Abstract
Background
Overactive bladder (OAB) is associated with considerable clinical and economic burden. Treatment of patients with OAB using anticholinergics is limited by tolerability issues and increased anticholinergic burden, which is associated with increased risk of dementia and falls/fractures. This analysis assessed the budget impact of introducing the β3-adrenergic agonist vibegron for the treatment of patients with OAB from US commercial payor and Medicare perspectives.
Methods
A budget impact model (BIM) with a 5-year time horizon was developed using a top-down, prevalence-based approach and projected market shares for 1-million-member US commercial and Medicare plans. The BIM included vibegron, mirabegron, and anticholinergics, incorporating changes in clinical outcomes (efficacy, drug–drug interactions, anticholinergic burden (ACB), OAB-related comorbidities, and adverse events (AEs)). Costs per member per month (PMPM) and per treated member per month (PTMPM) were determined. One-way sensitivity analyses quantified the impact of changes in key variables.
Results
The introduction of vibegron was associated with a modest increase in PMPM cost over 5 years of $0.12 (range for years 1‒5, $0.01‒$0.26) for commercial payors and $0.24 ($0.01‒$0.52) for Medicare (PTMPM cost: $2.70 ($0.17‒$4.85) and $3.15 ($0.19‒$5.82), respectively). Costs were partially offset by savings related to decreased third-line treatment use, yearly decreases in AE and comorbidity incidence, reduced drug–drug interactions, and reduced ACB associated with vibegron introduction. PMPM costs were most sensitive to vibegron market share assumptions, OAB prevalence, and vibegron persistence at 1 month for private payors and Medicare and additionally vibegron persistence at 12 months for Medicare.
Conclusions
Vibegron may address unmet needs in treating OAB and is a useful addition to health plans while minimizing risks of anticholinergic AEs, ACB, and drug–drug interactions, which may partially offset increased pharmacy costs.
Plain Language Summary
Adults with overactive bladder (OAB) experience frequent and sudden urges to urinate. OAB affects more than 100 million men and women in the USA. In 2020, the projected cost of OAB was $82.6 billion. One of the standard treatments for OAB includes a class of drugs called anticholinergics. Anticholinergic drugs can cause side effects such as dry mouth and constipation. Over time, taking a lot of anticholinergic drugs may lead to increased risk of cognitive impairment or dementia. Vibegron is from a different class of drug for the treatment of OAB known as β3-adrenergic receptor agonists. Adding a new drug to the market may have a financial impact on healthcare plans. This study assessed if adding vibegron for treating OAB is affordable in US commercial and Medicare plans. Adding vibegron to a health plan somewhat increased monthly costs over 5 years. For commercial insurance plans, monthly costs over 5 years increased $0.12 per person enrolled in the plan. For Medicare plans, monthly costs over 5 years increased $0.24 per person enrolled in the plan. However, adding vibegron to the market lowered overall costs not directly related to OAB by lowering healthcare costs related to taking a lot of anticholinergic drugs or costs of outpatient visits. Vibegron for treating OAB may be a helpful addition to health plans. Vibegron may reduce some healthcare costs for patients with OAB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Vibegron, a β3-adrenergic receptor agonist recently approved by the US Food and Drug Association for the treatment of overactive bladder (OAB), has a favorable drug–drug interaction profile and is not associated with anticholinergic-related side effects. |
The current budget impact model (BIM) is the first to incorporate comorbidities related to CYP2D6 drug–drug interactions and includes efficacy and safety. |
This BIM demonstrates that introducing vibegron to a health plan formulary is associated with moderate increases in costs for US commercial payors and Medicare; however, these costs are partially offset by decreases in costs associated with switching to different treatments, drug–drug interactions, and anticholinergic burden. |
1 Introduction
Overactive bladder (OAB) is highly prevalent in the USA [1] and is characterized by urinary urgency, often accompanied by frequency and nocturia, with or without urinary incontinence [2]. Bothersome symptoms of OAB are present in an estimated 30 million US adults ≥ 40 years of age; prevalence of OAB symptoms, which are more common in women, increases with age [1]. Beyond this clinical burden, OAB is also associated with notable socioeconomic burden in the USA, with reported mean insurer-paid annual all-cause total healthcare costs ranging from $8168 to $15,569 per patient and annual OAB-specific healthcare costs ranging from $656 to $860 per patient (2016 USD) [3]. In an analysis of administrative claims data from the Truven Health MarketScan databases (2007–2014), per-patient monthly direct healthcare costs adjusted for demographics and select clinical characteristics were significantly higher among adults with OAB ($3003; 2015 USD) compared with propensity score–matched controls without OAB ($1123, P < 0.0001) [4]. The total direct and indirect cost of OAB in the USA was estimated to be as high as $82.6 billion in 2020 [5].
