Abstract
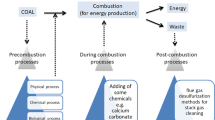
Coal still remains an important source of power generation world over. Along with its usage, comes unwanted generation of noxious gas emissions, toxic metal releases into wastewater and other pollutants which ultimately lead to environmental concerns. So cleaning of coal through physical or chemical processes becomes utmost important. There are several coals which cannot be cleaned by physical beneficiation techniques to produce low ash cleaner coals. Such coals can be cleaned only through chemical cleaning techniques. The present paper reviews the chemical demineralisation and desulphurisation of coals over the years using various inorganic and organic acids, alkalis, oxidants, leachants and various acids and alkali-acid combinations to reduce the ash and sulphur contents in coals. As high as 90% demineralisation and desulfurization could be achieved with the use of these cheap inorganic acids as compared to the expensive solvents used for solvent extraction processes, a parallel approach of cleaning and refining coals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Coal is still the largest contributor to the power generation and steel production. It still remains one of the most important fossil fuels and is likely to sustain that position over the years till suitable renewable replacements are not developed. However, the presence of inorganic mineral matter which is analysed as ash and sulfur limits the use of coal along with rise in the environmental concerns. Even in India, currently almost 55% of the power generation is still met by coal however; Indian coals are low grade coals because of the high ash contents (Meshram et al. 2015). The ash contents in Indian coals vary from 5% to as high as 55% in most of Indian coals with an average ash content of 35%. The washability characteristics of Indian coals are also poor, as coal mostly contains near gravity material and the inorganic mineral matter is finely dispersed in organic matter of these coals.
Physical beneficiation techniques such as dry fluidization, jigging, dense media/heavy media separations, hydroclone washings, magnetic separations, oil agglomerations, float and sink, air dense medium separations etc. can remove ash but to a limited extent (Rahman et al. 2017). These processes have their own limitations. The coal needs to be pulverised in order to effectively support the action of water, air, pressure or any magnetic separating device. The selective separation and not complete removal of mineral matter makes these processes ineffective with liberation yields. The coal obtained could also be required to be extensively dried for its maximum effective utilization in order to retain its calorific value.
The need to reduce ash in coal amounts from the fact of the disadvantages associated with its presence. Ash basically reduces the calorific value of coal and increases the transportation cost. Moreover, the presence of silicates and aluminosilicates largely result in fouling, slagging and clinkering of the furnance, heaters, turbines which ultimately corrode and erode the equipment thereby reducing its lifetime (Elliot 1981). Some of the volatile and extremely vulnerable toxic elements such as As, Se, Hg, B, cynates which when released into the atmosphere on coal combustion, result in acute health issues (Elliot 1981). The presence of certain metals such as Na and K which when present above the permissible limit have the ability to act as an adhesive between the ash particles and thereby making the process of ash deposition on the boilers etc. much faster (Elliot 1981). So ash removal should form an integral part of coal utilization through physical or chemical processes.
Mineral matter in coal has been classified by Speight (2012) as the (a) inherent mineral matter and (b) extraneous mineral matter. The inherent mineral matter that is associated with the plant material during coal formation which may or may not be derived from coal-forming plants. The extraneous mineral matter is the inorganic substances that were carried by the wind, water bodies and other mechanical means that were left on the coal beds or which gets added to the coal from over burden during mining operation. The inherent minerals are more difficult to be separated from the coal matrix because of their already developed extensive interactions with the organic matrix of coal as compared to the extraneous mineral matter. Almost all the elements present in the periodic table are present in coals. Coal ash is primarily composed of silicon, aluminium, iron oxides with variants of other oxides such as that of calcium, magnesium, sodium, potassium, titanium which are listed as the major constituents. The other minor elements or trace elements that are present are strontium, nickel, zinc, cadmium, mercury, lead, antimony, uranium, chromium, vanadium etc. (Deonarine et al. 2015).
