Abstract
The gas generation features of coals at different maturities were studied by the anhydrous pyrolysis of Jurassic coal from the Minhe Basin in sealed gold tubes at 50 MPa. The gas component yields (C1, C2, C3, i-C4, n-C4, i-C5, n-C5, and CO2); the δ13C of C1, C2, C3, and CO2; and the mass of the liquid hydrocarbons (C6+) were measured. On the basis of these data, the stage changes of δ13C1, δ13C2, δ13C3, and δ13CO2 were calculated. The diagrams of δ13C1–δ13C2 vs ln (C1/C2) and δ13C2–δ13C1 vs δ13C3–δ13C2 were used to evaluate the gas generation features of the coal maturity stages. At the high maturity evolution stage (T > 527.6 °C at 2 °C/h), the stage change of δ13C1 and the CH4 yield are much higher than that of CO2, suggesting that high maturity coal could still generate methane. When T < 455 °C, CO2 is generated by breaking bonds between carbons and heteroatoms. The reaction between different sources of coke and water may be the reason for the complicated stage change in \(\delta^{{{13}}} {\text{C}}_{{{\text{CO}}_{{2}} }}\) when the temperature was higher than 455 °C. With increasing pyrolysis temperature, δ13C1–δ13C2 vs ln (C1/C2) has four evolution stages corresponding to the early stage of breaking bonds between carbon and hetero atoms, the later stage of breaking bonds between carbon and hetero atoms, the cracking of C6+ and coal demethylation, and the cracking of C2–5. The δ13C2–δ13C1 vs δ13C3–δ13C2 has three evolution stages corresponding to the breaking bonds between carbon and hetero atoms, demethylation and cracking of C6+, and cracking of C2–5.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The natural gas generated from different origins provides its formation characteristics during its evolution (Behar et al. 1992, 2008, 2010; Wang et al. 2013). Coal-formed gas has been an important field of natural gas and plays an important role in China’s natural gas resources (Dai et al. 2014). The heterogeneity of coal is strong, and the evolution features of coal-formed gas are more complex than those of other source rocks (Sun et al. 2013). The kinetics of hydrocarbon generation extend the hydrocarbon generation of coal under laboratory conditions to geologic history (Butala et al. 2000; Ping'an et al. 2009; Shuai et al. 2006), which simplifies the gas evolution of the coal. δ13C is one of the most important properties of natural gas. The δ13C of coal-formed methane has obvious evolution stage features (Cramer 2004; Cramer et al. 1998; Liu and Xu 1999), and the δ13C of natural gas could be used to trace the gas origin (Schoell 1980; Song and Xu 2005). The process of gas formation and evolution could be reflected by the δ13C relationship of different carbon numbers (Chung et al. 1988; Peng et al. 2009). The diagram of δ13C2–δ13C1 versus δ13C3–δ13C2 could reflect the maturity of natural gas (Jenden et al. 1993), and the diagrams of ln (C2/C3) vs ln (C1/C2) and δ13Ci–δ13Cj vs ln (Ci/Cj) can be used to distinguish the gas origin (Prinzhofer and Huc 1995); these indicators are largely associated with thermal evolution (Tian 2006).
In recent years, there has been increasing attention on the CO2 generated during the thermal evolution of coal (Fu et al. 2019a; Killops et al. 1996; Kotarba and Lewan 2004; Lewan and Kotarba 2014; Shuai et al. 2013a, b). CO2 could be generated from organic matter at any maturity stage (Shuai et al. 2013b); at low maturity, CO2 could be generated from the cracking of kerogen itself, and the disproportionation between organic matter and water could generate CO2 at the high maturity stage (Seewald 2003; Seewald et al. 1998). The δ13C of CO2 in natural gas could be used for gas source contrast (Song and Xu 2005), and some post-reformation of natural gas can be reflected by δ13CO2 (Rice 1993; Zhang et al. 2004).
The evolution of coal-formed gas abundance and the δ13C of gas can be measured by pyrolysis experiments (Behar et al. 1995; Hill et al. 2007; Lewan et al. 1985). Coal has a favorable gas generation ability and low oil generation potential (Hill et al. 2003; Shuai et al. 2006), and because of its molecular sieve nature and chemical adsorption property, liquid hydrocarbons can be generated when Ro% = 0.7, but oil expulsion cannot occur (Cook 1988; Johnston et al. 1991). Therefore, a closed system simulation experiment could reflect the gas generation process from coal, and anhydrous pyrolysis in sealed gold tubes under pressure could simulate the geological condition very well (Behar et al. 2008; Hill et al. 2007; Tang et al. 1996).
