Abstract
In this paper, an efficient and product-oriented hydrometallurgical recycling process including pre-treatment is developed to handle the spent automotive Li–ion batteries. The possibility to recover the high-grade graphite, cathode metal salts and lithium carbonate is investigated. In the designed process, leaching, solution refining, cathode metals precipitation and lithium carbonate crystallisation are implemented. The leaching efficiencies of valuable metals (Co, Ni, Cu and Li) are in the range of 98.6–99.9 % under the optimum conditions: 80 °C, 50 g/L of hydrogen peroxide, 2 mol/L of sulphuric acid or 4 mol/L of hydrochloric acid in 2 h. Meanwhile, the filtered graphite with purity of 99.8 % is obtained. In the following Cu cementation, an optimum temperature of 60 °C is found and the calculated activation energy of the cementation reaction is 12.9 kJ/mol. In the hydroxide precipitation, pH 3.5–4 is suggested for Al and Fe removal and pH 10 is high enough for cathode metal (Co, Ni and Mn) salts precipitation. The carbonate and sulphide precipitation methods are also demonstrated to be successful. In all, several marketable products are obtained, such as graphite, Cu powder, cathode metal salts and lithium carbonate.
Similar content being viewed by others
Introduction
Nowadays, utilisation of clean energy gets more and more attention due to the large amount of the off-gas exhausted by vehicles and heavy industry. As known, the electric vehicles (EVs) have higher energy efficiency and less CO2 emission than the traditional vehicles. The production and sale of EVs might be widely promoted in the future, especially when the global warming becomes too obvious. For the electric vehicles, the Li–ion battery has the highest opportunity to be widely used due to its outstanding features in comparison with conventional batteries, such as high voltage, high energy density, low self-discharge rate and wide temperature range of operation. It is possible to forecast that the recycling market for automotive lithium–ion batteries (LiBs) will dramatically increase in the near future [1].
Different from a single Li–ion cell, the automotive Li–ion battery consists of a set of cells. Normally, a certain number of cells are packed together into a unit so-called “module” and the battery is composed of several modules. Therefore, the structure of automotive batteries is more complicated than portable Li–ion batteries. The electrical components, e.g. printed circuit board (PCB), are required to control different modules and cells in voltage and temperature [2].
In the European Union (EU), the directive 2006/66/EC regulates the management of batteries at the end of life. The disposal in landfills or incineration of waste industrial and automotive batteries and accumulators is prohibited. This legislative framework commits producers and traders of all kinds of batteries to take the spent batteries back and assure a state-of-the-art treatment and recycling. Additionally, the recycling rate of spent Li–ion batteries is obliged to reach at least 50 % by weight before September 29, 2010 [3].
The recycling processes of Li–ion batteries, which are currently operated in industrial scale in the world, can be divided into three types: pyrometallurgy, hydrometallurgy and pure mechanical treatment. More than 10 companies are recycling thousands of metric tons of spent portable and industrial Li–ion batteries annually. The companies, such as Umicore (Belgium), Xstrata Nickel (Canada), Accurec (Germany), Inmetco (USA), S.N.A.M (France) and Sony-Sumitomo (Japan) use pyrometallurgy as the main recycling process to recycle rechargeable portable and industrial Li–ion batteries also including NiMH and NiCd batteries. Valuable metals, such as Co and Ni, are fully recovered in the form of alloy at high temperature. The other easily vaporised or ignoble metals (e.g. Al, Li and Cd) enter in slag or flue dust [4–8].
Diversity in process design is presented in the hydrometallurgical processes. In the Recupyl (France) process, the valuable metals have been recycled as metal salts in the product. A pilot plant for the research project “Lithorec” was built in company Rockwood Lithium (Germany) in which an electrochemical membrane process (Electrodialysis) is used to synthesise LiOH from the solution. Especially, the Retriev Technologies/Toxco (USA) has applied a cryogenic freezing process to make the Li–ion batteries fragile, and then crush them before the hydrometallurgical separation [9–11]. Different from the above-mentioned processes, Batrec (Switzerland) focuses on the mechanical treatment technology. Through a complex mechanical treatment, the spent Li–ion batteries are converted to several marketable concentrates that can be used as raw material for other metallurgical processes [12].
