Abstract
Background
Overactive bladder (OAB) is characterized by the presence of bothersome urinary symptoms. Pharmacologic treatment options for OAB include anticholinergics and β3-adrenergic agonists. Use of β3-adrenergic agonists may result in similar treatment efficacy with a decreased side effect profile compared with anticholinergics because high anticholinergic burden is associated with cardiovascular and neurologic side effects. However, the β3-adrenergic agonist mirabegron, one of two approved drugs within this class, is a moderate cytochrome P450 (CYP) 2D6 inhibitor, and coadministration of drugs that are CYP2D6 substrates with mirabegron may lead to adverse drug effects.
Objective
The aim of this study was to quantify how often CYP2D6 substrates were dispensed in patients receiving mirabegron among adults of any age and among those ≥ 65 years of age.
Methods
In this retrospective descriptive analysis, a deidentified administrative claims database in the United States, IQVIA PharMetrics® Plus, was used to identify dispensing claims for CYP2D6 substrates and mirabegron from November 2012 to September 2019. Prevalence of CYP2D6 substrate dispensing was assessed in patients dispensed mirabegron among all adults ≥ 18 years old and additionally among a cohort of those ≥ 65 years old. Patient baseline profiles at the time of mirabegron and CYP2D6 substrate codispensing and at the time of mirabegron dispensing were compared. CYP2D6 substrates were categorized as those with the potential for increased risk of QT prolongation, with anticholinergic properties, with narrow therapeutic index (NTI), contraindicated or having a black box warning when used with CYP2D6 inhibitors, or used for depression or other psychiatric disease. Dispensing data and patient profiles were summarized descriptively.
Results
Overall, 68.5% of adults ≥ 18 years old dispensed mirabegron had overlapping dispensings for one or more CYP2D6 substrate; 60.6% and 53.6% had overlapping dispensings for CYP2D6 substrates with anticholinergic properties or risk of QT prolongation, respectively. CYP2D6 substrates with NTI, contraindicated with CYP2D6 inhibitors, or for psychiatric use were codispensed in 17.7%, 16.6%, and 38.0% of adult mirabegron users, respectively. Mirabegron users receiving one or more concurrent CYP2D6 substrate were more likely to be older, have more comorbidities and baseline polypharmacy, and have increased healthcare resource utilization compared with those without concurrent CYP2D6 substrates. Commonly codispensed CYP2D6 substrates included hydrocodone, oxycodone, tramadol, metoprolol, and tamsulosin. Findings were similar for patients in the older cohort (≥ 65 years old), with 72.1% receiving overlapping CYP2D6 substrates.
Conclusions
Codispensing of CYP2D6 substrates, especially those with anticholinergic properties or risk of QT prolongation, was common among adults and older adults receiving mirabegron. Results highlight the need for improved awareness of CYP2D6 substrate prescribing among patients receiving pharmacologic treatment for OAB that inhibits the CYP2D6 pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Previous evidence suggests concomitant use of drugs that are cytochrome P450 (CYP) 2D6 substrates with drugs that are CYP2D6 inhibitors may lead to increased concentration of the substrate and risk of drug‒drug interactions. |
Among patients receiving mirabegron, a moderate CYP2D6 inhibitor, codispensing with CYP2D6 substrates was common (~ 70%) among all adults and among patients ≥ 65 years old. |
Mirabegron users receiving one or more concurrent CYP2D6 substrate were more likely to be older, have more comorbidities and baseline polypharmacy, and have increased healthcare resource utilization compared with those without concurrent CYP2D6 substrate dispensings. |
1 Introduction
Overactive bladder (OAB) is a clinical diagnosis characterized by the presence of bothersome urinary symptoms including urgency, frequency, nocturia, and urge urinary incontinence [1]. Nearly one in four Americans aged ≥ 40 years has bothersome symptoms of OAB [2], and the proportion of adults with OAB increases with age in both men and women [2, 3]. In a large survey of adults in the United States, the percentage of respondents reporting frequency of OAB symptoms as at least “sometimes” rose from 17% for those 40‒45 years old to 49% for those ≥ 76 years old in male respondents and from 37 to 51%, respectively, in females [2]. Pharmacologic agents, including oral anticholinergics or β3-adrenergic receptor agonists, are recommended for the treatment of OAB [1]. While anticholinergics are commonly used treatments for OAB, it is generally recommended to avoid anticholinergics in older adults because increased use leads to greater anticholinergic burden (i.e., cumulative effects of taking one or more drug with anticholinergic properties) [1, 4] and associated risks [5, 6], including increased risk of dementia [6], as well as cardiovascular and neurologic side effects [5, 7].