In order to achieve maximum reduction in inorganic matter, chemical cleaning processes are used. This may also result in the deep cleaning of coals by removing a large portion of ash which is normally not possible by physical beneficiation techniques. Advanced techniques of beneficiation of coals have been reviewed by Meshram et al. (2015) some coals where only exterior ash is present mostly would show good liberation by physical beneficiation techniques whereas for others, chemical processes may have to be employed. It is essential to clean coals by removing the undesirable and non-combustible inorganic mineral matter from coal. There are two techniques of separation engineering which can be employed to clean the coal. In one of the techniques the organic matter is extracted from the coal through the solvent extraction leaving behind ash rich residual coal (Deonarine et al. 2015). In another method, the inorganic mineral matter is removed from the coal by chemical leaching by using inorganic chemicals. One obvious advantage of chemical leaching of coals over solvent extraction i.e. organo-refining of coal could be that here the yields of clean coal are much higher in comparison to organo-refining of coals (Deonarine et al. 2015). Moreover, inorganic minerals are separated from coals can lead to the recovery of value added minerals from the same. Table 1 summarises the disadvantages and advantages of the various leaching methods used for demineralisation of coals.
Parallel approach could also involve the use of bioleaching techniques by using microbes or their enzymes (Sharma and Wadhwa 1997; Bhatia 2007). Bhatia (2007) have reported the bioleaching and biodesulfurisation of coals by using Acidithiobacillus ferroodoxins. In fact, the extractability of coals in organic solvents under milder ambient pressure conditions is normally less than 25%–30%. Extraction yields can be enhanced by using higher pressure at elevated temperature. However, this leads to changes in the organic chemical structure of coals. Therefore, chemical leaching of coals by using inorganic chemicals seems to be a better option of cleaning the coals to obtain low ash cleaner coals. In this the coal is treated with inorganic acids and alkalis to reduce the ash as well as sulfur contents in coals. The most common reagents that have been used are NaOH, Na2CO3, CaO, Ca(OH)2, lime, KOH, mineral acids such as HCl, HF, HNO3, H2SO4,HI. Oxidizing agents such as H2O2, Fe2 (SO4)3, K2Cr2O7, NaOCl, have also been used by some researchers. The mild leachants such as EDTA, citric acids, pyroligenous acids have also been used. The presence of oxidizing agents in acids is also one method that has been used as this may also lead to the removal of pyritic sulfur. The reduction in mineral matter in chemical leaching of coals is defined by its degree of demineralisation. The degree of demineralisation (%) is calculated as follows:
Biochemicals are also used which may also be generated through by using microbes, enzymes or their metabolic compounds. Since bioleaching also involves the use of mostly chemicals, generated biochemically, therefore, presently authors have attempted to include some important biochemical cleaning processes under chemical leaching of coals. The first and the easiest washing that is performed is the water washing of coals. This would dissolve all the water soluble and vulnerable salts that are present as exterior components. Then ammonium acetate can be used to attack the ion-exchangeable cations which are generally present as carbonates in coals (Wijaya and Zhang 2011). Based upon the latest developments in the area of chemical leaching, the present paper categorizes the following methods for chemical demineralisation based upon the action of different inorganic reagents i.e. acids, alkalis and other chemicals:
-
Single acid leaching of coals
-
Stepwise acid leaching of coals
-
Use of oxidizing agents for leaching of coals
-
Alkali treatment of coals
-
Alkali-acid sequential treatment of coals
-
Chemical leaching of coals through biochemical route (Fig. 1).
2 Single acid leaching of coals
Most of the metal ions in coal are solubilised in acids generally at low pH. Therefore, single step acid washings have been used to remove the ash and sulfur in coals to a maximum extent possible using these acids. It has been reported by Steel et al. (2001a, b) that HCl has the ability to solubilise almost all minerals such as phosphates, carbonates, sulfates which are largely associated with major elements such as Ca, Mg, Fe, Al, K, Ti but fails to dissolve silicates, some aluminosilicates and pyrites in coal. Thus HF (Speight 2012) was proposed to be an effective acid to solubilise almost all minerals in coal but it still failed to remove pyrites. Therefore, subsequent treatments with HCl followed by HF were proposed to remove almost all the minerals. For pyrite removal, Steel and Patrick (2003) have suggested the use of HNO3, however, the action of nitric acid could only be proved effective if used above a certain concentration.