Many studies in laboratory scale were reported using hydrometallurgical methods. H2SO4, HCl and HNO3 are often used as leaching agents to extract metals in the electrode powder. Generally, an additive-like hydrogen peroxide (H2O2) is required to obtain the high dissolution efficiency. The temperature in the range of 50–85 °C and acid concentrations in the range of 2–6 mol/L were usually used with the solid–liquid ratio of 33:125 g/L. For the material, which has a high Al content, a basic leaching with NaOH was performed to recycle Al. As a result, the leaching efficiency of investigated metals can reach 80 % under certain leaching conditions. In term of solution purification, solvent extraction using PC88A and Cyanex272 is widely used. Cathode metals (Co, Ni and Mn) can be selectively extracted under different conditions. On the other hand, the precipitation methods also play an important role, such as the Fe precipitation as goethite, Co hydroxide and Co oxalate precipitation. A co-precipitation method of Co, Ni and Mn is published in the form of patent, in which the cathode material is synthesised from the co-precipitated metal salts [13]. Other methods, such as the crystallisation of Co sulphate (CoSO4) and electrolytic depositions of Co, have also recovered Co successfully [14–22].
Based on the above results and discussion, the presented solutions cannot satisfy the new requirement in the aspect of environment and recycling efficiency, therefore an efficient and product-oriented recycling process is highly needed to handle the foreseeable large amount of spent automotive Li–ion batteries. The motivations of this work are summarised below:
-
Development of a recycling process, which is efficient, economical and environmental friendly.
-
Recovery of the high-purity graphite as a marketable product under the optimised leaching conditions.
-
Recovery of the cathode metal salt as a precursor using different precipitation methods.
Design of Hydrometallurgical Recycling Process
A possibility to recycle the high-purity graphite as a marketable product is reported in this paper. In the industrial process, the leaching residual of electrode powder is mainly an impure graphite, which can only be treated as industrial waste. However, in the newly developed process, the purity of graphite is highly increased through optimising the leaching conditions.
Another novelty is the recovery of cathode metals avoiding separation of them. The traditional separation of Co and Ni metallurgy by solvent extraction is a very complicated and slow method. Moreover, the separated Co and Ni salts are mixed again during the synthesis of the new cathode material, such as LiCo1/3Ni1/3Mn1/3O2. Instead of separating Co and Ni, a new method is designed to precipitate cathode metals together. The obtained salts could serve as the precursor for synthesis of new cathode material.
The designed highly efficient hydrometallurgical recycling process is shown in Fig. 1. In the leaching step, two main objectives are set. One is to dissolve all the metals with a high efficiency and obtain graphite with high purity by the filtration. The other is to discover the optimum leaching conditions. Subsequently, Cu, Al and Fe are recovered in the solution-refining step. The cementation process is firstly operated to recover Cu. Secondly, the NaOH and H2O2 are added to adjust the pH of the solution and oxidise the Fe2+ to Fe3+ simultaneously. Al and Fe hydroxides are precipitated from the solution with an optimum pH value. The co-precipitation of other metals should be minimised under the optimum conditions.
In the following step, cathode metals (Co, Ni and Mn) are recovered by hydroxide, carbonate and sulphide precipitation, respectively. The obtained Li solution after filtration is ready for Li2CO3 crystallisation through adding Na2CO3 solution at 95 °C. To make the close loop of Li, the Li containing raffinate is fed back to Li2CO3 crystallisation step.
Theory of Applied Hydrometallurgical Processes
Leaching
The sulphuric acid (H2SO4) and hydrochloric acid (HCl) are chosen as acid leaching agents in this paper. The leaching reaction for LiCoO2 at the presence of hydrogen peroxide (H2O2) in sulphuric acid (H2SO4) is as follows [21]:
The dissolution of LiCoO2 involves the reduction of Co3+ in the solid species to Co2+ in the aqueous phase. Similarly, the reactions of LiNiO2 and LiMnO2 are consistent with the above reaction. In addition, H2O2 is employed to oxidise the metallic Cu to Cu2+ ions. The chemical reaction between H2O2 and Cu is as follows:
Cu Cementation
Cu cementation with Fe is in extensive use in the hydrometallurgy. In principle, the ignoble metals can reduce the noble metal ions according to the electromotive force (EMF) series. The larger the voltage gap between the two half-cell reactions, the higher is the driving force for the reaction. In the case of the Cu cementation with Fe powder, the reaction is written below [23]:
The Cu cementation mechanism can be described using the first-order reaction kinetic as follows [23, 24]:
where [Cu2+] is the Cu concentration, k is the rate constant and t is the time.