Compared with adults without OAB, adults with OAB often have more comorbid medical conditions, such as hypertension, depression, and dementia [8,9,10]. Likewise, older adults with OAB have higher rates of comorbidities, such as hypertension, psychiatric disorders, and urinary tract infections, than older adults without OAB [8]. Treatment of these comorbidities often necessitates pharmacologic management, leading to greater rates of polypharmacy. In a geriatric outpatient cohort study of 807 patients (mean age 81.7 years), in which 25.5% of patients had a history of or were currently experienced urinary incontinence, 84% were receiving more than one medication, with an average of approximately five medications prescribed per patient [11]. Polypharmacy in patients with OAB increases the risk of adverse health outcomes as each additional medication leads to a greater risk of drug‒drug interactions [11]. Treatment of common comorbidities of OAB often necessitates management with medications that also have anticholinergic properties [12], thereby leading to increased anticholinergic burden, especially among those receiving anticholinergics for OAB. In addition to anticholinergic properties, other drug properties can contribute to adverse health outcomes when polypharmacy is present. Drug-induced QT prolongation grows more concerning because increased risk factors for Torsades de Pointes (TdP), such as older age and female sex [13], are common attributes of the OAB population [2].
β3-adrenergic receptor agonists are also approved for the treatment of OAB and can avoid anticholinergic burden increases. However, mirabegron, a β3-adrenergic receptor agonist, moderately inhibits cytochrome P450 (CYP) 2D6 metabolism [14, 15]. Approximately 25% of drugs in clinical use in the United States are metabolized to some extent by CYP2D6 [16], and concomitant administration with drugs that inhibit CYP2D6 may lead to increased plasma concentrations of CYP2D6 substrates. Published research on the outcomes from concomitant use of mirabegron and CYP2D6 substrates is limited, with only small pharmacokinetic studies in healthy adults assessing concomitant dosing with desipramine, metoprolol, and tolterodine [14, 17]. A recent 5-year claims analysis of long-term care (LTC) facilities highlighted that among 159,785 residents identified as having OAB, 25,115 residents had one or more mirabegron dispensing, and of those, 92.5% were also dispensed a CYP2D6 substrate [18]. More research, including additional analyses using real-world data, is needed to better assess prescribing practices in patients with OAB.
Despite the US Food and Drug Administration documented drug interaction warning for prescribing mirabegron with a drug metabolized by CYP2D6, coadministration may still occur, and clinicians should be aware of the risk for drug‒drug interactions. The aim of the current study was to quantify how often CYP2D6 substrates were dispensed in patients receiving mirabegron among adults of any age and among those ≥ 65 years of age.
2 Methods
2.1 Study Design and Database
This retrospective database analysis used IQVIA PharMetrics® Plus Database, an administrative claims database of fully adjudicated medical and pharmacy claims in the United States that primarily includes national commercial health plans. Briefly, PharMetrics® Plus includes data from > 210 million commercially insured enrollees and > 75 million enrollees with ≥ 3 years of continuous enrollment and is representative of US patients < 65 years of age who are commercially insured. The database contains a longitudinal view of patient demographics, providers, diagnoses, pharmacy dispensing, inpatient and outpatient services, and costs [19]. The PharMetrics® Plus database, which uses deidentified insurance claims, is compliant with the Health Insurance Portability and Accountability Act to protect patient privacy.
The overall study period for this retrospective database analysis was defined as January 2011–December 2019, and the cohort entry period was defined as November 2012–September 2019.
2.2 Cohorts and Eligibility
2.2.1 Mirabegron Cohort
To form a base to derive study cohorts, an initial analysis was conducted to identify patients with OAB by selecting those with a record of receiving a dispensing of a drug approved for the treatment of OAB (i.e., mirabegron, darifenacin, fesoterodine, flavoxate, oxybutynin, solifenacin, tolterodine, or trospium).
To be included within the mirabegron cohort, patients had to have at least one dispensed prescription for mirabegron during the cohort entry period and be ≥18 years old at the time of mirabegron dispensing. Mirabegron prescription counts included only those claims during the continuous enrollment period. To enable comparison of patient baseline characteristics between patients with (codispensed group) and without (mirabegron-only group) concurrent CYP2D6 substrate dispensing, patients additionally had to have at least one mirabegron dispensing and ≥ 6 months of baseline continuous medical and pharmacy benefit enrollment; patients in the mirabegron-only group had to have at least one mirabegron dispensing, whereas patients in the codispensed group had to have at least one mirabegron dispensing overlapping with CYP2D6 substrate dispensings of interest (Fig. 1).