Behavioral therapy (e.g., bladder training, fluid management), either alone or in combination with pharmacologic treatment, is recommended as the first-line treatment for OAB; pharmacologic treatment (oral anticholinergics and/or β3-adrenergic agonists) is recommended as a second-line treatment approach [2]. Tolerability issues, such as dry mouth and constipation, can limit the use of anticholinergics in patients with OAB [6]. Additionally, long-term use of anticholinergics is associated with an increased risk of dementia [7], as well as falls/fractures [8]. However, for patients who fail to respond to first- or second-line treatment approaches, the American Urological Association recommends a third-line treatment such as intravesical onabotulinumtoxinA injection, percutaneous tibial nerve stimulation (PTNS), or sacral neuromodulation (SNM) [2].
Vibegron is a β3-adrenergic receptor agonist that was recently approved in December 2020 by the US Food and Drug Administration (FDA) for the treatment of adults with OAB [9]. In the phase 3, randomized, double-blind, placebo- and active-controlled 12-week EMPOWUR trial [10] and the 40-week double-blind extension trial [11], once-daily vibegron 75 mg demonstrated significant differences in efficacy versus placebo and was safe and well tolerated, with improvement in symptoms that were sustained over 12 months of treatment. Importantly, as a β3-adrenergic agonist, vibegron does not increase overall anticholinergic burden, nor is it associated with anticholinergic-related side effects [10, 11].
The current analysis was conducted to assess the budget impact of introducing vibegron for the treatment of patients with OAB from the perspectives of US commercial payors and Medicare.
2 Methods
2.1 Model Overview
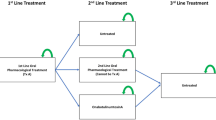
A budget impact model (BIM) with a 5-year time horizon was developed using a top-down, prevalence-based approach and projected market shares for 1-million-member US commercial and Medicare plans. The BIM was structured as a semi-Markov model with a 1-month cycle length. In each year of the time horizon, patients could persist, discontinue, or switch to a different pharmacologic treatment, reflecting real-life treatment patterns in OAB (Fig. 1). As in a previously reported model [12], treatment persistence was modeled at 1 month and 12 months, and patients who persisted at 12 months were assumed to be persistent for the remainder of the model horizon. In absence of real-world data, vibegron persistence was set equal to mirabegron 50 mg.
In the model, patients who discontinued first- or second-line pharmacologic treatment before reaching 12-month persistence did not reinitiate treatment until the start of the following year, at which point they either switched to a new oral pharmacologic treatment (to maintain projected market shares) or moved directly to third-line treatments (i.e., onabotulinumtoxinA, SNM, and PTNS) (Fig. 1), which are recommended for patients who are refractory to behavioral therapy and pharmacotherapy. The BIM assumed that discontinuers revert to their baseline severity and applied treatment costs only when patients were on treatment. Discontinuation followed a constant hazard rate between 1 month and 12 months using the following equation:
where P1 month and P12 months are persistence rates at 1 and 12 months, respectively. Any patient who discontinued oral pharmacotherapy and did not switch to third-line therapy was assumed to start new oral pharmacotherapy. The proportion of patients who started a third-line therapy was 2.2% based on conservative estimates from Linder et al. [13], and once initiated, patients remain on third-line therapy for the duration of the model time horizon. Efficacy is maintained for as long as patients remain on treatment. Additionally, the BIM assumed that the oral OAB treatment market remains constant over time; therefore, patients who progressed to a third-line treatment were replaced with an equal number of patients each year (see Online Supplementary Material (OSM) Table 1 for full model assumptions). At the start of each year, patients were distributed according to the yearly market share projections to each of the modeled OAB treatments.
2.2 Model Parameters
2.2.1 Overactive Bladder (OAB) Population
The target population consisted of all patients with OAB who were ≥ 25 years of age and who sought prescription medications for OAB. Age and gender distributions for enrollees with commercial insurance and Medicare coverage were derived from the March 2019 Current Population Survey's Annual Social and Economic Supplement by the US Census Bureau [14]. OAB prevalence was estimated using conservative estimates from Coyne et al. [15] for adults 25–70 years old and prevalence from Coyne et al. [1] for adults ≥ 70 years old, scaled down by applying a ratio of the prevalence for ages 60–70 years derived from Coyne et al. [15] to the prevalence for ages 66–70 years in Coyne et al. [1]. For patient inputs for prevalence by age and gender see OSM Table 2.