Previous hydrocarbon-generation kinetics research on Jurassic coal from the Minhe Basin shows that the mean values of the activation energies of CH4 and C1–5 are 64.55 kcal/mol and 63.93 kcal/mol, respectively, with a frequency factor of 1.0 × 1014 s−1 (Fu et al. 2019b). However, the stage evolution characteristics of gas generation have not been studied. In this study, the anhydrous pyrolysis of Jurassic coal from the Minhe Basin in sealed gold tubes was simulated. The gas component yields (C1, C2, C3, i-C4, n-C4, i-C5, n-C5, and CO2); the δ13C of C1, C2, C3, and CO2; and the mass of liquid hydrocarbons (C6+) were measured. Then, the stage δ13C values of C1, C2, C3, and CO2 were calculated, and the diagrams of δ13C1–δ13C2 versus ln (C1/C2) and δ13C2–δ13C1 versus δ13C3–δ13C2 were obtained to study the gas evolution of coal.
2 Experimental
2.1 Sample and closed system pyrolysis
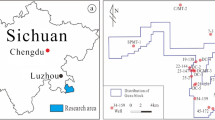
The immature Jurassic coal used in this study was from the Minhe Basin, Gansu Province, China (Fig. 1), and the basic geochemical characterization of the sample is listed in Table 1.
The coal was pulverized via a 100 mesh, and no other chemical treatment was conducted. The pyrolysis experiments were conducted in sealed gold tubes (40 mm × 5 mm). The sample (15–60 mg) was loaded in a gold tube with one end welded closed, and the air was replaced by argon. The open end was then welded closed by TIG (Pulse arc welding) with the closed end protected in room temperature water. The samples in sealed gold tubes were heated in one furnace in 12 separated stainless steel autoclaves. To avoid the influence of phase differentiation, the experiment was conducted at a constant pressure of 50 MPa, in which the fluid phase is basically in a single phase (Fu et al. 2019a, b). Two series of experiments were conducted, and the gold tubes were heated for 10 h from room temperature to 250 °C and then heated to 600 °C with heating rates of 20 °C/h and 2 °C/h. Additional descriptions of the experimental procedure can be found in (Pan et al. 2007; Shuai et al. 2013b; Wang et al. 2013).
2.2 Gas analysis
After the pyrolysis experiment, the gold tube was punctured in a closed system and connected to an Agilent 7890A gas chromatograph (GC) that has a Wasson ECE module for analyzing the gas composition (C1–5, CO2) using the external standard method to calculate the quantities of each gas component. The GC employed an HP-AL/S capillary column (25 m × 0.32 mm × 0.8 μm) and used helium as the carrier gas. The column temperature was programmed from 60 °C (held for 3 min) to 190 °C (held for 3 min) at 25 °C/min.
2.3 Stable carbon isotope analysis
The δ13C levels of C1, C2, C3, and CO2 analysis were conducted on an Isoprime 100 mass spectrometer that interfaced an Agilent 6890 GC. The GC was equipped with a CP-Poraplot Q column (27.5 m × 0.32 mm × 10 μm), and helium was used as the carrier gas. The column temperature was programmed from 50 °C (held for 3 min) to 190 °C (held for 5 min) at 25 °C/min. The δ13C of each sample was measured twice, and the average deviation was less than 0.3‰.
2.4 C6+ analysis
The C6+ composition was separated to C6–14 and C14+ for analysis. After the gas composition was analyzed, the C6–14 liquids were collected by a liquid nitrogen cold trap (4 mL, quartz bottle) (Behar et al. 1995) and injected with 3 mL of n-hexane. Then, the gold tube was cut into pieces and put into the bottle to ensure that the C6+ liquids could be dissolved completely. Deuterated-24-alkanes were used as the internal standard to determine the quantities of the liquids. The analysis was conducted on an Agilent 7890A GC that employed a DM-5 capillary column (30 mm × 0.32 mm × 0.25 μm) and used helium as the carrier gas. The initial oven temperature was 40 °C and held for 5 min, after which the oven was heated to 290 °C with a heating rate of 4 °C/min and held at 290 °C for 15 min. The C14+ was ultrasonically extracted by dichloromethane and weighed.