In one of the works demonstrated on single acid treatment of coal, the use of hydroiodic acid (HI) at 260 °C and 60 bar pressure on one of the Spanish coals could successfully completely remove in the first 10 min inorganic sulfur and 70% of the organic sulfur in the next 10 min of the acid treatment (Andres et al. 1996). Acidic ferric sulfate solution has also been used for the removal of pyritic sulfur which is commonly called as the Meyers Process (Meyers 1977).
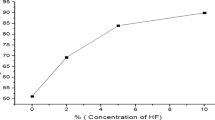
Treatment with nitric acid (almost 30%) has been demonstrated by some of the scientists (Rodriguez et al. 1996) on the Spanish coals. Almost 80%–90% of both pyritic and organic sulfur could be removed using HNO3 but on further studies it was concluded that rigorous treatment with HNO3 could alter the structure of coals, so single washing with HNO3 has not been encouraged. Steel et al. (2001a, b) have performed HCl treatment of the Australian bituminous coals and it was concluded that HCl could successfully dissolve mineral matter that was linked to carbonates, phosphates (mainly Ca, Mg, Fe, Na, Al, Ti) but preferably not silicates and some aluminosilicates (Si). The variations in the demineralisation have been studied by Gülen (2007) on Nallıhan lignite with 5% HCl, H2SO4, HNO3 and HF. Maximum deashing was reported by 5% HF treatment of the sample which was further verified using X-ray results and it also resulted in the subsequent increase in the calorific values from 16,652 to 23,950 kJ/kg. Then further ash reduction was observed by HNO3 > HCl > H2SO4 treatment which resulted in a lignite product that showed a higher calorific value. In fact, it is natural that the inorganic constituents in coal would vary depending upon the geological location of the mines and accordingly the action of these reagents on the coals differs due to their different chemical constitution. Another work (Hacifazlioglu 2016) also reports the production of ultra clean coal from a Turkish bituminous coal, Zonguldak coal where the ash contents have been brought down to 0.82% with aq.HF treatment. The optimization was carried out for varied temperatures, leaching time, acids (HCl, HF, HNO3, citric acid), acid concentration, coal particle size and the best results were obtained were when the coal was treated for 240 min, 25% HF treatment at 80 °C with coal particle size − 63 μm which resulted in the production of UCC (Ultra Clean coal) with the initial coal feed containing 8.84% ash.
3 Stepwise acid leaching of coals
Two stage leaching becomes necessary because of the inability of one acid to completely remove the inorganic matter from coals. The partial ability of these acids to selectively act upon the minerals requires the use of multiple acids on coals.
Steel et al. (2001a, b) and Steel and Patrick (2003) have studied the action of HF leaching followed by HCl treatment on Australian Black coals at varying concentrations and time. The resulting product showed less than 1% ash contents in washed coals. The collective action of HF on the silicates and aluminosilcates and the ability of HCl to act on Ca, Mg, Fe, Na, Al and Ti mostly as carbonates, phosphates could drastically reduce the mineral matter concentration in these coals.
In one such process by Steel et al. (Steel and Patrick 2001), a UK bituminous coal was treated with 3.5 M HCl solution followed by 1.36 M HNO3 at 65 °C for 3 h in each step. The ash content reduced from 7.9% to 0.63% and sulfur content from 2.6% to 1.4% in this two stage treatment. In another UK bituminous coal (Steel and Patrick 2003) treatment with 1.17 M HF and 1.4 M HNO3, the ash content in the coal could be reduced from 4.96% to 0.2% and S from 2.4% to 1.3% at 65 °C in 3 h. When the concentration of HF was increased to 3.5 M HF and treatment time for 0.5 h at 55 °C, the ash content in two plant coals, Zirab and Tabas coals (Jorjani et al. 2011) could be reduced to 0.69% from 8.31% to 0.39% from 10.36% respectively with prior microwave treatment. With 2 M HF-HNO3 treatment of Turkish, Australian and Polish coals (Kizgut et al. 2006), the ash contents could be reduced by 90% with 3 h treatment at 70 °C. Rubieira et al. (2002) investigated the action of 25% HF followed by 25% HNO3 treatment on a high volatile bituminous coal for 8 h and 16 h respectively at 60 °C and the resulting product contained ash of about 0.6%.