2.2.2 Treatments
Pharmacologic treatments included vibegron 75 mg, mirabegron 25 and 50 mg, and anticholinergics (i.e., darifenacin 7.5 and 15 mg, fesoterodine 4 and 8 mg, oxybutynin 5 and 10 mg extended release, oxybutynin 5 mg immediate release, solifenacin 5 and 10 mg, tolterodine 2 and 4 mg, and trospium 60 mg). The combination of mirabegron with solifenacin was not included in the analysis. The model focused on extended-release anticholinergic formulations because tolerability issues can limit the use of immediate-release anticholinergics; an exception was made for oxybutynin immediate release because prescription utilization data show that it is commonly used [16].
2.2.3 Market Share
The market share projections for vibegron from 2021 to 2025 were defined as part of the β3-adrenergic agonist market and as part of the overall oral treatment market for OAB (OSM Tables 3 and 4). Projections were proportionally distributed among the OAB treatments according to the baseline market share inputs, which were informed by an internal analysis of 12-month prescription data from January 2019 through December 2019 (National Prescription Audit®, IQVIA). In the market, both β3-adrenergic receptor agonists grew in market share over time, whereas the anticholinergics decreased in market share over time. Distributional inputs for allocation of patients to the third-line treatments were based on Jiang et al. [17], who used the MarketScan Commercial Claims and Encounters database to identify patients with ≥ one claim between January 2015 and December 2017 and reported that 12.9% of patients received PTNS, 48.8% received SNM, and 38.3% received onabotulinumtoxinA.
2.2.4 Additional Inputs
The model incorporated changes in clinical outcomes, which included treatment efficacy, cytochrome P450 (CYP) 2D6 drug–drug interactions, anticholinergic burden (ACB), OAB-related comorbidities, and adverse events (AEs). Because a subset of patients with OAB experience urinary incontinence (see Fig. 2), efficacy was defined by reduction in mean daily incontinence episodes at 3 and 12 months and varied among the different oral treatments (OSM Table 5); inputs were based on an internal network meta-analysis of randomized controlled trials and represent treatment difference versus placebo (OSM Methods). The incontinence episode assumption was applied to all patients in a treatment group and was used as a measure of treatment efficacy rather than micturitions as cost data were not available by the number of micturitions. To account for CYP2D6-related drug–drug interactions, the model assumed 40% of patients taking mirabegron (a moderate CYP2D6 inhibitor) also received a CYP2D6 substrate and thus were subject to increased exposure of the substrate, which in the case of anticholinergics increased their ACB and related hospitalizations. Of CYP2D6 substrates, 47% had ACB (based on internal analysis of CYP2D6 with ACB scores of > 0), with an assumed mean ACB score of 2. Based on the package insert of mirabegron and findings from Nomura et al. [18, 19], the model conservatively assumed that the mean ACB of these CYP2D6 substrate anticholinergic drugs is doubled due to CYP2D6 drug–drug interaction. The model assumed that 2.4% of patients with potential drug–drug interactions required hospitalization [20]. ACB was defined as the cumulative effect of taking multiple anticholinergic drugs. Each anticholinergic drug for OAB (darifenacin, fesoterodine, oxybutynin, solifenacin, tolterodine, and trospium) was assigned a score of 3 (definite anticholinergic activity) based on the Anticholinergic Cognitive Burden Scale [21], and vibegron and mirabegron, which were not scored in the above scale as they are both β3-adrenergic agonists, were assigned a score of 0 (no anticholinergic activity), as were third-line therapies. The BIM starts with a baseline ACB score and models the ACB score of each patient based on his/her current OAB treatment. This approach represents a conservative approach of modeling ACB via an instantaneous burden defined by the current OAB treatment as opposed to a cumulative burden. The BIM follows the approach in Perk et al. [22] to model consequences of ACB in terms of healthcare resources (outpatient and emergency department visits; OSM Table 6). A one-unit increase in ACB score resulted in an additional 1.135 outpatient visits and 0.114 emergency department visits. Annual baseline ACB score was 0.6 for adults < 65 years old and 1.2 for adults ≥ 65 years old based on a report by Szabo et al. [8]. OAB-related comorbidities were modeled as a function of OAB incontinence severity and included monthly rates of depression, urinary tract infection, and skin infection (OSM Tables 6 and 7), and AEs included monthly rates of blurred vision, dry mouth, constipation, and tachycardia (OSM Tables 6 and 8).