3 Results
3.1 Liquid (C6+) yield
The yields of C6+ should be the sum of C6–14 and C14+. Table 2 and Fig. 2a show the cumulative yields of the liquid hydrocarbons (C6+) in the experiments. As Fig. 2a shows, the yields of C6+ increased rapidly at low temperature (T < 410 °C), and the maximum yield was 78.17 mg/g TOC (20 °C/h, 406.9 °C). The yield began to decrease rapidly when the temperature > 410 °C because of cracking.
3.2 Gas yields
The yields of C1, C2, C3, C4, C5, and CO2 generated from coal pyrolysis are shown in Fig. 2 and Table 2. As a whole, C2-5 (Fig. 2b) peaked at 431 °C (2 °C/h) and 478 °C (20 °C/h), which is close to the end of the C6+ liquids cracking. The volumetric productivities of C2, C3, C4 and C5 were in descending order, and the relationship with temperature was similar. However, the cracking temperature was different and decreased with increasing carbon number; in addition, the cracking temperature was different at different heating rates, which manifested as the temperature became lower during the slow heating rate than during the rapid heating rate.
The yields of methane were low before 410 °C and increased rapidly after 410 °C, which was close to the cracking temperature of the C6+ liquids. The maximum yield of methane was 257.35 mL/g TOC (599.3 °C, 2 °C/h), which was much higher than that of all the other gases. At the same temperature, the gas yield during the slow heating rate was higher than during the rapid heating rate, which was the result of the complementary relationship between time and temperature on the chemical kinetics (Connan 1974).
CO2 is the most important nonhydrocarbon gas of the coal thermal maturity process (Shuai et al. 2013b; Tang et al. 1996). Figure 2h shows that the yield of CO2 was significant at 336 °C and much higher than that of the hydrocarbon gases. The yield of methane exceeded that of CO2 when the temperature was higher than 455 °C (20 °C/h) and 431 °C (2 °C/h), and the maximum CO2 was 112.13 mL/g TOC (599.3 °C, 2 °C/h). The XRD results (Table 3) indicated that the sample contains very small amounts of carbonate minerals, and the detection of liquid HCl showed the same results. The carbonate minerals contained in the sample could not generate such a large amount of CO2, which means that the CO2 was mainly from an organic origin.
3.3 The δ13C of gases
The δ13C values of C1, C2, C3, and CO2 are shown in Fig. 3 and Table 2. The δ13C of C1–3 decreased with increasing pyrolysis temperature and then began to enrich 13C, manifesting as δ13C1 < δ13C2 < δ13C3 at the same temperature and heating rate. Taking 2 °C/h as an example, the δ13C1 decreased over the range of 334.8–383.5 °C and began to enrich the 13C after 383.5 °C and the lightest δ13C1 was −37.71‰. Many other pyrolysis experiments of kerogen and crude oil samples have similar changes (Tang et al. 2000; Tian et al. 2007; Wang et al. 2013; Xiong et al. 2004). The δ13C of CO2 ranged from −24.03‰ to 20.62‰, and this is much less than that of hydrocarbon gases and is different from the report of Shuai et al. (2013b), showing that CO2 became enriched in 13C with increasing temperature but increased to −20.74‰ at 358.7 °C and then fluctuated between –20.63‰ and –21.03‰ to 479.0 °C; in addition, with increasing temperature, it became lighter after 479.0 °C.
4 Discussion
4.1 Stage δ13C values of C1, C2, C3, and CO2
The instantaneous changes in δ13C could not be obtained because of the closed pyrolysis system. To solve this problem, the following formula was used to calculate the stage value of δ13C according to the law of isotopic conservation and based on the gas yields and δ13C (Shuai et al. 2013b):
where, δ13CTi’ is the stage value of δ13C from temperature Ti−1 to Ti; δ13CTi and δ13CTi−1 are the cumulative δ13C values of gases at temperatures Ti and Ti−1, respectively; and VTi and VTi−1 are the cumulative volumetric yields of gases at temperature Ti and Ti−1, respectively.
The stage δ13C values of C1, C2, C3, and CO2 are shown in Fig. 4. Ethane and propane are generated and cracked during pyrolysis, so δ13CTi’, calculated by Eq. (1), can be divided into two processes. There is a certain temperature overlap between ethane and propane at the end of the formation and at the beginning of the cracking (Shuai et al. 2006; Tian 2006), so the calculated values that were close to the temperature when cracking began were discarded. As shown in Fig. 4, with increasing pyrolysis temperature, the stage δ13C values of C2 and C3 first decreased and then increased during the generation stage. During the cracking processes, the light carbon ethane and propane were cracked prior because the bond energy of 12C–13C is higher than that of 12C–12C (Arneth and Matzigkeit 1986; Stevenson et al. 1948; Tang et al. 2000). Therefore, the cumulative values of δ13C2 and δ13C3 in Fig. 3 began to increase when the cracking temperature was reached.