The cohort entry date for the codispensed group was defined as the date of first eligible overlapping dispensing (the later dispensing date of mirabegron and CYP2D6 substrate). When comparing patient characteristics between the two groups, patients entered the cohort with eligible codispensing on a particular number of mirabegron dispensings (e.g., third mirabegron dispensing) and were 1:1 matched with patients only dispensed mirabegron at that number of mirabegron dispensings (e.g., third mirabegron dispensing) and any previous mirabegron dispensings with no concurrent CYP2D6 substrate (Fig. 2). Matching was performed in ascending order of number of mirabegron dispensings without replacement (see Sect. 2.3 for details). The cohort entry date for the mirabegron-only comparator group was the dispensing date of matched mirabegron. One patient could contribute to both groups, even on the same number of mirabegron dispensings, as long as the cohort entry date for the mirabegron-only group was before the cohort entry date for the codispensed group. However, once the patient had entered the codispensed group, the patient could not contribute further to the mirabegron-only group.
For comparing patient profiles, cohorts were generated separately for overall CYP2D6 substrates, for five groups of CYP2D6 substrates based on drug properties (see Sect. 2.3), for nine individual substrates with narrow therapeutic index (NTI; aripiprazole, desipramine, donepezil, flecainide, imipramine, propafenone, thioridazine, tramadol, venlafaxine), for the five individual most commonly prescribed substrates among patients with OAB (hydrocodone, oxycodone, ondansetron, tolterodine, tramadol), and for prespecified distinct number of concurrent CYP2D6 substrates (see Sect. 2.3).
2.2.2 Older Adult (≥ 65 Years) Mirabegron Cohort
Eligibility criteria and cohort entry dates followed the same criteria as the mirabegron cohort, with age criterion set to ≥ 65 years old.
2.3 CYP2D6 Substrate Identification and Definition of Codispensing and Exposure
Within each cohort, CYP2D6 substrate dispensings were identified using National Drug Codes (NDCs) for 106 of the 108 drugs defined as CYP2D6 substrates in a publication by Rutman et al. [20]. CYP2D6 substrates were further classified into non-mutually exclusive groups, including those (1) with risk of QT prolongation or TdP [21], (2) having reported anticholinergic properties [12], (3) with NTI and prohibited in a phase IV trial of mirabegron in older adults with OAB [22], (4) with a contraindication or black box warning for coadministration with a CYP2D6 inhibitor, and (5) indicated for the treatment of depression or other psychiatric disease.
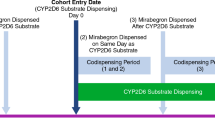
Codispensing (Fig. 3) was defined as either (1) if there was a CYP2D6 substrate dispensing during the mirabegron exposure window when the CYP2D6 substrate dispensing was on or after the mirabegron dispensing or (2) if there was a mirabegron dispensing during a CYP2D6 substrate use window when the CYP2D6 substrate dispensing was before the mirabegron dispensing. The mirabegron exposure window started on the date of dispensing (‘index date’) and extended to 1.5 times the number of days supplied for mirabegron; for example, for a 30-day supply, the exposure began on the date of dispensation and extended to 45 days following the date of dispensation. Based on the average day supply for each CYP2D6 substrate NDC, five CYP2D6 substrate use windows were defined. The longest window (for a 90-day supply) began 135 days before mirabegron dispensing, whereas the shortest window (for a ≤ 7-day supply) began 10 days before mirabegron dispensing. These broad windows of medication use were included in this study to ensure identification of potential codispensings between mirabegron and CYP2D6 substrates.
The number of concurrent CYP2D6 substrates was counted based on distinct CYP2D6 substrates overlapping with a single mirabegron dispensation. While one patient could increase the number of codispensed CYP2D6 substrates across multiple mirabegron dispensings, only the first date for each count in ascending order was captured as the cohort entry date of that concurrent number of CYP2D6 substrates because the patient was considered to retain the risk of multiple codispensings as described above (Supplementary Figure 1, see electronic supplementary material [ESM]). Each distinct concurrent number of CYP2D6 substrates meeting the prespecified criteria for cohort population sizing was included for assessment.
2.4 Statistical Analyses
The number of patients who had codispensed mirabegron and CYP2D6 substrates was assessed in multiple cohorts: overall CYP2D6 substrates, five prespecified groups of CYP2D6 substrates, and 106 individual CYP2D6 substrates. The number of patients in each codispensing cohort is presented using descriptive statistics.
Demographic variables (age, sex, US census region) were assessed at the index date (Fig. 1). Length of continuous medical and pharmacy enrollment, chronic comorbidities (identified using International Classification of Diseases codes; see Supplementary Table 1 in the ESM), comorbidity scores, and the 10 most common diagnoses (identified across all patients with OAB in the study period) were assessed using all available history within the same continuous enrollment window before the cohort entry date; healthcare resource utilization, acute comorbidities, urogynecologic procedures, OAB-related and CYP2D6 substrate medications, and the 10 most common prescription drugs (identified across all patients with OAB in the study period) were assessed during the 183 days before the cohort entry date. Within each matched cohort, patient characteristics were compared between those with and without CYP2D6 substrate codispensing using standardized mean differences (SMDs) and were considered unbalanced if the |SMD| was ≥ 0.2 [23]. Categorical covariates were collapsed to binary variables for each category, and a positive instance was coded as ‘1’ for the category.