By separation of variables and integration of the concentration and time, the result in a logarithmic relationship between the ion concentration and the time is as follows:
The reaction rate constants (k) for different temperatures can be determined, which allow a determination of the Arrhenius activation energy in the following reaction:
where k is the rate constant, E A is the activation energy (J/mol), R = 8.314 J*K−1*mol−1 is the gas constant, T is the temperature (K) and A is the stoichiometric coefficient
Taking the logarithm of Eq. 6, a linear relationship between the rate constants (k) and 1/T is obtained. However, a plot to 1000/T is frequently selected since the values of the x axis are more manageable. Finally, the activation energy (E A) can be calculated from the slope of this straight line. The equation is then as follows:
Experimental
Pre-treatment of Spent Automotive Li–ion Batteries
Different from portable Li–ion batteries, the automotive Li–ion batteries must be dismantled at the beginning because of the large size and weight. The industrial partner has gathered the samples of spent Li–ion automotive batteries from the manufactures. Figure 2 has shown the procedure of pre-treatment. The steel casing, Cu cables, plastics and electrical components (PCB) were manually separated from the cells in the dismantling step. In the following vacuum-thermal deactivation step, the battery cells were treated under thermal and vacuum conditions to deactivate the cells and vaporise the electrolyte. In an inductive heating vacuum furnace, the pressure of the furnace was decreased from 1 bar to 100 mbar. After that, the temperature was increased to 500 °C slowly. At this temperature, the volatile components like organics, halides and cracking products would evaporate and leave the cell through a pressure vent and were recovered in a condenser. After the vacuum–thermal treatment, the deactivated cells were granulated and sieved. By this way, the Cu and Al foils were separated from electrode powder. At last, the electrode powder was collected and applied as the raw material for leaching in the hydrometallurgical process.
Characterisation of the Electrode Powder
The chemical composition of the electrode powder is assayed by ICP–OES method and shown in Table 1. The contents of Co and Ni are about 22 and 9.9 %, respectively. Al, Fe, Mn and Cu have low contents, which are in the range of 0.2–1.18 %. However, Li content is about 3.7 % in the raw material. It has a very small variability in many kinds of electrode powder. As the main component in the powder, graphite takes more than one-third of the whole weight of powder.
Particle size analysis was performed by Sympatec’s laser diffraction sensor HELOS. The particle size distribution of three random samples in terms of cumulative passing percentage versus size in microns was assayed. In the result, about 10 % of the particles are smaller than 5 μm and 90 % of the particles are smaller than 60 μm. The d 50 equals to 20 μm.
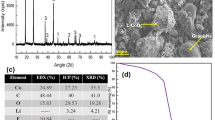
Furthermore, the SEM photograph of the electrode powder is shown in Fig. 3, and the graphite particles, metallic particles and metal compounds are mixed homogeneously. As the dismantled cells are completely crushed and sieved in the pre-treatment process, the shape of particles is relatively inerratic.
Laboratory Tests
The electrode powder obtained by pre-treatment was used in the leaching tests. The effects of parameters, such as temperature (25–70 °C), hydrogen peroxide concentration (0–100 g/L) and sulphuric acid concentration (2–4 mol/L), were investigated with 100 g/L solid liquid ratio and 75 min retention time. Since H2O2 can easily decompose, 50 % of the required amount of H2O2 was added at the beginning, and the rest of H2O2 was added continuously throughout the whole retention time using a burette. When the leaching process was finished, the leaching solution was filtered using a vacuum filter. The filtered graphite was washed with deionized water to remove the acid traces and dried in an oven at 90 °C for 10 h before it was weighed. Additionally, the verification experiments were carried out in sulphuric acid (2 mol/L) and hydrochloric acid (4 mol/L) at the presence or absence of hydrogen peroxide (50 g/L) with 100 g/L solid liquid ratio and 120 min retention time. The photography of filtered graphite was characterised by the SEM method.
