaces—making the most of modern research and development techniques. In: Davis B, Moats M, Wang S (eds) Extraction 2018: Proceedings of the first global conference on extractive metallurgy. Springer, New York, pp 103-125. https://doi.org/10.1007/978-3-319-95022-8_8
Shishin D, Hidayat T, Chen J, Hayes PC, Jak E (2019) Combined experimental and thermodynamic modeling investigation of the distribution of antimony and tin between phases in the Cu-Fe-O-S-Si system. Calphad 65:16–24
Shishin D, Hidayat T, Chen J, Hayes PC, Jak E (2018) Integrated experimental study and thermodynamic modelling of the distribution of arsenic between phases in the Cu-Fe-O-S-Si system. J Chem Thermodyn 135:175–182
Shishin D, Hidayat T, Chen J, Hayes PC, Jak E (2018) Experimental Investigation and thermodynamic modelling of the distributions of Ag and Au between slag, matte and metal in the Cu-Fe-O-S-Si system. J Sustain Metal 5:240–249. https://doi.org/10.1007/s40831-019-00218-w
Shevchenko M, Jak E (2019) Experimental liquidus studies of the Pb-Fe-Si-O system in air. J Phase Equilib Differ 40:319–355. https://doi.org/10.1007/s11669-019-00727-x
Shevchenko M, Jak E (2018) Experimental liquidus studies of the Pb-Fe-Si-O system in equilibrium with metallic Pb. Metall Mater Trans B 49:159–180. https://doi.org/10.1007/s11663-017-1136-0
Shevchenko M, Jak E (2019) Thermodynamic optimization of the PbO-FeO-Fe2O3-SiO2 system. Calphad. https://doi.org/10.1016/j.calphad.2019.101670
Moon N, Hino M, Lee Y, Itagaki K (1999) Composition region of homogeneous liquid phase in lead-containing slas related to QSL process. Metal Rev MMIJ 16:3–14
Moon N, Hino M, Lee Y, Itagaki K (1998) Phase equilibrium and minor elements distribution between metallic lead and PbO-FeOx-CaO-SiO2 or PbO-FeOx-CaO-SiO2-ZnO Slag at 1423K. Metal Rev MMIJ 15:38–62
Jak E (2012) Integrated experimental and thermodynamic modelling research methodology for metallurgical slags with examples in the copper production field. In: Proceedings of Paper presented at the 9th International Conference on Molten Slags, Fluxes and Salts (MOLTEN12), Beijing, China,
Jak E, Hayes PC, Lee H-G (1995) Improved methodologies for the determination of high temperature phase equilibria Korean. J Miner Mater Inst (Seoul) 1:1–8
Shevchenko M, Jak E (2019) Experimental liquidus study of the binary PbO-ZnO and ternary PbO-ZnO-SiO2 systems. Ceram Int 45:6795–6803. https://doi.org/10.1016/j.ceramint.2018.12.172
Bale CW et al (2016) FactSage thermochemical software and databases, 2010–2016. CALPHAD 54:35–53. https://doi.org/10.1016/j.calphad.2016.05.002
Fallah-Mehrjardi A, Hidayat T, Hayes PC, Jak E (2017) Experimental Investigation of gas/slag/matte/tridymite equilibria in the Cu-Fe-O-S-Si system in controlled gas atmospheres: development of technique. Metall Mater Trans B 48:3002–3016. https://doi.org/10.1007/s11663-017-1073-y
Chen J, Allen CM, Azekenov T, Ushkov LA, Hayes P, Jak E Quantitative determination of trace/ultra trace elements concentration in slag and matte generated in copper smelting using microanalysis techniques. In: Yamaguchi K (ed) Copper 2016, Kobe, Japan, 13-16 November 2016. MMIJ and JMIA, pp 1096-110
Pelton AD, Decterov SA, Eriksson G, Robelin C, Dessureault Y (2000) The Modified quasichemical model: I—binary solutions. Metall Mater Trans B 31:651–659
Pelton AD, Chartrand P (2001) The Modified Quasichemical Model: II–multicomponent solutions. Metall Mater Trans A 32:1355–1360
Hillert M (2001) The compound energy formalism. J Alloys Compd 320:161–176
Redlich O, Kister AT (1948) Thermodynamics of nonelectrolytic solutions: algebraic representation of thermodynamic properties and the classification of solutions. Ind Ing Chem 40:345–348