3 Results
3.1 Mirabegron Cohort
3.1.1 Patient Attrition
A total of 838,844 adult patients were identified as having an OAB medication during the cohort entry period, and 665,502 (79.3%) had a dispensing of one or more CYP2D6 substrate of any type at some point in the follow-up period after OAB medication dispensing. Of the 106,908 patients identified as having a mirabegron dispensing during the cohort entry window, 106,260 were ≥ 18 years old, and 91,835 had ≥ 6 months of continuous enrollment before dispensing (Supplementary Figure 2A, see ESM). To enable comparison of baseline characteristics, 40,769 patients with mirabegron and CYP2D6 substrate codispensing were matched to 40,769 patients with mirabegron without codispensation after matching on number of mirabegron dispensations.
3.1.2 CYP2D6 Substrate Dispensing Patterns
Among the 106,260 patients in the mirabegron adult cohort, 72,825 (68.5%) had dispensings for one or more CYP2D6 substrate that overlapped with mirabegron dispensing (Table 1). CYP2D6 substrates with anticholinergic properties or with risk of QT prolongation were codispensed in 60.6% and 53.6% of patients in the mirabegron cohort, respectively. CYP2D6 substrates indicated for the treatment of depression or psychiatric diseases, with a black box warning or contraindication for codispensing with a CYP2D6 inhibitor, and with NTI were also codispensed in ≥ 15% of patients (Table 1). The most commonly codispensed CYP2D6 substrates were hydrocodone (20.0% of patients), oxycodone (11.2%), tramadol (10.9%), metoprolol (9.9%), and tamsulosin (9.2%) (Supplementary Table 2, see ESM).
3.1.3 Demographics and Baseline Characteristics
After matching on number of mirabegron dispensations, the median age of the overall CYP2D6 substrate codispensed group was 59 years and of the mirabegron-only group was 56 years; 74% and 78%, respectively, were female (Supplementary Table 3, see ESM). Compared with the mirabegron-only group, patients in the overall CYP2D6 substrate codispensed group had greater baseline overall comorbidity scores (i.e., Charlson Comorbidity Index, Elixhauser Comorbidity Index). Patients in the codispensed group were also more likely to have diagnoses for a greater number of individual baseline comorbidities, including cardiovascular (e.g., hypertension, chronic pulmonary disease, congestive heart failure) and neurologic or psychiatric (e.g., depression) disorders, and more common baseline medication use, including dispensings for hydrocodone, gabapentin, and simvastatin (Fig. 4; Supplementary Table 3, see ESM). Compared with the mirabegron-only group, patients in the overall CYP2D6 substrate codispensed group had more frequent baseline use of outpatient visits and emergency department visits. Observations were similar across predefined subgroups of CYP2D6 substrates from each cohort (Fig. 4). As the number of CYP2D6 substrates codispensed with mirabegron increased, patients were more likely to have a poorer overall medical condition, greater medication use, and increased use of healthcare services during the baseline period (data not shown).
Comparison of demographics and baseline clinical characteristics among patients with vs without CYP2D6 substrate prescriptions in the mirabegron cohort. Shown are results for CYP2D6 substrates overall and CYP2D6 substrates with risk of QT prolongation, anticholinergic properties, NTI (as an overall group and as individual substrates [aripiprazole, desipramine, donepezil, flecainide, imipramine, propafenone, thioridazine, tramadol, venlafaxine]), contraindicated or with warning for prescribing with a CYP2D6 inhibitor, and indicated for the treatment of depression or other psychiatric disorder. Profile comparisons for the cohorts are also provided for the five most commonly dispensed CYP2D6 substrates among patients with OAB (hydrocodone, oxycodone, ondansetron, tolterodine, tramadol). As tramadol was included within the NTI group, it is displayed separately from the other most commonly dispensed CYP2D6 substrates. SMD values indicate the difference in proportions for a characteristic between those with and without codispensing, adjusted for the large patient population, with colors representing SMD value ranges, as shown on the right. Colors in the yellow–red range indicate that a higher proportion of patients with codispensing experience have that characteristic, whereas colors in the blue–green range indicate a higher proportion in the mirabegron-only group. |SMDs| ≥ 0.2 were considered unbalanced between patients who received a CYP2D6 substrate vs those who did not receive a CYP2D6 substrate. CYP cytochrome P450, ER emergency room, IQR interquartile range, NTI narrow therapeutic index, OAB overactive bladder, SMD standardized mean difference
3.2 Older Adult Mirabegron Cohort (≥ 65 Years Old)
3.2.1 Patient Attrition
Among the patients dispensed one or more mirabegron prescription, 27,445 were ≥ 65 years of age and 23,463 had ≥ 6 months of continuous enrollment before dispensing (Supplementary Figure 2B, see ESM). After matching on number of mirabegron dispensations, 8570 patients ≥ 65 years of age who had mirabegron and CYP2D6 substrate codispensing were matched to 8570 patients ≥ 65 years of age who had mirabegron dispensings without CYP2D6 codispensation.