Wu and Steel (2007) investigated the HF-FeNO3 action on a Haworth coal and concluded the ash could be reduced up to 990 ppm when the coal was treated with 3.51 M HF for 4 h at 65 °C followed by FeNO3 treatment at 100 °C for 6 h. The major advantage of HF treatment is in the use of milder conditions under ambient pressure conditions. It has been reported that the HF can be recovered by following a cycle of reactions which will discussed in detail later in the text (Steel and Patrick 2004). Authors feel that the HF treatments were used to study leaching behaviour mostly on low ash coals which resulted in the production of UCC with ultra low ash contents. The use HF, HCl and HNO3 would require special and rugged materials of construction of reactors. Process economics may discourage the use of these acids on high ash coals.
4 Oxidizing agents and chelating agents for leaching of coals
Apart from the inorganic reagents, the use of oxidizing agents such as H2O2, K2Cr2O7, NaOCl, Fe2(SO4)3 etc. (Steel and Patrick 2004) have been tried on a number of coals. The reduction in ash contents along with effective desulfurization was demonstrated by a number of scientists over the years. Two Turkish coals (Karaca and Ceylan 1997) were treated with 15% H2O2 for 60 min at 30 °C and the ash contents in the two coals were found to be 21.4% and 16.71% which was effectively reduced from 65% to 31% respectively. About 90% pyritic sulfur could also be removed with just 15% H2O2 treatment. Inorganic sulfur could be completely removed with little reduction in ash content when H2O2 was used with H2SO4 for demineralization (Vasilakos and Clinton 1984). Mukherjee and Srivastava (2004) have performed kinetic studies with H2O2 and 0.1 N H2SO4 treatment of Indian coals and concluded that H2SO4 acts as a catalyst to bring pyrtic sulfur and oxygen together in order to carry out effective desulfurisation. Other oxidizing agents such as K2Cr2O7 have also resulted in 24% sulfur and 61% mineral matter removal from one of the Indian coals, i.e. Ledo coal (Ali et al. 1992). NaOCl treatment followed by hydrolysis with NaOH has also been tried by Li and Cho (2005) on Piitsburg No. 8 and Illionis No. 6 coal. About 70% of pyritic sulfur and 37.8% of the organic sulfur could be removed with 0.4 M hypochlorite, 0.4 M NaOH at 90 °C in Pittsburg No. 8 coal.
The basic disadvantage of using mineral acids is their ability to alter the coal structure and its morphological surface properties. So a number of other reagents have been tried. Ohki et al. (2004) have studied the effect of various leachants at different concentrations on the effective removal of metals. A number of treatments with HNO3 and EDTA have been tried and Ca, Mg and Mn could be completely removed. The use of pyroligeneous acid and citric acid has been reported for demineralisation of coal where these acids have good ability to act as chelating agents and thus these could effectively make coordination complexes with inorganic mineral matter of coal which ultimately bring down the ash contents. Pyroligenous acid produced by the pyrolysis of woody biomass and this can have pH as low as 2–3. Citric acid may be produced chemically or biochemically or from fruits and plants. EDTA being a good chelating agent has been used with various acids such as HF, HCl, HNO3 which further promotes the reduction of ash in coals (Shakirullah et al. 2006). The chemical demineralisation studies at room temperature using EDTA along with different organic acids have also been performed by Manoj (2012) where the ash contents were brought down to 2% and 1.8% of an Indian coal with citric acid and EDTA treatments. Aluminium, silica and calcium could be completely removed from the coal using acetic, citric and gluconic acids with EDTA. Citric acid has three –COOH groups which can form carboxylates with metal cations. Wijaya et al. (2011) treated two Victorian brown coals with 1 M pyroligenous acid and citric acid followed by treatment with Na-EDTA for 3 h and the resulting coals contained 0.08% and 1.5% ash as compared to 1.65% and 2.35% ash in the feed coals. The use of strong chelating agent, disodium ethylenediamine tetra acetic acid (Na-EDTA) was also studied along with the combination of dilute acid (1 M HNO3) and the combination resulted in breaking of the metallic complexes in the coal matrix (Wijaya et al. 2012). In this study, the mass transfer effects were also studied. The effect of coal particle size was studied and it was observed that the coal deashing did not significantly improve because of intra particle diffusion resistance whether it was fine or coarse particle and these Victorian brown coals treatments were also independent of temperature used for demineralisation.