Monthly treatment costs (OSM Table 6) were derived from GoodRx for all treatments except mirabegron, vibegron, and fesoterodine, where median wholesale acquisition cost was used as there was no generic option at the time of analysis. Third-line treatment costs (OSM Table 6) were derived following the approach used in Yehoshua et al. [23], in which, briefly, the cost of onabotulinumtoxinA, SNM, and PTNS included the drug/device costs as well as administration costs, follow-up visits, and costs from commonly associated AEs from the US commercial payer perspective. All costs were inflated to 2020 USD via the Medical Care Component of Consumer Price Index [24]. Private payer costs (non-drug portion) were converted from Medicare costs by applying a multiplier of 1.35 based on the 2020 Medicare Payment Advisory Commission Report of Commercial to Medicare Payment Rates [25].
2.3 Outcomes and Analyses
Economic outcomes are provided as costs per member per month (PMPM) and per treated member per month (PTMPM); total costs were also assessed. The impact of adding vibegron to a health plan formulary was determined for clinical outcomes and healthcare resource utilization (HCRU). One-way sensitivity analyses (OWSAs) were performed to quantify the impact in response to changes in key variables (OSM Table 9; i.e., changes in treatment persistence, number of incontinence episodes, CYP2D6-related drug–drug interactions, cost of comorbidities, and cost of AEs). OWSAs were run with an input variable set to either its low (20% decrease) or its high (20% increase) value (a range commonly used in BIMs; for a recent example, see Schultz et al. [26]), while the remaining variables remained unchanged. Findings from the OWSAs are summarized in tornado diagrams in terms of the PMPM. Due to uncertainties associated with the base-case values, scenario analyses were performed to examine changes in key input variables (OSM Table 10; i.e., changes in vibegron persistence, percentage of patients receiving mirabegron and a CYP2D6 substrate, ACB HCRU, and vibegron market share uptake).
3 Results
3.1 Overactive Bladder (OAB) Population
For a 1-million-member plan, the estimated number of patients with OAB treated with prescription medication was 31,456 from the commercial payor perspective (Fig. 2A) and 56,684 from the Medicare perspective (Fig. 2B). The estimated number of patients with incontinence, a subset of patients with OAB, receiving prescription medication for OAB was 20,936 and 37,276 from the US commercial payor and Medicare perspectives, respectively.
3.2 Budget Impact Model (BIM) Outcomes
3.2.1 Budget Impact
Adding vibegron to a health plan formulary was associated with a modest increase in PMPM cost over 5 years of $0.12 (range for years 1‒5, $0.01‒$0.26) for commercial payors and $0.24 (range for years 1‒5, $0.01‒$0.52) for Medicare (Table 1; see OSM Tables 11 and 12 for raw costs). The total incremental increase in PTMPM cost was $2.70 (range for years 1‒5, $0.17‒$4.85) for commercial payors and $3.15 (range for years 1‒5, $0.19‒$5.82) for Medicare. Higher pharmacy costs were partially offset by savings related to a decreased use of third-line treatments, yearly decreases in the incidence of AEs and comorbidities, reduced drug–drug interactions, and a reduced ACB associated with the introduction of vibegron.
3.2.2 Clinical and Healthcare Resource Utilization (HCRU) Outcomes
Adding vibegron to a formulary was associated with a slightly lower number of comorbid events and lower HCRU for both commercial payors (Fig. 3) and Medicare (Fig. 4). Among comorbid conditions, the rate of urinary tract infections per year had the most marked lowering, whereas among HCRU parameters the rate of outpatient visits per year had the most pronounced lowering; for both outcomes, the magnitude of reduction was greater with each modeled year.
3.2.3 Sensitivity and Scenario Analyses
The OWSAs indicated that PMPM costs were most sensitive to OAB prevalence and vibegron persistence at 1 month for both commercial payors (Fig. 5A) and Medicare (Fig. 5B), as well as vibegron persistence at 12 months for Medicare. For the commercial payor perspective, the range (i.e., difference between 20% decrease and 20% increase from base case) was $0.05 and $0.04 PMPM for OAB prevalence and vibegron persistence at 1 month, respectively. For the Medicare perspective, the range was $0.09, $0.07, and $0.05 PMPM for OAB prevalence, vibegron persistence at 1 month, and vibegron persistence at 12 months, respectively.