The stage δ13C value of C1 is significantly different from its cumulative value. Cramer (2004) explained the evolution of the δ13C value of coal-generated methane according to kinetics and divided methane generation into the following four reaction stages: (1) cleavage of heteroatoms, (2) demethylation reactions, (3) second cracking of long-chain alkanes and cyclic compounds generated, and (4) polycondensation reactions. Taking 2 °C/h as an example, with the increase of pyrolysis temperature, the stage δ13C1 value decreased to −38.85‰ at 383.5 °C, which means it was close to the end of the reaction stage (1) (Cramer 2004; Liao et al. 2007). After that, the stage value of δ13C1 began to increase, and the peak value of −17.59‰ was reached at 527.6 °C. This stage corresponded to the second cracking of liquid hydrocarbon (C6+) and wet gas (C2–5) one after another (Fig. 2a, b), which reflects that reactions stage (2) and (3) occurred (Cramer 2004). Then, the stage value of δ13C1 started to decrease with increasing temperature, and the yield continued to increase which corresponding to the reaction stage (4). However, the changes in δ13C2 and δ13C3 (Figs. 3, 4) and their remaining amounts (Fig. 2) showed that the cracked gases were almost complete and that δ13C became heavier, which means that the methane generated at this stage came from not only the cracking of C2-5 but also other reactions that may be the main source of methane. The reaction between water and coke in the higher evolution stage could generate some of the CH4, but as Eq. (2) below shows
The gas generation yields of CO2 and CH4 of this evolution stage should be equal; however, as Table 2 shows, the increase in CH4 is much higher than that in CO2. Therefore, there must be another origin of CH4. Cramer (2004) points out that the polycondensation reaction is the reason that δ13C1 decreases during this stage and that coal has gas generation potential at this stage.
The stage δ13C value of CO2 was complex and changed between −37.04‰ and −10.67‰, and it is generally believed that the δ13CO2 of an organic origin is less than −10‰ (Dai et al. 1996; Wycherley et al. 1999); in addition, the calculated stage δ13C value of CO2 shows that the CO2 in the experiment was of an organic origin and that the XRD result (Table 3) also supported this view. Shuai et al. (2013b) suggest that the δ13C of organic origin CO2 could be as high as 18‰. However, in this paper, the data do not support this conclusion, which may be due to the complex composition of coal. The stage δ13C value of CO2 changed smoothly before 455 °C (remaining at approximately −20‰), which was significantly higher than the stage δ13C value of methane at the same stage. This is because during this stage, the CO2 is mainly sourced from the cleavage of the heteroatom reaction of coal (Seewald et al. 1998), and the carbon that is attached to the heteroatom is more enriched in 13C than the aliphatic side chains (Cheng et al. 2009). At the high maturity stage, CO2 is generated from the reaction between H2O and organic matter (Lewan 1997; Seewald et al. 1998). Because of anhydrous pyrolysis, H2O may originate from the pyrolysis of coal or from the bond water in clay minerals (Wang et al. 2013). When the temperature was higher than 455 °C, the stage δ13C value of CO2 changed substantially, which may be related to the different causes of coke (cracking of aromatization carbon or liner carbon) reacting with H2O. In fact, as the experimental temperature increases, the number of free radicals in the reaction system increases accordingly. The generated CO2 should result of the reaction of oxygen free radicals and carbon free radicals. The oxygen free radicals and carbon free radicals here are from the cracking of H2O and coal, respectively. At the same time, we believe that the production of C radicals is mainly affected by temperature and pressure, and the isotope fractionation process is more complicated, which directly leads to the complex characteristics of the δ13C value of CO2.
4.2 δ13Ci–δ13Cj vs ln Ci/Cj
The δ13C of hydrocarbon gases has a good correlation with thermal maturity and source rock (Dai and Qi 1989; Schoell 1983), and the gas composition is very sensitive to thermal maturity (Wang et al. 2013). Therefore, the δ13Ci–δ13Cj vs ln Ci/Cj diagram can be used to indicate the thermal maturity and source rock of natural gas (Hill et al. 2003; Prinzhofer and Huc 1995; Tian et al. 2010). The δ13C2–δ13C3 vs ln C2/C3 diagram based on the pyrolysis experimental data (Fig. 5) shows a significant feature of kerogen pyrolysis gas (Prinzhofer and Huc 1995), which is characteristic of coal.