In Cu cementation tests, a metallic Fe powder with particle size “200 Mesh” was used to reduce Cu2+ in the leaching solution. Firstly, a 300 mL of solution was poured into a 600-mL beaker that was placed on the heating plate. Following, the solution was stirred at 150 RPM and heated up to the target temperatures (25, 40, 60 and 80 °C) before adding the powder.
For the metal hydroxide and carbonate precipitation tests, titration experiment with a 400 mL of initial solution was instructed by adding NaOH solution (200 g/L) and Na2CO3 solution (200 g/L) drop by drop using a 50-mL burette at 40 °C. The pH was increased from pH 1 to 10 during the experiment. Solution samples were taken using a 15-mL pipette at specific pH levels. For the metal sulphide precipitation, experiment started by addition of a Na2S·9H2O solution (100 g/L) using a 50-mL burette. The concentration of sulphide ions in solution was increased from 0 to 10.5 g/L. Figure 4 has shown the filtration of cathode metal salts with the three methods.
Li2CO3 crystallisation by titration with Na2CO3 (200 g/L) was implemented. 500 mL of initial solution generating from metal hydroxide precipitation was evaporated at 95 °C within 3 h until the volume of solution reduced to about 100 mL. At this time, a solution of Na2CO3 (200 g/L) was titrated into the solution until attaining 1.2 times (stoichiometric equivalent) the moles of Li ions. After 10 min, the crystallised white powder was filtered and prepared for chemical assay.
Results and Discussion
Leaching
Effect of Temperature
The extraction rates of Li, Co, Cu, Ni, Al, Fe and Mn in the leaching process at different temperatures are shown in Fig. 5. Apparently, Ni dissolution increases from 50 to 97 % (∆ = 47 %) as the temperature increases from 22 to 70 °C. Similarly, the extraction rate of Fe has also increased a lot with increasing the temperature. About 5 % increment for the Co extraction rate is found in the same range of temperature.
However, the effect of the temperature on other metals is not obvious. The extraction rates of Li, Al and Cu are larger than 88 % at 22 °C. As the temperature increases from 22 to 70 °C, the dissolved Li changes gradually from 95 to 98.5 % (∆ = 3.5 %), meanwhile the extraction rate of Cu increases from 96 to 98.6 % (∆ = 2.6 %). Almost 100 % of Al is dissolved since 60 °C. The Mn has an average extraction rate of 85 % in all trials.
Accordingly, the optimum temperature of the leaching is 70 °C among the 4 tested temperatures, since it exhibits the highest metal extraction rates for almost all the metals.
Effect of Hydrogen Peroxide (H2O2) Concentration
The extraction rates of the Li, Co, Ni, Cu, Al, Fe and Mn are shown in Fig. 6 at different H2O2 concentrations of 0, 25, 50, 75 and 100 g/L. The strongest response to the H2O2 addition is revealed on the Cu extraction rate, which goes from 7.9 % sharply up to 96.6 % (∆ = 88.7 %) as the H2O2 concentration increases from 0 to 25 g/L. It can be explained by the noble nature of metallic Cu in the electrode powder; hence, a strong oxidising reagent is needed to dissolve Cu. The extraction rate of Ni rises from 48.9 to 83.7 % (∆ = 34.8 %) as the H2O2 concentration increases from 0 to 25 g/L. However, when the H2O2 concentration is further increased to 100 g/L, the extraction rates of Cu and Ni increase only by 2 %.
On the other hand, Li, Co, Al and Mn have shown insignificant improvement of the extraction rates with the addition of H2O2. By increasing the H2O2 concentration from 0 to 25 g/L, the extraction rate of Li increases from 95.7 to 97.7 % (∆ = 2 %) and Co extraction rate rises from 94.3 to 98.4 % (∆ = 4.1 %). Furthermore, the extraction of Al and Mn has increased by 10.2 and 8.4 %, respectively. The extraction of metallic Fe is not affected by the H2O2; however, it is very helpful for the oxidation of bivalent iron. According to the above results, it can be concluded that 25 g/L of H2O2 is efficient for Cu and Ni leaching. Nevertheless, 50 g/L of H2O2 is recommended to ensure a high extraction rate for all metals.