Top ten variables from OWSAs* for 5-year incremental PMPMs from A US commercial payor and B Medicare perspectives. CYP cytochrome P450, DDI drug–drug interaction, OAB overactive bladder, OWSA one-way sensitivity analysis, PMPM per member per month. *Each bar represents the results of the model with a single variable set to its high and low values and all other inputs at their base-case value; bars are sorted in descending order of impact
Scenario analyses for vibegron persistence indicated that the highest impact arose from the assumption regarding vibegron market share uptake. The scenario assuming the introduction of vibegron impacts only the market share of mirabegron resulted in approximately 90% lower PMPM and PTMPM costs relative to the base case (OSM Table 13) owing to lower incremental pharmacy costs for both commercial payors and Medicare. When assuming that the introduction of vibegron impacted only the market share of anticholinergics, PMPM and PTMPM costs for both commercial payors and Medicare increased by approximately 45% relative to the base case owing to higher incremental total costs as a result of higher incremental pharmacy costs and smaller cost savings in most other categories. The scenario assuming 10% increase in persistence at both 1 month and 12 months for commercial payors and Medicare resulted in slightly higher PMPM and PTMPM costs relative to the base case owing to higher incremental pharmacy costs, which were partially offset by greater cost savings in all other categories (OSM Table 13). When a scenario with an alternate dataset for the proportion of patients receiving mirabegron and CYP2D6 substrates (from an abbreviated list of substrates) was considered, the scenario resulted in comparable to slightly higher PMPM and PTMPM costs relative to the base case for both commercial payors and Medicare. For the scenario in which ACB-related HCRU was reduced, the introduction of vibegron was associated with higher budget impact overall relative to base case owing to lower cost offsets for both commercial payors and Medicare.
4 Discussion
This BIM was developed to assess the impact of introducing vibegron for the treatment of OAB in the USA from both commercial payor and Medicare perspectives. The findings suggest that adding vibegron to the health plan formulary would be associated with modest increases in PMPM and PTMPM costs over a 5-year time frame for commercial payors ($0.12 and $2.70, respectively) and Medicare ($0.24 and $3.15). Costs were higher for Medicare partially because the target population for the Medicare perspective is much larger than the private payor perspective and because the Medicare population is older, with a higher baseline ACB score. Reducing costs related to the use of third-line treatments, AEs, ACB, comorbidities, and drug–drug interactions may partially offset the higher pharmacy costs associated with the introduction of vibegron. OWSAs revealed that the BIM for both commercial payors and Medicare was most sensitive to vibegron market share assumptions, OAB prevalence, and vibegron persistence.
Although anticholinergics are commonly used in the treatment of OAB, their use can be limited by tolerability issues [6]. In addition, long-term anticholinergic treatment is associated with increased ACB, characterized by an increased risk of dementia and falls/fractures [7, 8]. Use of anticholinergics can therefore impact not only direct pharmacy costs but also indirect costs associated with hospitalizations related to adverse effects and long-term sequelae of anticholinergic use rather than the impact of OAB itself. β3-adrenergic agonists can provide efficacious control of OAB symptoms while minimizing the risk of anticholinergic-related AEs [10, 27,28,29], and thus represent important alternatives to anticholinergics.
The current BIM was fully comprehensive: It modeled treatment persistence, discontinuation, and switches, and included third-line treatment options, reflecting real-world treatment pathways. Efficacy and AE rates were based on an internal network meta-analysis of results from randomized controlled trials (OSM Methods). Model inputs used treatment differences versus placebo and assumed that patients reverted to their baseline disease severity after discontinuing treatment, therefore representing a conservative approach. Because anticholinergics for the treatment of OAB contribute to ACB, the BIM incorporated the real-world impact of ACB, which was modeled via increased HCRU (outpatient and emergency department visits), an approach used in other published OAB economic models [12, 22, 30]. The largest offsets upon addition of vibegron to the formulary were regarding ACB, owing to patients who may have previously received an anticholinergic for OAB now receiving vibegron, a β3-adrenergic agonist with no anticholinergic activity. Additionally, patients who may have previously received mirabegron and a CYP2D6 substrate with anticholinergic properties and now are receiving vibegron would additionally have reduced ACB.