The δ13C1–δ13C2 versus ln C1/C2 diagram could be used to reflect the maturity of natural gas, the leakage of natural gas and the mixing of thermogenic gas and biogenic gas (Jenden et al. 1993; Prinzhofer and Huc 1995). The closed system pyrolysis experiment could exclude the influences of leakage and biogenic gas, which should only be correlated with the thermal maturity and the sample. The δ13C1–δ13C2 versus ln C1/C2 diagram based on the pyrolysis experimental data (Fig. 6) shows obvious stage changes. Taking 2 °C/h as an example, the first stage was 334.8–358.7 °C, and C1/C2 decreased with increasing temperature, which means that the increasing ratio of ethane was higher than that of methane (Wang et al. 2013). The δ13C1–δ13C2 decreased slightly, which was possibly because both methane and ethane were generated from cleavage of the heteroatom side chain, and the isotope fractionation effect of methane was higher than that of ethane. The second stage was from 358.7 to 407.2 °C, corresponding to the end of the cleavage heteroatom reaction. The C1/C2 almost remained unchanged, and the δ13C1–δ13C2 decreased rapidly because of high hereditability from the source rock of ethane (James 1983; Xie et al. 1999). The next stage was from 407.2 to 455.3 °C, both the methane and ethane yields were increased because of the cracking of C6+ (Fig. 2) during this interval, and the δ13C1 and δ13C2 were also increased. C1/C2 increased, which means that the increase ratio of methane was higher than that of ethane, and the δ13C1–δ13C2 increased slightly, showing that the degree of increasing δ13C1 heaviness was larger than that of δ13C2. When the temperature was higher than 455.3 °C, ethane began to crack (Fig. 2), and C1/C2 increased rapidly with decreasing δ13C1–δ13C2.
δ13C1–δ13C2 vs ln C1/ln C2 ((1) the increase ratio of ethane was higher than that of methane, and the isotope fractionation effect of methane was higher than that of ethane; (2) the end of the cleavage heteroatom reaction, where C1/C2 remained almost unchanged and δ13C1–δ13C2 decreased rapidly; (3) both the methane and ethane yields increased because of C6+ undergoing cracking; (4) the ethane begins to crack)
4.3 δ13C3–δ13C2 vs δ13C2–δ13C1
The δ13C3–δ13C2 and δ13C2–δ13C1 values decrease with increasing thermal maturity (Jenden et al. 1993; Prinzhofer and Huc 1995), so the δ13C3–δ13C2 vs δ13C2–δ13C1 diagram can be used to reflect the maturity of natural gas (Jenden et al. 1993). However, many simulation experiments show the opposite change (Guo et al. 2009, 2011; Tian 2006). The indexes above largely depend on the maturity of natural gas (Tian 2006). As Fig. 7 shows, in this experiment, the δ13C3–δ13C2 vs δ13C2–δ13C1 diagram shows obvious stage changes. Taking 2 °C/h as an example, when the temperature was lower than 383.5 °C, the δ13C3–δ13C2 increased slightly from 2.16‰ to 2.94‰, and the δ13C2–δ13C1 increased rapidly from 5.36‰ to 9.82‰. This is because during this stage, δ13C1 decreased, but δ13C2 first decreased and then increased, and δ13C3 remained almost unchanged (Figs. 2, 3). Then, when the temperature was between 383.5 and 431.2 °C, the δ13C3–δ13C2 first decreased and then increased, changing over the range of 2‰ to 3‰, and the δ13C2–δ13C1 remained almost unchanged. This result indicates that the variations in δ13C1, δ13C2 and δ13C3 were basically the same, and the main sources of these gases were the same during this stage. In contrast to Fig. 2, we conclude that the cracking of C6+ was the main source of these gases. The last stage was from 431.2 to 455.3 °C, where δ13C2–δ13C1 remained at approximately 10‰ and δ13C3–δ13C2 increased rapidly from 1.95‰ to 5.08‰ because of propane undergoing cracking before ethane.