Effect of Sulphuric Acid Concentration
The effects of the sulphuric acid concentrations (1, 1.33, 2 and 4 mol/L) on the extraction rates of Li, Co, Ni, Cu, Al, Fe and Mn are shown in Fig. 7. An increment of Ni extraction (∆ = 21.4 %) is observed when the concentration of H2SO4 acid rises from 1 to 4 mol/L. Similarly, the extraction of Fe was also increased from 41.9 % (1 mol/L) to 88.1 % (4 mol/L).
It can be seen that the effect of sulphuric acid concentration on dissolution of Li, Co, Cu, Mn and Al is not obvious. Cu extraction rate is kept at a high level and increases by 4.1 % as the acid concentration increases from 1 to 4 mol/L. The extraction rates of Li and Co increase by 1.7 and 2 %, respectively. The extraction rate of Mn fluctuates at 90 %.
Verification Tests of Leaching
After investigating the effects of different parameters, 80 °C, 50 g/L of H2O2, 2 mol/L of sulphuric acid or 4 mol/L of hydrochloric acid are recommended for the subsequent verification leaching. Leaching experiments with different acids (2 mol/L H2SO4 and 4 mol/L HCl) and different concentrations of additive reagent (0 and 50 g/L H2O2) were carried out at 80 °C with solid liquid ratio of 100 g/L. The retention time was extended to 2 h to ensure a high leaching efficiency.
The extraction rates of Co, Ni, Li, Cu, Al, Fe and Mn are shown in Fig. 8. The extraction rates of Co in the four trails are all higher than 99.9 %. Similarly, the extraction rates of Li have reached 99.3 %. At the presence of H2O2 (50 g/L), the Cu dissolution is higher than 99.9 % in both HCl and H2SO4 acids. However, at the absence of H2O2, the extraction rate of Cu is only 73.2 % in H2SO4 media and 87.1 % in HCl media. On the other hand, the extraction of Ni mainly depends on the types of acid. Ni is easier dissolved in HCl than in H2SO4. It has reached 99.9 % in HCl regardless of H2O2; yet, 98.6 % of Ni is leached in H2SO4 media at the presence of H2O2, and it is even lowered to 97.9 % at the absence of H2O2. The extraction rates of Mn and Al in all trails are over 99 and 98 %, respectively. The Fe has a relative wide extraction range (94.8 % in “HCl + H2O2” and 99.8 % in “H2SO4 + H2O2”). Considering the very low composition of Fe (0.31 %), this extraction rate can be accepted. As a summing up, both acids are able to leach Al, Li, Fe, Mn and Co efficiently. The Ni and Cu are easier be leached in HCl than H2SO4. The adding of H2O2 in acid significantly increases the dissolution of Cu.
In addition, the chemical compositions of the filtered graphite are assayed and shown in Table 2. The carbon contents in the residues are above 99.8 % after leaching at the presence of H2O2 in both acids. However, in the experiments at the absence of H2O2, the purities of graphite are only 98.5 % in sulphuric acid and 99.5 % in hydrochloric acid, respectively. From the SEM photographs of filtered graphite (from trail “HCl + H2O2”) comparing with the electrode powder in Fig. 3, it can be observed that the shape and the size of graphite are not changed before and after leaching. The fine morphology and high purity ensure the feasibility of reutilisation as the battery-level graphite.
Cu Cementation
The Cu contents in solution by time at different temperatures are shown in Fig. 9. Apparently, the concentrations of Cu in solution decrease sharply within the first 5 min. Nearly, all Cu ions are reduced in 40 min. It is clear that the test at room temperature (25 °C) shows the slowest cementation kinetic. Cu precipitates faster at higher temperature. On the other hand, the oxidised Fe has also demonstrated the reaction kinetics, as shown in Fig. 10. It is found that Fe powder dissolves fast at high temperature, especially at 80 °C.
Additionally, the activation energy of Cu cementation is calculated based on the Arrhenius plot. According to Eq. 5, the rate constants (k) at different temperatures are obtained from the slope of the regression line. Following, an Arrhenius plot of ln(k) to 1000/T is shown in Fig. 11. At last, the activation energy (E A) is computed from the slope of this straight line based on Eq. 7.