3.2.2 CYP2D6 Substrate Dispensing Patterns
Among the older adult cohort, 72.1% of patients were dispensed one or more overlapping CYP2D6 substrate (Table 1). CYP2D6 substrates with anticholinergic properties or with risk of QT prolongation were codispensed in 61.3% and 52.4% of patients, respectively. CYP2D6 substrates for the treatment of depression or psychiatric diseases, with NTI, or with a black box warning/contraindication were codispensed in 35.4%, 20.2%, and 16.8% of patients, respectively. The most commonly codispensed CYP2D6 substrates were hydrocodone (19.2% of patients), metoprolol (17.3%), tamsulosin (14.0%), simvastatin (12.5%), and tramadol (11.7%).
3.2.3 Demographics and Baseline Characteristics
After matching on number of mirabegron dispensations, the median age of the older adult overall CYP2D6 substrate codispensed group was 71 years and of the older adult mirabegron-only group was 69 years; 60% and 65%, respectively, were female (Supplementary Table 3, see ESM). Within the older adult overall CYP2D6 substrate codispensed group, those who had one or more CYP2D6 substrate dispensing had greater baseline comorbidity scores than those in the mirabegron-only group (Supplementary Table 3, see ESM). Likewise, patients in the overall codispensed group had a greater number of diagnosed baseline comorbidities, including cardiovascular disorders (e.g., hypertension, cardiac arrhythmia, congestive heart failure) and depression, and had greater baseline use of hydrocodone, gabapentin, and simvastatin. Patients in the codispensed group also had higher baseline use of outpatient and emergency department visits compared with those in the mirabegron-only group (Supplementary Table 3, see ESM). Observations were similar across predefined subgroups of CYP2D6 substrates from each cohort.
4 Discussion
Management of patients with OAB, particularly older adults, is complicated by polypharmacy and the potential for drug‒drug interactions [11]. Overlapping dispensings of mirabegron, a moderate CYP2D6 inhibitor, with CYP2D6 substrates occurred frequently (~ 70%) in patients overall and among patients ≥ 65 years old. Across all analyses, the most commonly codispensed CYP2D6 substrates included hydrocodone, oxycodone, tramadol, metoprolol, tamsulosin, and simvastatin. Those receiving one or more CYP2D6 substrate were more likely to be older, have more comorbidities and baseline polypharmacy, and have increased healthcare resource utilization than patients dispensed mirabegron without CYP2D6 substrate prescriptions. These patterns were observed with or without codispensing of specific CYP2D6 substrate groups (e.g., risk of QT prolongation, anticholinergic properties, NTI, or contraindications/warnings). The differences seen with versus without codispensing were generally heterogeneous across specific CYP2D6 substrates. For example, of the NTI substrates evaluated, thioridazine, propafenone, and donepezil were more commonly prescribed compared with other NTI CYP2D6 substrates. As the number of CYP2D6 substrates codispensed with mirabegron increased, patients generally had worse overall medical condition, greater polypharmacy, and increased healthcare resource utilization compared with those who had the same number of mirabegron dispensings without overlapping CYP2D6 substrate dispensing.
Consistent with these results, a high prevalence (> 90%) of CYP2D6 substrate prescribing has been reported among residents of LTC facilities who have OAB [18]. In the prior analysis of residents of LTC, approximately two-thirds of residents were prescribed CYPD26 substrates indicated for the treatment of depression/psychiatric diseases; > 86% and > 78% of residents had received CYP2D6 substrate prescriptions that had anticholinergic properties or risk of QT prolongation, respectively [18]. Furthermore, nearly 25% of residents with OAB who had received mirabegron also had one or more claim for a CYP2D6 substrate with a boxed warning and/or a contraindication for use with CYP2D6 inhibitors during the 5-year analysis window. Although these findings parallel our study, the analysis among residents of LTC facilities did not specifically assess codispensing rates but instead assessed whether prescribing occurred at any time during an extended 5-year analysis window, thus limiting the ability to detect potentially inappropriate prescribing practices.