5 Use of alkalis for demineralisation of coals
Alkali treatment of coals helps in the conversion of silicates and aluminosilicates in coal ash into sodium silicates like sodalite or sodium aluminosilicate and thus the ash contents can be reduced by following treatments after the action of alkali on coals.
Friedman and Warzinski (1977) studied the action of sodium hydroxide solution on the mineral matter of coal at 300 °C that resulted in complete removal of pyritic sulfur and about 40% organic sulfur from coal. Around 91% demineralisation could be achieved by Kara and Ceylan (1988) using aqueous NaOH solution for demineralization of Turkish lignites. The effect of grinding of coal on ash reduction and 5% aq.NaOH leaching was studied by Balaz et al. (2001) that resulted in ash reduction to 1.5 and 0.9% from 28.2% and 7% respectively in two coals. Mukherjee and Borthakur (2003) demonstrated the effect of alkali treatment for ash and sulfur removal from an Indian coal i.e. Assam coal and concluded that effective deashing and desulfurization could be achieved at 95 °C with caustic solution treatments. The aqueous caustic leaching has also been reported by Saydut et al. (2011) where a Turkish asphaltite sample was treated under varying conditions with aqueous NaOH solution from 0.1 to 1 M concentration with variations in temperature from 100 to 180 °C and time 4–16 h. The ash content of the asphaltite reduced from 32.49% to 18% and the total sulfur also dropped to 2.68% from 7.02%.
In one of the processes, called as the TRW Gravimelt process where a mixture of NaOH and KOH was heated at 350 °C for 2–3 h at room temperature which could (> 90%) desulfurize the coal (Kusakabe et al. 1989). In this process, sulfur is converted to H2SO4, CaSO4 or elemental sulfur after it is removed as Na2S. The used caustic can be regenerated and reused. Inorganic sulfur could be completely removed and 70% of the organic sulfur could also be removed with Na2CO3 treatment at 120–150 °C with one of the coal samples (Markuszewski et al. 1978). The treatment of a Nigerian coal (Adeleke et al. 2011) with Na2CO3 instead of sodium hydroxide was studied and it was found that ash reduced to 19.90% from 32.55%. Because of the less corrosiveness of lime on the equipments, CaO and lime Ca (OH)2 have also been used instead of NaOH on coals to reduce their ash contents. Stambaugh et al. (1975) and Stambaugh (1977a, b) made use of 10% aq.NaOH and 2%–3% Ca(OH)2 as a leaching agent at temperature of 250–300 °C and pressure of 3.9–8.4 MPa. Wang and Tomita (1997) have also rigorously studied the chemistry of hydrothermal treatment of aq.Ca(OH)2 with pure quartz and pure kaolinite. The use of lime though is not as effective as that of NaOH, but still it results in the reduction of cost of the leaching process. There is a wide scope of using stronger basic solutions for the demineralisation of coals. Studies in this direction can be extended in the future. An environment friendly Coal-Ca(OH)2–NaOH roasting process has been suggested by Zheng et al. (2017) which when applied on Bayan Obo tailings improved the leaching ratio of rare earth elements by 90.14% and magnetic separation of haematites 2.37. The optimized conditions of the process were found to be 650 °C roasting temperature, 60 min roasting time, 2% coal dosage, 4% aq.Ca(OH)2 dosage and 2% aq.NaOH dosage. Table 2 summarizes some of the processes of desulphurisation of coal (Edgar 1983). However, these processes were developed in the past. Authors feel soda-lime process could be an interesting process even presently. Some research work on the development of soda lime or lime treatment followed by acid treatment under milder ambient pressure conditions may also be extended (Sharma and Wadhwa 1997).