δ13C3–δ13C2 vs δ13C2–δ13C1 ((1) δ13C1 decreased, but δ13C2 first decreased and then increased, and δ13C3 remained almost unchanged; (2) the variations in δ13C1, δ13C2 and δ13C3 were basically the same, and the main sources of these gases were generated from C6+ cracking; (3) propane cracked before ethane)
5 Conclusions
Anhydrous pyrolysis experiments were conducted in sealed gold tubes with Jurassic coal from the Minhe Basin. The hydrocarbon composition and CO2 yields and the δ13C of gas compositions were obtained. The following conclusions were drawn:
The stage δ13C value of methane shows a change trend of the “S” type that first decreased, then increased and finally decreased. The stage δ13C values of ethane and propane first decreased and then increased during the generating stage and became enriched in 13C during the cracking stage because the bond energy of 12C–13C was higher than that of 12C–12C. The stage δ13C value of CH4 decreased when T > 520 °C, but the δ13C values of cracked ethane and propane increased, which proved that coal still had the potential to generate CH4 at high maturity. The stage δ13C value of CO2 changes when T > 455 °C since the difference caused coke to be reacted with H2O.
According to the diagram of δ13C1–δ13C2 versus ln C1/C2 and δ13C3–δ13C2 versus δ13C2–δ13C1, five stages of coal gas generation could be determined: (1) early stage of heteroatom cleavage reaction, (2) later stage of heteroatom cleavage reaction, (3) demethylation reaction and second cracking of C6+, (4) cracking of wet gases (C2–5), and (5) polycondensation reaction.
References
Arneth J, Matzigkeit U (1986) Laboratory-simulated thermal maturation of different types of sediments from the Williston Basin, North America—effects on the production rates, the isotopic and organo-geochemical composition of various pyrolysis products. Chem Geol 58:339–360. https://doi.org/10.1016/0168-9622(86)90022-9
Behar F, Kressmann S, Rudkiewicz JL, Vandenbroucke M (1992) Experimental simulation in a confined system and kinetic modelling of kerogen and oil cracking. Org Geochem 19:173–189. https://doi.org/10.1016/0146-6380(92)90035-V
Behar F, Vandenbroucke M, Teermann S, Hatcher P, Leblond C, Lerat O (1995) Experimental simulation of gas generation from coals and a marine kerogen. Chem Geol 126:247–260
Behar F, Lorant F, Lewan M (2008) Role of NSO compounds during primary cracking of a Type II kerogen and a Type III lignite. Org Geochem 39:1–22. https://doi.org/10.1016/j.orggeochem.2007.10.007
Behar F, Roy S, Jarvie D (2010) Artificial maturation of a Type I kerogen in closed system: mass balance and kinetic modelling. Org Geochem 41:1235–1247. https://doi.org/10.1016/j.orggeochem.2010.08.005
Butala SJM, Medina JC, Taylor TQ, Bartholomew CH, Lee ML (2000) Mechanisms and kinetics of reactions leading to natural gas formation during coal maturation. Energy Fuels 14:235–259. https://doi.org/10.1021/ef990076k
Cheng Q, Fan M, Huang J, Chen Z (2009) The two-stage fractionation model of methane carbon isotope from the thermao simulation experiments of source rocks in the Kuqa depression of the Tarim Basin. Pet Geol Exp 31:101–104
Chung H, Gormly J, Squires R (1988) Origin of gaseous hydrocarbons in subsurface environments: theoretical considerations of carbon isotope distribution. Chem Geol 71:97–104. https://doi.org/10.1016/0009-2541(88)90108-8
Connan J (1974) Time-temperature relation in oil genesis: geologic notes. AAPG Bull 58:2516–2521
Cook R (1988) Interpretation of the geochemistry of oils of Taranaki and West Coast region, western New Zealand. Energy Explor Exploit 6:201–212
Cramer B (2004) Methane generation from coal during open system pyrolysis investigated by isotope specific, Gaussian distributed reaction kinetics. Org Geochem 35:379–392. https://doi.org/10.1016/j.orggeochem.2004.01.004
Cramer B, Krooss BM, Littke R (1998) Modelling isotope fractionation during primary cracking of natural gas: a reaction kinetic approach. Chem Geol 149:235–250. https://doi.org/10.1016/S0009-2541(98)00042-4
Dai J, Qi H (1989) The δ13C-Ro relationship of coal generated gas in China. Chin Sci Bull 09:690–692