Anticholinergics are commonly prescribed for OAB, and patients with OAB may also receive drugs with anticholinergic properties for the treatment of common comorbid conditions. Despite recommendations to minimize the use of anticholinergics in older adults [4], our analysis showed that a high percentage of older adults (≥ 65 years of age) received CYP2D6 substrates that had some degree of anticholinergic activity. These results are in line with a study that showed high rates of polypharmacy and anticholinergic burden, as well as potentially inappropriate prescribing practices, in residents of LTC facilities [24]. While a previous retrospective study reported that anticholinergic polypharmacy (i.e., ≥ 30 days of ≥ 2 unique anticholinergics) was uncommon among Medicare beneficiaries with OAB (occurring in < 2% of patients in 2017), patients with anticholinergic polypharmacy were shown to have a significantly greater number of adverse outcomes (e.g., falls, fractures, altered mental status) and higher healthcare resource utilization versus those without anticholinergic polypharmacy [25]. Anticholinergics are also increasingly being associated with increased risk of incident dementia [6], and older adults may be particularly vulnerable for cognitive dysfunction with increased anticholinergic burden [26].
CYP2D6 substrates with an NTI also present a potential risk in the older adult population, especially if the substrate is coadministered with CYP2D6 inhibitors such as mirabegron. Pharmacokinetic studies of CYP2D6 substrates commonly prescribed with mirabegron—including tolterodine, metoprolol, and desipramine—have shown increases in maximum plasma concentration and area under the curve of the substrate [14, 17]. Individuals who are poor CYP2D6 metabolizers and are prescribed a CYP2D6 inhibitor have a compounded risk of experiencing potentially harmful adverse drug reactions owing to increased substrate concentration [27]. Alternatively, prodrugs that require CYP2D6 metabolism for activity (e.g., hydrocodone) may lose effectiveness if coadministered with CYP2D6 inhibitors.
This high proportion of patients receiving overlapping mirabegron and CYP2D6 substrate prescriptions is concerning, as is the high codispensing of metoprolol and tramadol in the mirabegron cohorts given the warnings for prescribing with CYP2D6 inhibitors. Because the risk of drug‒drug interactions and associated adverse drug reactions is increased in older adults owing to the higher prevalence of polypharmacy [28], identifying potential drug‒drug interactions may lead to improved patient safety and reduced healthcare resource utilization. Indeed, drug‒drug interactions can result in toxicity that can lead to hospital admissions [29]. A recent case crossover study showed that concomitant use of antidepressants, many of which are CYP2D6 substrates, with CYP2D6-inhibiting drugs was associated with an increased risk of fall injuries [30]. Furthermore, several studies evaluating polypharmacy and drug‒drug interactions have shown that optimizing treatment plans can reduce healthcare and medication costs [31, 32]. Deprescribing of or changing some medications may be beneficial for patients with OAB who are receiving multiple medications, particularly CYP2D6 substrates with anticholinergic properties, to prevent or minimize clinically relevant drug‒drug interactions.
The findings from this analysis are subject to typical limitations of real-world studies of administrative insurance claims data analysis, such as the potential for misclassification and residual confounding. PharMetrics® Plus is an administrative claims database that primarily consists of commercial health plan enrollees and contains limited data for patients ≥ 65 years old. Therefore, those included in the older adult (≥ 65 years) cohort may not be representative of all patients ≥ 65 years of age. Additionally, prescription dispensing does not guarantee administration of medication, especially for as-needed medications, or that administration of medication was aligned with indications for use, and the reason for prescribing is not captured in the database. Evaluation of adverse outcomes associated with codispensing was not captured by this study. Medications taken as needed can be taken over an extended time and may not be well understood within the data; and over-the-counter medications (e.g., oxybutynin patch for women) are not captured in claims data. Additional study-specific limitations include the potential for over- or underestimating actual days supplied for CYP2D6 substrates based on average days’ supply. Intentional gaps in medication use are also not clearly specified within claims data. Despite these limitations, this study defined codispensing of mirabegron and CYP2D6 substrates, which implies the potential for coadministration, and serves as a first step toward understanding the patterns of OAB medication use with concomitant CYPD26 substrates.
5 Conclusions
In this retrospective analysis of a US administrative claims database, codispensing mirabegron, a moderate CYP2D6 inhibitor, and CYP2D6 substrates was common among all adults and among older adults (≥ 65 years old). These results highlight the need for improved awareness when prescribing CYP2D6 substrates for patients with OAB who are receiving mirabegron. Healthcare providers should consider a patient’s entire medical profile to better mitigate potentially inappropriate prescribing when selecting OAB therapy.
References
Gormley EA, Lightner DJ, Burgio KL, Chai TC, Clemens JQ, Culkin DJ et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline: American Urological Association. 2019.
Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology. 2011;77:1081–7. https://doi.org/10.1016/j.urology.2010.08.039.
Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–36. https://doi.org/10.1007/s00345-002-0301-4.
American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67:674–94. https://doi.org/10.1111/jgs.15767.
Fox C, Smith T, Maidment I, Chan WY, Bua N, Myint PK, et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age Ageing. 2014;43:604–15. https://doi.org/10.1093/ageing/afu096.