The final calculated activation energy is 12.92 kJ/mol as shown in Eq. 8, which is basically consistent with other researchers, such as El-Batouti (25–39 kJ/mol) and Miller (36 kJ/mol). It indicates that the reaction of Cu cementation by Fe is mainly controlled by the boundary layer diffusion. Therefore, the high temperature is favoured for the Cu cementation; hence, 60 °C is efficient among the four tested temperatures and avoids too much dissolution of Fe [24, 25].
Metal Hydroxide Precipitation (Fe, Al and Cathode Metals)
After the removal of Cu, metal hydroxide precipitation is implemented to precipitate Fe and Al, afterwards, Co, Ni and Mn. In principle, Fe is precipitated in the form of goethite (α-FeO·OH) according to Eq. 9. The final concentration of Fe3+ in the solution is in general no more than 1 g/L. Al is precipitated as a metal hydroxide in Eq. 10 [23, 26].
It should be stressed that the performance of precipitation is highly dependent on the pH value of the solution. The removal of Fe and Al is not complete when pH value is lower than the demanded value. On the other hand, Co, Ni and Mn will co-precipitate when pH value is too high. Therefore, an optimum pH value is required to make sure more removal of Fe and Al and simultaneously less loss of other valuable metals. Figure 12 shows the metal precipitation diagram at 40 °C by titration with NaOH. The contents of Fe, Al and Cu in the solution decrease rapidly from pH 2.3 to pH 4.8. Obviously, Cu precipitates less than Fe and Al at the same pH value. When pH 4.8 is attained, 99.75 % of Fe has already been precipitated, while 6.9 % of Al and 43.8 % of the Cu are still in solution. At the end of Fe and Al precipitation, the co-precipitation (loss) of Co and Ni has reached a relatively high level, which cannot be ignored. It has a high risk to lose the valuable Co and Ni metals in this step if the pH value is not concisely controlled. Hence, the optimum ending pH value should be 3.5–4 to ensure the sufficient removal of Fe and Al and also minimum loss of Co, Ni and Mn. Until pH 5.8, nearly all Fe, Al and Cu are precipitated. The precipitation rates have reached 21.4 % for Ni, 17.3 % for Co and 14.1 % for Mn, respectively. At the same pH value, Mn presents a lower precipitation rate than Co and Ni. At pH 8, more than 90 % of Co and Ni have been precipitated. However, 59 % of Mn is still in solution. At pH 9, more than 99.8 % of Co and Ni metals are removed from solution. Finally, Mn is completely (>99.9 %) removed from the solution at pH 10.37. In addition, Li in solution decreases by 20 % at the end of the experiment because of the absorption.
Metal Carbonate Precipitation
The cathode metal precipitation by titration with Na2CO3 is presented in Fig. 13. From pH 2 to 3.5, the precipitation of Fe and Al proceed very fast. In contrast, the other metals exhibit very low precipitation rates in this range of pH value. When pH 4.5 is attained, nearly all Fe has been precipitated, while 40 % of the Al and 83 % of the Cu are still in the solution. Until pH 5.7, 99.6 % of Fe, 96.7 % of Al and 90.5 % of Cu are removed from the solution. However, the percentages of Ni, Co and Mn in the solution are still 80, 89 and 87 %, respectively. It can be seen that Ni, Mn and Co drastically precipitate since pH 6.5 and more than 99 % at pH 8.5. At last, Ni, Mn and Co contents in solution are lower than 0.7 % when pH 9.8 is reached. Different from the cathode metals, Li in the solution decreases slowly as the pH value increases. At the end of the experiment, the Li content in the solution has decreased by 21 %.
Metal Sulphide Precipitation
Metal precipitation using sodium sulphide (Na2S·9H2O) is assessed as an effective method to precipitate Cu and cathode metals. Figure 14 shows the metal contents in solution by changing the concentration of S2− ions with the titration of Na2S. Due to the very low solubility of CuS, Cu concentration in solution decreases extremely fast and 82.4 % of Cu has been precipitated when [S2−] is only 1.28 g/L. When changing [S2−] from 2.04 to 4.5 g/L, the Co in solution decreases from 88 to 56 % and Ni decreases from 87 % to 35 %. Furthermore, only 24 % of Co and 3 % of Ni remain in solution at 7.40 g/L of [S2−]. However, Mn precipitates slowly and there is still 56 % of Mn in solution. When [S2−] has reached 10.33 g/L, nearly all Co and Ni have been precipitated and 38 % of Mn is still in solution. To further precipitate Mn, more S2− ions are required.