It has been shown that CYP2D6-related drug–drug interactions can indirectly impact ACB, and we therefore incorporated CYP2D6 drug–drug interactions and the potential impact on ACB among patients taking mirabegron and CYP2D6 substrates with anticholinergic properties. To the best of our knowledge, this aspect has not been modeled in any other published OAB economic models. CYP2D6 is involved in the metabolism of approximately 25% of all prescribed drugs, with substrates found in many common therapeutic classes including those for the treatment of pain, depression, and psychiatric and cardiovascular disorders. This is of interest because adults with OAB have greater numbers of comorbid medical conditions (e.g., depression, hypertension) and associated polypharmacy than adults without OAB [31,32,33], and are thus at an increased risk of drug–drug interactions and subsequent adverse outcomes and/or hospitalizations. In a systematic review and meta-analysis of 13 observational studies, the median prevalence of drug–drug interactions at hospital admission was 1.1% [20]. Drug–drug interactions may also exacerbate polypharmacy as additional drugs are added to counteract the interaction-related side effects. Vibegron is not an inhibitor of any hepatic isoenzymes (e.g., CYP2D6) [9, 34], whereas mirabegron is a moderate inhibitor of CYP2D6 [35].
Our model was performed similarly to an analysis by Perk et al. [22], which estimated that with mirabegron introduction the PMPM costs incurred for years 1–3 from the commercial payor perspective was increased by $0.005, $0.016, and $0.031, respectively, and for the Medicare Advantage perspective by $0.010, $0.034, and $0.065. However, it is difficult to do a full comparison because the model assessed by Perk et al. did not look at drug–drug interactions, did not have patients going to third-line therapy, used a different target population and size, and used different market shares. The current analyses are limited by the assumption that the oral OAB medication market is constant over time and that patients switching to third-line treatment are replaced by an equal number of patients initiating oral therapies. A recent analysis, however, suggests that introduction of new OAB treatments may actually result in an increase in overall prescribing of OAB medications [16]. The assumption that patients on third-line treatment do not discontinue treatment may be unrealistic; indeed, a prior study showed that treatment with transcutaneous nerve stimulation (TNS) has high discontinuation rates ranging from 71% at ≥ 12 months to 84% at ≥ 18 months [36]. Because patients remain on third-line therapy for the duration of the model horizon when discontinuation rates range from approximately 9–61%, costs attributed to third-line therapy may be overestimated. However, because only 2.2% of patients initiate third-line therapy, we anticipate that any change due to discontinuation would be minor (approximately $0.01 for both US commercial payor and Medicare perspectives) [13]. In addition, because of the absence of trials lasting longer than 52 weeks, long-term efficacy inputs were assumed to be sustained beyond 12 months. Another limitation is the absence of real-world data, and therefore patients who persisted on treatment after 1 year were assumed to persist indefinitely. For vibegron persistence, because real-world data are not available, the base case assumed vibegron to have the same persistence as mirabegron. Also, there is uncertainty in the model inputs related to the association between CYP2D6 drug–drug interactions and ACB owing to the lack of clinical studies that directly examine such associations. Despite these limitations, the current BIM is comprehensive in that it includes efficacy, safety, and drug–drug interactions and is the first BIM to incorporate CYP2D6-related comorbidities; thus, this model provides a fuller representation of the total disease burden and actual experience of the OAB population. Future directions could include studies involving untreated patients, as vibegron could expand treatment access to the untreated population.
5 Conclusions
Results from this BIM for the introduction of vibegron into a health plan formulary from US commercial payor and Medicare perspectives shows that adding vibegron was associated with modest increases in monthly costs over 5 years, which were partially offset by savings related to decreased use of third-line treatments, yearly decreases in incidence of AEs and comorbidities, reduced drug–drug interactions, and reduced ACB. Vibegron may therefore address an unmet need in treating patients with symptoms of OAB and may be a useful addition to a health plan by improving clinical outcomes and reducing indirect costs.
References
Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology. 2011;77(5):1081–7.
Gormley EA, Lightner DJ, Burgio KL, Chai TC, Clemens JQ, Culkin DJ, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline: American Urological Association; 2019.
Powell LC, Szabo SM, Walker D, Gooch K. The economic burden of overactive bladder in the United States: a systematic literature review. Neurourol Urodyn. 2018;37(4):1241–9.
Durden E, Walker D, Gray S, Fowler R, Juneau P, Gooch K. The direct and indirect costs associated with overactive bladder within a commercially-insured population in the United States. J Occup Environ Med. 2018;60(9):847–52.
Ganz ML, Smalarz AM, Krupski TL, Anger JT, Hu JC, Wittrup-Jensen KU, et al. Economic costs of overactive bladder in the United States. Urology. 2010;75(3):526–32 (32.e1–18).
Rai BP, Cody JD, Alhasso A, Stewart L. Anticholinergic drugs versus non-drug active therapies for non-neurogenic overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2012;12(12):CD003193.