Dmochowski RR, Thai S, Iglay K, Enemchukwu E, Tee S, Varano S, et al. Increased risk of incident dementia following use of anticholinergic agents: a systematic literature review and meta-analysis. Neurourol Urodyn. 2021;40:28–37. https://doi.org/10.1002/nau.24536.
Arana A, Margulis AV, McQuay LJ, Ziemiecki R, Bartsch JL, Rothman KJ, et al. Variation in cardiovascular risk related to individual antimuscarinic drugs used to treat overactive bladder: a UK cohort study. Pharmacotherapy. 2018;38:628–37. https://doi.org/10.1002/phar.2121.
Ganz ML, Liu J, Zou KH, Bhagnani T, Luo X. Real-world characteristics of elderly patients with overactive bladder in the United States. Curr Med Res Opin. 2016;32:1997–2005. https://doi.org/10.1080/03007995.2016.1226167.
Asche CV, Kim J, Kulkarni AS, Chakravarti P, Andersson KE. Presence of central nervous system, cardiovascular and overall co-morbidity burden in patients with overactive bladder disorder in a real-world setting. BJU Int. 2012;109:572–80. https://doi.org/10.1111/j.1464-410X.2011.10436.x.
Zarowitz BJ, Allen C, O’Shea T, Tangalos E, Berner T, Ouslander JG. Clinical burden and nonpharmacologic management of nursing facility residents with overactive bladder and/or urinary incontinence. Consult Pharm. 2015;30:533–42. https://doi.org/10.4140/TCP.n.2015.533.
Tulner LR, Frankfort SV, Gijsen GJ, van Campen JP, Koks CH, Beijnen JH. Drug-drug interactions in a geriatric outpatient cohort: prevalence and relevance. Drugs Aging. 2008;25:343–55. https://doi.org/10.2165/00002512-200825040-00007.
Hanlon P, Quinn TJ, Gallacher KI, Myint PK, Jani BD, Nicholl BI, et al. Assessing risks of polypharmacy involving medications with anticholinergic properties. Ann Fam Med. 2020;18:148–55. https://doi.org/10.1370/afm.2501.
Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55:934–47. https://doi.org/10.1016/j.jacc.2010.01.001.
Krauwinkel W, Dickinson J, Schaddelee M, Meijer J, Tretter R, van de Wetering J, et al. The effect of mirabegron, a potent and selective β3-adrenoceptor agonist, on the pharmacokinetics of CYP2D6 substrates desipramine and metoprolol. Eur J Drug Metab Pharmacokinet. 2014;39:43–52. https://doi.org/10.1007/s13318-013-0133-1.
Takusagawa S, Miyashita A, Iwatsubo T, Usui T. In vitro inhibition and induction of human cytochrome P450 enzymes by mirabegron, a potent and selective β3-adrenoceptor agonist. Xenobiotica. 2012;42:1187–96. https://doi.org/10.3109/00498254.2012.700140.
Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5:6–13. https://doi.org/10.1038/sj.tpj.6500285.
Nomura Y, Iitsuka H, Toyoshima J, Kuroishi K, Hatta T, Kaibara A, et al. Pharmacokinetic drug interaction study between overactive bladder drugs mirabegron and tolterodine in Japanese healthy postmenopausal females. Drug Metab Pharmacokinet. 2016;31:411–6. https://doi.org/10.1016/j.dmpk.2016.08.002.
Stefanacci RG, Horn JR, Yeaw J, Shah D, Carrera A, Goti N et al. High utilization of CYP2D6 substrate prescriptions among long-term care residents with overactive bladder. Ann Long-Term Care. 2022. https://doi.org/10.25270/altc.2022.10.001.
IQVIA. IQVIA PharmMetrics® Plus: Fact Sheet. 2020. https://www.iqvia.com/library/fact-sheets/iqvia-pharmetrics-plus. Accessed 22 Feb 2022.
Rutman MP, Horn JR, Newman DK, Stefanacci RG. Overactive bladder prescribing considerations: the role of polypharmacy, anticholinergic burden, and CYP2D6 drug-drug interactions. Clin Drug Investig. 2021;41:293–302. https://doi.org/10.1007/s40261-021-01020-x.
Woosley RL, Heise CW, Gallo T, Tate J, Woosley D, Romero KA. www.CredibleMeds.org QTdrugs List. AZCERT, Inc. 2020. https://www.crediblemeds.org/. Accessed 17 Apr 2020.
Astellas Pharma US Inc. Study to evaluate the efficacy, safety, and tolerability of mirabegron in older adult subjects with overactive bladder (OAB) (PILLAR) (NCT02216214). Trial Protocol. 2015. https://clinicaltrials.gov/ct2/show/NCT02216214. Accessed 29 Jan 2021.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed.: Lawrence Erlbaum Associates; 1988.