Li2CO3 Crystallisation
Lithium carbonate exhibits the lowest solubility among the possible formed salts as shown in Table 3. The solubility of Li2CO3 is 13.3 g/L at 25 °C and decreases to 7.2 g/L at 100 °C [23]. The lithium carbonate crystallisation is described in Eq. 11:
Evidently, the XRD pattern (s. Fig. 15) has indicated the existence of Li salt in the residue as Li2CO3. The purity of crystallised Li2CO3 has reached 90.9 %, which could be computed from the weight percentage of Li (17.2 %) in the product. The compositions of other metals are quite low. Regarding to the efficiency, 59.7 % of Li is crystallised, while the rest remains in the raffinate.
Evaluation of Recycling Products, Efficiency and Feasibility
In the developed hydrometallurgical process, several products are obtained, such as graphite, cemented copper powder, Al and Fe hydroxide, cathode metal (Co, Ni and Mn) salts and Li2CO3. In order to evaluate the process, the comprehensive recycling rates of products, purity, marketable value and application of products are summarised in Table 4. Additionally, some representable compositions of the products are shown in Table 5.
The high purity and fine graphite is obtained with high recycling efficiency above 98 %. It can be seen that it has a high possibility to be reused in the anode of Li–ion batteries since the purity of recycled graphite is higher than 99.5 %. Therefore, the marketable value is apparently higher than the impure graphite. The cemented copper is another valuable product of the recycling process, which has an efficiency of 94–99 % under optimum operating conditions. Hence, it is a very good raw material for the Cu smelting furnace.
It is found that the Al and Fe hydroxide has a moderate purity. The composition of Co is above 4 % showing that the risk to lose the valuable Co and Ni metals in this step is high. Considering the high price of Co and Ni, the Fe and Al hydroxide should be further treated.
The cathode metal salt is a very valuable product obtained from the precipitation process. The recycling rates of Co, Ni and Mn can reach 95 % in the developed recycling process. Regarding to the application, the cathode metal salt could serve as the raw material (precursor) to synthesise the new cathode material. At last, the crystallised Li2CO3 is a very important raw material for Li industry. Li composition in crystallised Li2CO3 ranges from 16.8 to 19.2 % under different operating conditions. Meanwhile, the recovery efficiency ranges from 48 to 64 % in lab scale.
Conclusions
A highly efficient hydrometallurgical recycling process including pre-treatment for the spent automotive Li–ion batteries has been developed, showing the possibility of using a unique process to recover the high-grade graphite, cathode metal salts and lithium carbonate.
In the leaching step, the temperature of 80 °C, H2O2 concentration of 50 g/L, 2 mol/L of sulphuric acid or 4 mol/L of hydrochloric acid and 2 h of retention time are recommended for the industrial operation. The recycling rates of valuable metals (Co, Ni, Li and Cu) are above 98.6 % in the verification test. In addition, the recycled graphite has a quite high purity (>99.5 %) and very fine grain size.
In solution-refining step, the optimum temperature for Cu cementation is 60 °C. Even though a better kinetic of cementation can be obtained at 80 °C, the dissolution of Fe powder is at high level and increases the burden of following Fe removal. In addition, the calculated activation energy of copper cementation is 12.92 kJ/mol. Regarding the Fe and Al precipitation, more than 60 % of Al and 92 % of Fe are recovered. The losses of cathode metals (Co, Ni and Mn) are 3–5 % in average. The range of pH 3.5–4 is recommended to make sure the sufficient removal of Fe and Al and minimum loss of Co, Ni and Mn.
In the cathode metal precipitation step, the hydroxide, carbonate and sulphide precipitation methods are demonstrated to be successful. Nearly, all cathode metals can be precipitated by hydroxide precipitation and carbonate precipitate through increasing pH value to 10. In the trail of Li2CO3 crystallisation, a product with high Li2CO3 content above 90 % was obtained.
All in all, the utilisation of many extraction and recovery methods in hydrometallurgy overcomes the complexity of spent automotive Li–ion batteries and produces valuable products. Those products, such as battery-level graphite, cathode metal (Co, Ni and Mn) salts and lithium carbonate, could be applied in production of new automotive Li–ion batteries. The close loop of many elements could support the automotive industry in a long run.