Dai J, Song Y, Dai C, Wang D (1996) Geochemistry and accumulation of carbon dioxide gases in China. AAPG Bull 80:1615–1625
Dai J, Yu C, Huang S, Gong D, Wu W, Fang C, Liu D (2014) Geological and geochemical characteristics of large gas fields in China. Pet Explor Dev 41:1–13
Fu D, Zhou S, Ma Y, Li J, Xu G, Tian T, Yang F (2019a) Petroleum accumulation history of Nanbaxian belt—study of gas generation and fluid phase, northern margin of Qaidam Basin. West China J Pet Sci Eng 178:449–458. https://doi.org/10.1016/j.petrol.2019.03.060
Fu D, Zhou S, Xu G, Tian T, Ma Y (2019b) Phase characteristics and geological significance of coal-generated hydrocarbon: take the Minhe Basin as an example. Energy Explor Exploit 37:1306–1319. https://doi.org/10.1177/0144598718823956
Guo L, Xiao X, Tian H, Song Z (2009) Distinguishing gases derived from oil cracking and kerogen maturation: insights from laboratory pyrolysis experiments. Org Geochem 40:1074–1084
Guo L, Xiao X, Tian H (2011) Laboratory studies of differences between oil-derived and kerogen maturation gases. Pet Geol Exp 33:428–436
Hill RJ, Tang Y, Kaplan IR (2003) Insights into oil cracking based on laboratory experiments. Org Geochem 34:1651–1672. https://doi.org/10.1016/S0146-6380(03)00173-6
Hill RJ, Zhang E, Katz BJ, Tang YC (2007) Modeling of gas generation from the Barnett Shale, Fort Worth Basin, Texas. AAPG Bull 91:501–521. https://doi.org/10.1306/120606063
James AT (1983) Correlation of natural gas by use of carbon isotopic distribution between hydrocarbon components. AAPG Bull 67:1176–1191
Jenden P, Drazan D, Kaplan I (1993) Mixing of thermogenic natural gases in northern Appalachian Basin. AAPG Bull 77:980–998
Johnston J, Collier R, Maidment A (1991) Coals as source rocks for hydrocarbon generation in the Taranaki Basin, New Zealand: a geochemical biomarker study. J Southeast Asian Earth Sci 5:283–289
Killops SD, Allis RG, Funnell RH (1996) Carbon dioxide generation from coals in Taranaki Basin, New Zealand: implications for petroleum migration in southeast Asian Tertiary basins. AAPG Bull 80:545–568
Kotarba MJ, Lewan MD (2004) Characterizing thermogenic coalbed gas from Polish coals of different ranks by hydrous pyrolysis. Org Geochem 35:615–646
Lewan MD (1997) Experiments on the role of water in petroleum formation. Geochim Cosmochim Acta 61:3691–3723. https://doi.org/10.1016/s0016-7037(97)00176-2
Lewan M, Kotarba M (2014) Thermal maturity limit for primary thermogenic gas generation from humic coals as determined by hydrous pyrolysis. AAPG Bull 98:2581–2610
Lewan MD, Spiro B, Illich H, Raiswell R, Mackenzie AS, Durand B, Manning DAC, Comet PA, Berner RA, De Leeuw JW (1985) Evaluation of petroleum generation by hydrous pyrolysis experimentation [and discussion]. Philos Trans R Soc Lond A 315:123–134
Liao Y, Geng A, Liu D, Lu J (2007) Carbon isotopic fractionation effect caused by maturity during the generation of coal-pyrolysis hyrocarbons. Pet Geol Exp 6:583–588
Liu W, Xu Y (1999) A two stage model of carbon isotopic fractionation in coal gas. Geochimica 4:359–366
Pan C, Geng A, Zhong N, Liu J, Yu L (2007) Kerogen pyrolysis in the presence and absence of water and minerals. 1. Gas components. Energy Fuels 22:416–427
Peng Pa, Zou Y, Fu J (2009) Progress in generation kinetics studies of coal-derived gases. Pet Explor Dev 36:297–306
Prinzhofer AA, Huc AY (1995) Genetic and post-genetic molecular and isotopic fractionations in natural gases. Chem Geol 126:281–290. https://doi.org/10.1016/0009-2541(95)00123-9
Rice DD (1993) Composition and origins of coalbed gas Hydrocarbons from coal. AAPG Stud Geol 38:159–184
Schoell M (1980) The hydrogen and carbon isotopic composition of methane from natural gases of various origins. Geochim Cosmochim Acta 44:649–661
Schoell M (1983) Genetic characterization of natural gases. AAPG Bull 67:2225–2238
Seewald JS (2003) Organic-inorganic interactions in petroleum-producing sedimentary basins. Nature 426:327–333. https://doi.org/10.1038/nature02132