6 Alkali-acid leaching of coals
The alkali leaching and acid washing together play a great role in drastically reducing the ash in coals as the formed sodalites and sod-aluminosilicates which are produced by the reaction with NaOH and are mostly soluble in acids. So sometimes, mild acid washing also reduces the extra sodium compounds along with acid soluble minerals in the ash of coals. Not only Kaolinites and quartz, pyrites and sometimes the organic sulfur content is also reduced.
Out of the successive caustic-HF, caustic-HCl–HNO3 and caustic-HCl–H2SO4 leaching methods (Kumar and Shankar 2000), the caustic-HF leaching method has been found to be the most effective method for coal demineralization. However, the use of HF would increase the cost of the process. Duz et al. (2008) studied the action of NaOH at 400 °C for 45 min followed by acid washing of some asphaltite samples. 100% inorganic sulfur, 70% organic sulfur and ash contents could be reduced as a result of the combined sequential treatments. Yang et al. (1985) have investigated the three step treatment process of coals in which the aqueous NaOH solution followed by the action of sulfuric and nitric acid was studied. It was inferred that NaOH resulted in the formation of sodalites and sod. aluminosilicates whereas action of sulfuric acid dissolved most aluminates and silicates and nitric acid could further reduce the sulfur and iron by dissolving the pyrites. Wang et al. (1996) used 5% CaO at 340 °C for 120 min followed by hydrochloric acid washing for an Australian Newstan coal and this resulted in about 76% of ash removal. Mukherjee and Borthakur (2003) showed that on action of 16% aq.NaOH solution followed by 10% HCl at 90–95 °C about 43%–50% ash reduction, complete inorganic sulfur removal and 10% organic sulfur reduction in two Assam coals containing 8.4% and 10.4% ash in feed coals and total sulfur 4.3% respectively could be achieved. Kumar and Gupta carried out demineralisation of Assam coking coal at 120 °C with 500 g/L NaOH and reported that 70% demineralization could be obtained through this treatment. In one of the processes by CSIRO, Australia (Waugh and Bouling 1984) to achieve 90% demineralisation, a bituminous coal was treated with 10% NaOH at 200–300 °C under pressure followed by acid treatment. Bolat et al. (1998) treated a Turkish bituminous coal with 0.5 N aq.NaOH followed by 10% HCl treatment which resulted in 46.8% of the demineralisation whereas on treatment of another Indian coking coal (33.6% ash) (Waugh and Bouling 1984) with 20% NaOH and 10% HCl, as high as 75%–80% demineralisation could be obtained.
Sharma and Gihar (1991) have reported the alkali-acid leaching of Indian coals under milder ambient pressure conditions. These authors used 5 wt% to 40 wt% NaOH solution under reflux conditions in the first step. The alkali-treated coal samples were treated with 10% aq.HCl and 10% aq.H2SO4 in the second stage under reflux conditions. About 75% degree of demineralisation of Indian coals was reported by these authors. Sharma and Singh (1995) treated a low grade Indian coal, Talcher with 2%–20% aq.NaOH solution, refluxed for 24 h followed by 2% aq.HCl washing resulting in 75% deashing of coal under ambient pressure conditions. Nabeel et al. (2009) have studied the stepwise alkali-acid leaching of Indian coals. These authors have used 1% aq. alkali and 1% aq. HCl in the successive alkali-acid treatments which would lead to the reduction in the cost of alkali and acid through this stepwise alkali-acid treatment. It is possible to reduce the cost of alkali-acid treatments. These authors have reported that the ash contents in coals could vary world over and the reactivity of dilute aqueous alkali solutions would vary accordingly especially under milder ambient pressure conditions. Degree of demineralisation of high ash, low grade coals would be lesser. Chemical leaching of low ash coals has more chances of producing ultra clean coal having less than 1% ash or may be even almost zero ash. Recently, Behera et al. (2017a, b) have reported batch studies (2.5 L) of caustic-H2SO4 leaching of a low grade Indian coal where on treatment with NaOH initially at 100 °C, 26% ash could be removed and along with H2SO4 leaching resulted in 27.5% demineralisation however, on caustic-H2SO4 leaching, 48% demineralisation could be achieved. Therefore, the reactivity of aqueous NaOH or aq.KOH or of aq. Ca(OH)2 can vary depending upon the nature of the constituents present in coal ash. As low as 3.3% ash content was obtained from the Tuncbilek lignite which was treated with 30% aq.NaOH followed