References
Nishi Y (2001) Lithium ion secondary batteries; past 10 years and the future. J Power Sour 100:101–106
Wakihara M (2001) Recent developments in lithium ion batteries. Mater Sci Eng 33:109–134
Official Journal of the European Union (2006) Directive 2006/66/EC. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:266:0001:0014:en:PDF. Accessed on 17.03.15
Espinosa D, Bernardes A, Tenório J (2004) An overview on the current processes for the recycling of batteries. J Power Sour 135:311–319
Müller T, Friedrich B (2006) Development of a recycling process for nickel-metal hydride batteries. J Power Sour 158:1498–1509
Georgi-Maschler T, Friedrich B, Weyhe R, Heegn H, Rutz M (2012) Development of a recycling process for Li-ion batteries. J Power Sour 207:173–182
Chéret D (2004) Recycling of rechargeable batteries Li-ion −NiMH. In: Proceeding of International Congress for Battery Recycling, Como, Italy
Henrion P (2008) Recycling Li-ion batteries at Xstrata nickel. In: Proceeding of International Congress for Battery Recycling, Düsseldorf
Tedjar F (2007) Methode for the mixed recycling of lithium-based anode batteries and cells. US Patent No. 20070196725
Lain M (2001) Recycling of lithium ion cells and batteries. J Power Sour 97:736–738
Report of LithoRec Project (2012) Braunschweig, Germany
A. Krebs (2005) ICBR—International Congress for Battery Recycling
Wang Y, Apelian D, Zou H (2013) Method and apparatus for recycling lithium-ion batteries. US Patent No. 20130302226
Ferreira D, Prados L, Majuste D, Mansur M (2009) Hydrometallurgical separation of Al Co, Cu and Li from spent Li-ion batteries. J Power Sour 187:238–246
Chen L, Tang X, Zhang Y, Li L, Zeng Z, Yi Zhang (2011) Process for the recovery of Co oxalate from spent Li-ion batteries. Hydrometallurgy 108:80–86
Contestabile M, Panero S, Scrosati B (2001) A laboratory-scale Li-ion battery recycling process. J Power Sour 92:65–69
Dorella G, Mansur M (2007) A study of the separation of Co from spent Li-ion battery residues. J Power Sour 170:210–215
Lee C, Rhee K (2003) Reductive leaching of cathodic active materials from Li ion battery wastes. Hydrometallurgy 68:5–10
Mantuano D, Dorella G, Elias R, Mansur M (2006) Analysis of a hydrometallurgical route to recover base metals from spent rechargeable batteries by liquid–liquid extraction with Cyanex 272. J Power Sour 159:1510–1518
Shin S, Kim N, Sohn J, Yang D, Kim Y (2005) Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 79:172–181
Swain B, Jeong J, Lee J, Lee G, Sohn J (2007) Hydrometallurgical process for recovery of Co from waste cathodic active material generated during manufacturing of Li ion batteries. J Power Sour 167:536–544
Freitas M, Garcia E (2007) Electrochemical recycling of cobalt from cathodes of spent lithium-ion batteries. J Power Sour 171:953–959
Gupta C, Mukherjee T (1990) Hydrometallurgy in extraction processes. CRC Press, USA
Miller J, Beckstead L (1973) Surface deposit effects in the kinetics of copper cementation by iron. Metall Trans 4:1969–1973
El-Batouti M (2005) Removal of copper metal by cementation using a rotating iron cylinder. J Colloid Interface Sci 283:123–129
Ismael M (2003) Iron recovery from sulphate leach liquors in zinc hydrometallurgy. Miner Eng 16:31–39
Acknowledgments
The authors are very grateful for the German Federal Ministry of Education and Research BMBF (Bundesministerium für Bildung und Forschung) for the financial support for the Project No. 03X4601E “Lithiumbatterie Verbundstrukturen”, as well as the European Union for the Project “HELIOS—High Energy Lithium–Ion Storage Solutions”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Friedrich, B. Development of a Highly Efficient Hydrometallurgical Recycling Process for Automotive Li–Ion Batteries. J. Sustain. Metall. 1, 168–178 (2015). https://doi.org/10.1007/s40831-015-0016-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-015-0016-6