Seewald JS, Benitez-Nelson BC, Whelan JK (1998) Laboratory and theoretical constraints on the generation and composition of natural gas. Geochim Cosmochim Acta 62:1599–1617
Shuai Y, Peng P, Zou Y-R, Zhang S (2006) Kinetic modeling of individual gaseous component formed from coal in a confined system. Org Geochem 37:932–943. https://doi.org/10.1016/j.orggeochem.2006.04.006
Shuai Y, Zhang S, Gao Y, Lu H, Chen J, Mi J, Liu J, Hu G (2013a) Effect and quantitative evaluation of CO2 derived from organic matter in coal on the formation of tight sandstone reservoirs. Sci China Earth Sci 56:756–762. https://doi.org/10.1007/s11430-012-4565-2
Shuai Y, Zhang S, Peng P, Zou Y, Yuan X, Liu J (2013b) Occurrence of heavy carbon dioxide of organic origin: evidence from confined dry pyrolysis of coal. Chem Geol 358:54–60
Song Y, Xu Y (2005) Origin and identification of natural gases. Pet Explor Dev 32:24–29
Stevenson D, Wagner C, Beeck O, Otvos J (1948) Isotope effect in the thermal cracking of propane-1-C13. J Chem Phys 16:993–994
Sun Y, Yang Z, Cramer B (2013) Multiple stages of gas generation from coal organic matter as revealed by molecular isotopic geochemistry and implications for gas generation potential assessment of highly to over mature kerogen. Geochimica 42:97–102
Tang Y, Jenden P, Nigrini A, Teerman S (1996) Modeling early methane generation in coal. Energy Fuels 10:659–671
Tang Y, Perry JK, Jenden PD, Schoell M (2000) Mathematical modeling of stable carbon isotope ratios in natural gases. Geochim Cosmochim Acta 64:2673–2687. https://doi.org/10.1016/S0016-7037(00)00377-X
Tian H (2006) A study on the kinetic modeling of natural gas generation and gas accumulation in the platform area of Tarim basin. Ph.D. Thesis
Tian H, Xiao XM, Wilkins RWT, Li XQ, Gan HJ (2007) Gas sources of the YN2 gas pool in the Tarim Basin: evidence from gas generation and methane carbon isotope fractionation kinetics of source rocks and crude oils. Mar Pet Geol 24:29–41. https://doi.org/10.1016/j.marpetgeo.2006.10.001
Tian H, Xiao X, Wilkins RWT, Gan H, Guo L, Yang L (2010) Genetic origins of marine gases in the Tazhong area of the Tarim basin, NW China: implications from the pyrolysis of marine kerogens and crude oil. Int J Coal Geol 82:17–26. https://doi.org/10.1016/j.coal.2010.01.012
Wang Q, Lu H, Greenwood P, Shen C, Liu J, Peng P (2013) Gas evolution during kerogen pyrolysis of Estonian Kukersite shale in confined gold tube system. Org Geochem 65:74–82. https://doi.org/10.1016/j.orggeochem.2013.10.006
Wycherley H, Fleet A, Shaw H (1999) Some observations on the origins of large volumes of carbon dioxide accumulations in sedimentary basins. Mar Pet Geol 16:489–494. https://doi.org/10.1016/S0264-8172(99)00047-1
Xie Z, Li J, Lu X (1999) An approach to the changing origin and classification of ethane carbon isotope in marine gas of Tarim Basin. Pet Explor Dev 26:27–29
Xiong Y, Geng A, Liu J (2004) Kinetic-simulating experiment combined with GC-IRMS analysis: application to identification and assessment of coal-derived methane from Zhongba Gas Field (Sichuan Basin, China). Chem Geol 213:325–338
Zhang X, Tao M, Wang W, Duan Y, Shi B (2004) Generation of biogenic coalbed gases and its significance to resources. Bull Mineral Pet Geochem 23:166–171
Acknowledgements
The authors would like to thank Prof. Liu Jinzhong and Dr. Xu An for their great help on experiments analysis. This work is financially supported by the Major science and technology projects of Shaanxi Coal Geology Group Co., Ltd. (SMDZ-2019ZD-1), Independent subject of the Key Laboratory of Coal Exploration and Comprehensive Utilization, Ministry of Nature and Resources (ZP2019-3), the “Enterprise top innovative young talents support plan” (20190412), the “Shaanxi Provincial Postdoctoral Science Foundation (No. 2018M633642XB)” and China Postdoctoral Science Foundation (No. 2018M633642XB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fu, D., Xu, G., Ma, L. et al. Gas generation from coal: taking Jurassic coal in the Minhe Basin as an example. Int J Coal Sci Technol 7, 611–622 (2020). https://doi.org/10.1007/s40789-020-00318-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-020-00318-z