by 10% HCl treatment (Karaca and Yildiz 2007). Treatment with 40% NaOH concentration on heating at 124 °C and followed by 10% HCl treatment and then alkali followed by dilute acid treatment (10% NaOH, 170 °C, 0.8 MPa pressure and 10% HCl) was reported by Chong et al. (1995) on two Chinese coals, Pingxiang and Yangquan coals resulting in 81.0% and 90.0% demineralisation respectively. Baruah et al. (2006) studied the action of chemical leaching for 120 h at 45 °C for 120 h on two high sulfur Indian coals, Ledo and Baragorai coals from Assam (the ash content of 10.35% and 5.70%, total sulfur content of 3.57% and 5.30%, respectively). About 89.7% and 77.05% sulfur in Ledo and Baragorai coals could be removed. Behera et al. (2017a, b) reported > 70% demineralisation with a low grade Indian coal with 40% NaOH leaching at 100 °C for 3 h followed by leaching with 20% H2O2, H2SO4, HCl and HF separately where HF leaching proved to be most effective resulting in such high degree of demineralisation. The reason for such high demineralisation was found to be the high interaction of OH− and F− ions in the reagents with the mineral matter in coal (Table 3).
In one of the studies the treatment with aq. KOH alone at 95 °C and 150 °C and also followed by acid washing were compared (Behera et al. 2017a, b). At 150 °C, action of coal with 18% KOH and 10% HCl led to 52.7% desulfurization with complete removal of inorganic sulfur and 37% removal of organic sulfur. Baruah and Khare (2007) demonstrated the removal of pyritic and organic sulfur and minerals through solvent extraction and caustic treatment of the coal oxidized by H2O2–HCOOH leaching.
The effect of coal particle size, reaction time, temperature and change in alkali and acid concentration have also been studied on various Indian coals to study their effect on the degree of demineralisation and desulfurisation. Variations in the particle size helped in the studies on the effect of mass transfer during chemical leaching of coals. The effective removal of mineral matter increased on increasing the time, temperature and alkali concentration as explained by Dash et al. (2013). The acid treatment resulted in the reduction of the silica, alumina and phosphorous content. Phosphorous particularly was noticed to be reduced after the acid treatment. The complexity in the association of the P in coal has been discussed by where the occurrence of P as the apatite, crandallite and other phosphate minerals has been reported. P has also been found to be associated with Kaolinite (Saikia et al. 2013; Beck et al. 2005).
Other parameters that could be changed are the pre-treatment studies of the coal samples through solvent extraction, change in pressure, oxidation conditions and coal rank. Three different methods are have been used to carry out the kinetic studies and then on the determination of activation energy and K0 for the coal leaching processes in one of the works reported. Parametric estimation through rate equation, non-linear regression and parametric estimation through shrinking core model have been used to study the mechanism behind the chemical leaching of coals by Srirmajou et al. (2014) concluding that reaction of silica and alumina was found to be second order and exponential polynomial was found to be the best fit for the chemical demineralisation of coals. Demineralisation using NaOH and HCl was carried out by Choudhury (2013) using fractional factorial design under the optimum conditions of 0.22 mm particle size, 90 °C in 2 h extraction time. The maximum deashing was observed under this condition which was verified using statistical analysis. Not only demineralisation, but the characterisation of the product also forms an important aspect to study the process of leaching at various stages which has been reported by Dash et al. (2013) using FTIR, SEM, XRD and microscopic studies. The mechanism of alkali-acid leaching at a 50 kg batch pilot plant study has also been demonstrated by Sriramoju et al. (2017) and 150 °C was found to be the critical temperature for precipitation of sodalite during alkali treatment with silica and alumina in coal ash. The 60% recovery of the spent acid with almost 99% silica and 80% alumina removal has been reported through the optimization of the process. In another work reported by Srirmajou et al. (2018), the effect of increased alkali-concentration, higher temperature and lower reaction time on an Indian coal has been studied that resulted in about 68% ash reduction with the decrease in the alumina to silica ratio from 0.53 to 0.25 and almost 90% phosphorous reduction.