Eriksson G, Pelton AD (1993) Critical evaluation and optimization of the thermodynamic properties and phase diagrams of the CaO-Al2O3, Al2O3-SiO2, and CaO-Al2O3-SiO2 systems. Metall Trans 24:807–816
Hidayat T, Shishin D, Jak E, Decterov S (2015) Thermodynamic reevaluation of the Fe-O system. CALPHAD 48:131–144
Decterov SA, Jak E, Hayes PC, Pelton AD (2001) Experimental study and thermodynamic optimization of the Fe-Zn-O system. Metall Mater Trans B 32:643–657
Shishin D, Prostakova V, Jak E, Decterov S (2016) Critical assessment and thermodynamic modeling of the Al–Fe–O system. Metall Mater Trans B 47:397–424
Hidayat T, Shishin D, Decterov SA, Jak E (2017) Experimental study and thermodynamic re-evaluation of the FeO-Fe2O3-SiO2 system. J Phase Equilib Diffus 38:477–492. https://doi.org/10.1007/s11669-017-0535-x
Shevchenko M, Jak E (2019) Thermodynamic optimization of the binary systems PbO-SiO2, ZnO-SiO2, PbO-ZnO, and ternary PbO-ZnO-SiO2. Calphad 64:318–326. https://doi.org/10.1016/j.calphad.2019.01.011
Shevchenko M (2019) Integrated experimental and thermodynamic modelling research on the multicomponent Pb-Cu-Fe-Zn-Ca-Si-O system. PhD Thesis, School of Chemical Engineering, The University of Queensland
Lee B-Z, Oh C-S, Lee DN (1994) A thermodynamic evaluation of the Ag-Pb-Sb system. J Alloys Compd 215:293–301
Wang J, Liu HS, Jin ZP (2004) Thermodynamic assessment of the Au-Pb system. CALPHAD 28:91–95. https://doi.org/10.1016/j.calphad.2004.05.003
David N, Hertz J, Fiorani JM (2003) Thermodynamic assessment of the Pb-Zn system. Z Metallkd 94:8–11. https://doi.org/10.3139/146.030008
Gierlotka W, Tung Y-C (2017) A new thermodynamic description of the Bi-Pb-Sn system. J Phase Equilib Diffus 38:814–828. https://doi.org/10.1007/s11669-017-0572-5
Hidayat T, Chen J, Hayes PC, Jak E (2018) Distributions of Ag, Bi, and Sb as minor elements between iron-silicate slag and copper in equilibrium with tridymite in the Cu-Fe-O-Si system at T = 1250 & #xB0;C and 1300 & #xB0;C (1523 K and 1573 K). Metall Mater Trans B. https://doi.org/10.1007/s11663-018-1448-8
Nagamori M, Mackey PJ, Tarassoff P (1975) Distribution of arsenic, antimony, bismuth, selenium, and tellurium between molten copper and white. Metal Metall Trans B 6B:197–198
Chen C, Wright S (2016) Distribution of Bi between slags and liquid copper. Metall Mater Trans B. https://doi.org/10.1007/s11663-016-0610-4
Takeda Y, Ishiwata S, Yazawa A (1983) Distribution equilibriums of minor elements between liquid copper and calcium ferrite slag. Trans Jpn Inst Met 24:518–528
Jimbo I, Goto S, Ogawa O (1984) Equilibria between silica-saturated iron silicate slags and molten copper-arsenic, copper-antimony, and copper-bismuth alloys. Metall Trans B 15B:535–541. https://doi.org/10.1007/bf02657385
Assal J, Hallstedt B, Gauckler LJ (1997) Thermodynamic assessment of the silver-oxygen system. J Am Ceram Soc 80:3054–3060
Shevchenko M, Shishin D, Jak E (2019) Experimental study and thermodynamic modelling of the Ag2O-PbO-SiO2 slag in equilibrium with silver metal Internal report. The University of Queensland, Pyrometallurgy Innovation Centre
Borisov A, Palme H (1996) Experimental determination of the solubility of Au in silicate melts. Miner Petrol 56:297–312. https://doi.org/10.1007/bf01162608
Han YS, Swinbourne DR, Park JH (2015) Thermodynamics of gold dissolution behavior in CaO-SiO2-Al2O3-MgOsat slag system. Metall Mater Trans B 46:2449–2457. https://doi.org/10.1007/s11663-015-0421-z
Swinbourne DR, Yan S, Salim S (2005) The solubility of gold in metallurgical slags. Trans Inst Min Metall C 114:C23–C29. https://doi.org/10.1179/037195505x28429