Dmochowski RR, Thai S, Iglay K, Enemchukwu E, Tee S, Varano S, et al. Increased risk of incident dementia following use of anticholinergic agents: a systematic literature review and meta-analysis. Neurourol Urodyn. 2021;40(1):28–37.
Szabo SM, Gooch K, Schermer C, Walker D, Lozano-Ortega G, Rogula B, et al. Association between cumulative anticholinergic burden and falls and fractures in patients with overactive bladder: US-based retrospective cohort study. BMJ Open. 2019;9(5): e026391.
Urovant Sciences, Inc. GEMTESA® (vibegron). Full Prescribing Information. Irvine, CA: 2020.
Staskin D, Frankel J, Varano S, Shortino D, Jankowich R, Mudd PN Jr. International phase III, randomized, double-blind, placebo and active controlled study to evaluate the safety and efficacy of vibegron in patients with symptoms of overactive bladder: EMPOWUR. J Urol. 2020;204(2):316–24.
Staskin D, Frankel J, Varano S, Shortino D, Jankowich R, Mudd PN Jr. Once-daily vibegron 75 mg for overactive bladder: long-term safety and efficacy from a double-blind extension study of the international phase 3 trial (EMPOWUR). J Urol. 2021;205(5):1421–9.
Wielage RC, Perk S, Campbell NL, Klein TM, Posta LM, Yuran T, et al. Mirabegron for the treatment of overactive bladder: Cost-effectiveness from US commercial health-plan and Medicare Advantage perspectives. J Med Econ. 2016;19(12):1135–43.
Linder BJ, Gebhart JB, Elliott DS, Van Houten HK, Sangaralingham LR, Habermann EB. National patterns of filled prescriptions and third-line treatment utilization for privately insured women with overactive bladder. Female Pelvic Med Reconstr Surg. 2021;27(2):e261–6.
US Census Bureau Population Division. Current Population Survey, Annual Social and Economic Supplement. Published March 2019. https://data.census.gov/mdat/#/. Accessed 6 Apr 2020.
Coyne KS, Sexton CC, Bell JA, Thompson CL, Dmochowski R, Bavendam T, et al. The prevalence of lower urinary tract symptoms (LUTS) and overactive bladder (OAB) by racial/ethnic group and age: results from OAB-POLL. Neurourol Urodyn. 2013;32(3):230–7.
Kinlaw AC, Jonsson Funk M, Conover MM, Pate V, Markland AD, Wu JM. Impact of new medications and $4 generic programs on overactive bladder treatment among older adults in the United States, 2000–2015. Med Care. 2018;56(2):162–70.
Jiang J, Patil D, Traore EJ, Hammett J, Filson CP. Contemporary patterns of third-line treatments for privately insured individuals with overactive bladder in the United States. Urology. 2020;142:87–93.
Nomura Y, Iitsuka H, Toyoshima J, Kuroishi K, Hatta T, Kaibara A, et al. Pharmacokinetic drug interaction study between overactive bladder drugs mirabegron and tolterodine in Japanese healthy postmenopausal females. Drug Metab Pharmacokinet. 2016;31(6):411–6.
Astellas Pharma US Inc. MYRBETRIQ® (mirabegron extended-release tablets). Full Prescribing Information. Northbrook, IL: 2018.
Dechanont S, Maphanta S, Butthum B, Kongkaew C. Hospital admissions/visits associated with drug–drug interactions: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2014;23(5):489–97.
Aging Brain Program of the Indiana University Center for Aging Research. Anticholinergic Cognitive Burden Scale 2012 Update. https://corumpharmacy.com/wp-content/uploads/2020/08/Anticholinergic-cognitive-burden-scale.pdf. Accessed 6 Apr 2020.
Perk S, Wielage RC, Campbell NL, Klein TM, Perkins A, Posta LM, et al. Estimated budget impact of increased use of mirabegron, a novel treatment for overactive bladder. J Manag Care Spec Pharm. 2016;22(9):1072–84.
Yehoshua A, Murray BP, Vasavada SP, Sand PK. Comparing direct medical costs of onabotulinumtoxinA with other common overactive bladder interventions. Am J Pharm Benefits. 2018;10(1):11–7.
US Department of Labor. CPI Inflation Calculator—Medical Care Component (2020) Medical care in U.S. city average, all urban consumers, seasonally adjusted. https://beta.bls.gov/dataViewer/view/timeseries/CUSR0000SAM. Accessed 22 June 2020.