Bourrel C, Zacarin A, Rousseau V, Montastruc JL, Bagheri H. Are potentially inappropriate and anticholinergic medications being prescribed for institutionalized elderly subjects? Fundam Clin Pharmacol. 2020;34:743–8. https://doi.org/10.1111/fcp.12560.
Campbell NL, Hines L, Epstein AJ, Walker D, Lockefeer A, Shiozawa A. A 12-year retrospective study of the prevalence of anticholinergic polypharmacy and associated outcomes among Medicare patients with overactive bladder in the USA. Drugs Aging. 2021;38:1075–85. https://doi.org/10.1007/s40266-021-00901-2.
Campbell NL, Perkins AJ, Bradt P, Perk S, Wielage RC, Boustani MA, et al. Association of anticholinergic burden with cognitive impairment and health care utilization among a diverse ambulatory older adult population. Pharmacotherapy. 2016;36:1123–31. https://doi.org/10.1002/phar.1843.
Blake CM, Kharasch ED, Schwab M, Nagele P. A meta-analysis of CYP2D6 metabolizer phenotype and metoprolol pharmacokinetics. Clin Pharmacol Ther. 2013;94:394–9. https://doi.org/10.1038/clpt.2013.96.
Jokanovic N, Tan ECK, Dooley MJ, Kirkpatrick CM, Bell JS. Prevalence and factors associated with polypharmacy in long-term care facilities: a systematic review. J Am Med Direct Assoc. 2015;16:535. https://doi.org/10.1016/j.jamda.2015.03.003.
Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652–8. https://doi.org/10.1001/jama.289.13.1652.
Dahl ML, Leander K, Vikstrom M, Frumerie C, Nordenmalm S, Moller J, et al. CYP2D6-inhibiting drugs and risk of fall injuries after newly initiated antidepressant and antipsychotic therapy in a Swedish, register-based case-crossover study. Sci Rep. 2021;11:5796. https://doi.org/10.1038/s41598-021-85022-x.
Arnold RJG, Tang J, Schrecker J, Hild C. Impact of definitive drug-drug interaction testing on medication management and patient care. Drugs Real World Outcomes. 2018;5:217–24. https://doi.org/10.1007/s40801-018-0143-z.
Kojima G, Bell C, Tamura B, Inaba M, Lubimir K, Blanchette PL, et al. Reducing cost by reducing polypharmacy: the polypharmacy outcomes project. J Am Med Dir Assoc. 2012;13(818):e11–5. https://doi.org/10.1016/j.jamda.2012.07.019.
Acknowledgements
Funding for this analysis was provided by Urovant Sciences (Irvine, CA). Medical writing and editorial support was provided by Krystina Neuman, PhD, CMPP, and Jory Fleischauer, PharmD, of The Curry Rockefeller Group, LLC (Tarrytown, NY), and was funded by Urovant Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this analysis and for medical writing and editorial assistance was provided by Urovant Sciences (Irvine, CA).
Competing Interests
MER was a consultant to CERobs Consulting, LLC, a consulting firm that was contracted by Urovant Sciences and consults with other pharmaceutical companies, at the time the work was conducted. JW and RC are employees of Sumitovant Biopharma, Inc. JCY is a consultant to CERobs Consulting, LLC, a consulting firm that was contracted by Urovant Sciences and consults with other pharmaceutical companies. AC is an employee of Urovant Sciences and of Duke Health. NG is an employee of Urovant Sciences. JRH is a co-author and publisher of The Top 100 Drug Interactions: A Guide to Patient Management and a consultant to Urovant Sciences and Seegnal US. CJG is the President of CERobs Consulting, LLC, a consulting firm that consults with pharmaceutical companies and was contracted by Urovant Sciences.
Compliance With Ethical Standards
This retrospective database analysis used deidentified claims data from the IQVIA PharMetrics® Plus database, which is compliant with the Health Insurance Portability and Accountability Act to protect patient privacy.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code availability
Not applicable.
Availability of Data and Material
Datasets generated and/or analyzed during the current study are available from Sumitovant Biopharma, Inc. (Jingjun Wang; monica.wang@sumitovant.com), through licensing with IQVIA, Inc, and requests will be considered from qualified researchers on a case-by-case basis with the condition of IQVIA approval.
Authors’ Contribution
MER, JW, JCY, RC, AC, NG, and CJG developed the protocol. JW and RC analyzed the data. All authors contributed to the interpretation of data, revised the manuscript for intellectual content, and read and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ritchey, M.E., Wang, J., Young, J.C. et al. CYP2D6 Substrate Dispensing Among Patients Dispensed Mirabegron: An Administrative Claims Analysis. Drugs - Real World Outcomes 10, 119–129 (2023). https://doi.org/10.1007/s40801-022-00339-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-022-00339-x