Avarmaa K, O’Brien H, Taskinen P (2016) Equilibria of gold and silver between molten coppe -SiO2-Al2O3 slag in WEEE smelting at 1300 °C. In: Ramana G. Reddy PC, P. Chris Pistorius, and Uday Pal (eds) Advances in molten slags, fluxes, and salts: proceedings of The 10th international conference on molten slags, fluxes and salts (MOLTEN16). The Minerals, Metals & Materials Society, pp 193-202
Sukhomlinov D, Taskinen P (2017) Distribution of Ni, Co, Ag, Au, Pt, Pd between copper metal and silica saturated iron silicate slag. In: Proceedings of EMC 2017, Leipzig, Germany, pp 1-10
Jak E, Decterov SA, Wu P, Hayes PC, Pelton AD (1997) Thermodynamic optimisation of the systems PbO-SiO2, PbO-ZnO, ZnO-SiO2 and PbO-ZnO-SiO2. Metall Mater Trans B 28B:1011–1018
Shevchenko M, Jak E (2019) Experimental Liquidus Studies of the Zn-Fe-Si-O System in air. IJMR 110:600–607. https://doi.org/10.3139/146.111779
Hidayat T, Hayes PC, Jak E (2018) Phase equilibria in the ZnO-”FeO”-SiO2 system in reducing atmosphere and in the ZnO-”FeO”-SiO2-”Cu2O” system in Equilibrium with liquid copper metal at 1250°C (1523 K). Metall Mater Trans B 49:1766–1780. https://doi.org/10.1007/s11663-018-1285-9
Risold D, Hallstedt B, Gauckler LJ, Lukas HL, Fries SG (1995) The bimuth-oxygen system. J Phase Equilib 16:223–234. https://doi.org/10.1007/bf02667306
Diop I, David N, Fiorani JM, Podor R, Vilasi M (2009) Experimental investigations and thermodynamic description of the PbO-Bi2O3 system. J Chem Thermodyn 41:420–432. https://doi.org/10.1016/j.jct.2008.10.012
Onderka B, Fitzner K, Kopyto M, Przybyło W (2017) Thermodynamics of Bi2O3-SiO2 system. J Mining Metal B 53:223–231
Lu J, Qiao LJ, Fu PZ, Wu YC (2011) Phase equilibrium of Bi2O3-Fe2O3 pseudo-binary system and growth of BiFeO3 single crystal. J Cryst Growth 318:936–941. https://doi.org/10.1016/j.jcrysgro.2010.10.181
Maitre A, Francois M, Gachon JC (2004) Experimental study of the Bi2O3-Fe2O3 pseudo-binary system. J Phase Equilib Diffus 25:59–67. https://doi.org/10.1007/s11669-004-0171-0
Phapale S, Mishra R, Das D (2008) Standard enthalpy of formation and heat capacity of compounds in the pseudo-binary Bi2O3-Fe2O3 system. J Nucl Mater 373:137–141. https://doi.org/10.1016/j.jnucmat.2007.05.036
Yazawa A, Nakazawa S, Takeda Y Distribution behavior of various elements in copper smelting systems. In: Proceedings of International Sulfide Smelting Symposium, San Francisco, USA, 1983. Metall. Soc. AIME, pp 99-117
Hollitt MJ, Willis GM, Floyd JM (1984) Thermodynamics of the silica-saturated Pb-Fe-O-SiO2 system at 1200oC. 2nd Int Symp Metal Slags Fluxes. 10.1016/j.calphad.2019.01.011
Yazawa A, Takeda Y, Waseda Y (1981) Thermodynamic properties and structure of ferrite slags and their process implications. Can Metall Q 20:129–134
Acknowledgements
The authors acknowledge the financial support and technical guidance by the consortium of lead producers: Aurubis, Kazzinc Glencore, Umicore, Nystar, Peñoles, and Boliden through Australian Research Council Linkage Program LP180100028. The present study would not be possible without the facilities and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis in The University of Queensland. Dr. Charlotte Allen at the Centre of Analytical Research Facilities at Queensland University of Technology, Brisbane, Australia provided valuable contribution to the development of the LA-ICP-MS technique.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Markus Reuter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shishin, D., Hidayat, T., Sultana, U. et al. Experimental Study and Thermodynamic Calculations of the Distribution of Ag, Au, Bi, and Zn Between Pb Metal and Pb–Fe–O–Si slag. J. Sustain. Metall. 6, 68–77 (2020). https://doi.org/10.1007/s40831-019-00257-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-019-00257-3