Medicare Payment Advisory Commission. March 2020 Report to the Congress: Medicare Payment Policy. Washington, DC: MedPAC; 2020.
Schultz NM, O’Day K, Sugarman R, Ramaswamy K. Budget impact of enzalutamide for nonmetastatic castration-resistant prostate cancer. J Manag Care Spec Pharm. 2020;26(4):538–49.
Nitti VW, Khullar V, van Kerrebroeck P, Herschorn S, Cambronero J, Angulo JC, et al. Mirabegron for the treatment of overactive bladder: a prespecified pooled efficacy analysis and pooled safety analysis of three randomised, double-blind, placebo-controlled, phase III studies. Int J Clin Pract. 2013;67(7):619–32.
Kelleher C, Hakimi Z, Zur R, Siddiqui E, Maman K, Aballea S, et al. Efficacy and tolerability of mirabegron compared with antimuscarinic monotherapy or combination therapies for overactive bladder: a systematic review and network meta-analysis. Eur Urol. 2018;74(3):324–33.
Mitcheson HD, Samanta S, Muldowney K, Pinto CA, Rocha BA, Green S, et al. Vibegron (RVT-901/MK-4618/KRP-114V) administered once daily as monotherapy or concomitantly with tolterodine in patients with an overactive bladder: a multicenter, phase IIb, randomized, double-blind, controlled trial. Eur Urol. 2019;75(2):274–82.
Parise H, Espinosa R, Dea K, Anaya P, Montoya G, Ng DB. Cost effectiveness of mirabegron compared with antimuscarinic agents for the treatment of adults with overactive bladder in Colombia. Pharmacoecon Open. 2020;4(1):79–90.
Andersson KE, Sarawate C, Kahler KH, Stanley EL, Kulkarni AS. Cardiovascular morbidity, heart rates and use of antimuscarinics in patients with overactive bladder. BJU Int. 2010;106(2):268–74.
Asche CV, Kim J, Kulkarni AS, Chakravarti P, Andersson KE. Presence of central nervous system, cardiovascular and overall co-morbidity burden in patients with overactive bladder disorder in a real-world setting. BJU Int. 2012;109(4):572–80.
Ganz ML, Liu J, Zou KH, Bhagnani T, Luo X. Real-world characteristics of elderly patients with overactive bladder in the United States. Curr Med Res Opin. 2016;32(12):1997–2005.
Edmondson SD, Zhu C, Kar NF, Di Salvo J, Nagabukuro H, Sacre-Salem B, et al. Discovery of vibegron: a potent and selective β3 adrenergic receptor agonist for the treatment of overactive bladder. J Med Chem. 2016;59(2):609–23.
Takusagawa S, Miyashita A, Iwatsubo T, Usui T. In vitro inhibition and induction of human cytochrome P450 enzymes by mirabegron, a potent and selective beta3-adrenoceptor agonist. Xenobiotica. 2012;42(12):1187–96.
Leroux PA, Brassart E, Lebdai S, Azzouzi AR, Bigot P, Carrouget J. Transcutaneous tibial nerve stimulation: 2 years follow-up outcomes in the management of anticholinergic refractory overactive bladder. World J Urol. 2018;36(9):1455–60.
Acknowledgements
Medical writing and editorial support was provided by Kulvinder Katie Singh, PharmD, for The Curry Rockefeller Group, LLC (CRG; Tarrytown, NY), and Krystina Neuman, PhD, CMPP, of CRG, and was funded by Urovant Sciences (Irvine, CA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Urovant Sciences (Irvine, CA). Urovant Sciences was involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflict of interest
Jing Voon Chen was an employee of Medical Decision Modeling at the time of analysis. James C. Gahn is an employee of Medical Decision Modeling. Jeffrey Nesheim is an employee of Urovant Sciences. Paul N. Mudd, Jr. was an employee of Urovant Sciences at the time the work was conducted.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Request for data from Urovant Sciences (email: medinfo@urovant.com) will be considered from qualified researchers on a case-by-case basis.
Code availability
Not applicable.
Author contributions
All authors participated in study conception and design, data collection and analysis, and review and critical revisions of the manuscript and approved the final version of the manuscript.
Additional information
Jing Voon Chen and Paul N. Mudd, Jr.: at the time the work was conducted.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chen, J.V., Gahn, J.C., Nesheim, J. et al. Budget Impact Analysis of Vibegron for the Treatment of Overactive Bladder in the USA. PharmacoEconomics 40, 979–988 (2022). https://doi.org/10.1007/s40273-022-01163-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-022-01163-5