Authors feel that there is a wide scope of using super acids and super bases in the chemical leaching of coals. Some of the reactive ionic liquids may also be used to demineralise the coals. Lime and carbonic acid (from the CO2) seem to be the cheaper chemicals however; there may be a need to use elevated temperature and high pressure conditions in order to carry out effective demineralisation. Some of the low rank coals such as lignites or brown coals are soluble in aq. NaOH and aq. KOH solutions even milder conditions. Therefore, it would be difficult to demineralise such low rank coals through alkali-acid leaching process. Research work on alkali-acid leaching of Indian coals from Sharma’s laboratory (Bolat et al. 1998; Sharma and Gihar 1991; Sharma and Singh 1995) has been carried out under milder ambient pressure conditions. A comparison of the degree of demineralisation by using aq. NaOH alone and then followed by HCl or H2SO4 treatments has also been reported. The use of lime in place of NaOH was also made. Some of the Indian coals have higher ash contents (> 45%) and the inorganic mineral matter of some of these coals was found to show lesser reactivity towards aq. NaOH under milder ambient pressure conditions. With some Indian coals, the treatment with aq. NaOH at higher temperature led to the formation of sodiumsilicate salts having varied leaching tendencies with HCl or H2SO4.
7 Emerging options in chemical leaching of coals
Sharma and Gihar (1991) have reported that the use of aqueous NaOH or aqueous Ca(OH)2 followed by the acid leaching using either HCl or H2SO4 can bring about more than 75% degree of demineralisation of coals including high ash coals. Sharma’s group (Sharma and Singh 1995; Sharma and Gihar 1991; Nabeel et al. 2009) has also demonstrated that it is possible to reduce the concentration of aq. NaOH and HCl to the minimum extent through stepwise alkali-acid leaching of coals. The schemes for the recovery and recycling of chemicals used have also been proposed. The major advantage of this alkali-acid leaching process is that this is carried out under milder ambient pressure conditions and can be said to be the most effective method out of all discussed for the demineralisation of coals. The alkali-acid leaching process has been found to be most effective besides HF leaching. However, HF leaching is costlier in comparison to alkali-acid leaching under ambient pressure conditions and recovery and recycling of HF can be difficult and may involve a number of steps. The authors have carried out the studies on organo-chemical structure of coal and FTIR spectral analysis of the coal products and it was found that the organo-chemical structure is not affected with chemical leaching of coals as the process involves the action of chemicals on the mineral matter in coals not on the condensed polyaromatic network. Dash et al. (2015) employed high pressure and elevated temperature conditions in the alkali-acid leaching of coals showing large degree (> 70%) of demineralisation. In fact, the use of severe conditions and treatments such as microwave or ultrasonic treatments also result in increasing the degree of demineralisation of coals. The water, caustic S and other ash components absorb microwave radiations more readily than other constituents. Microwave treatments (Al-Harahsheh and Kingman 2004) have also been used in the extraction of metals such as copper, gold, nickel, cobalt, lead, zinc and manganese and coal desulfurisation. However, the understanding of the process still requires rigorous understanding of the interaction of the solids with microwave energy. Work can be extended in this direction in the future.
The use of Box-Beign experimental design for chemical demineralisation using NaOH–HCl of a low ash coal floatation tailings has also been studied by Suresh et al. (2015) where the optimization of the process conditions such as coal particle size, reaction time, reagent concentration %, reaction temperature have been studied and the STATISTICA software has been used to carry out regression analysis to develop the model. The most optimized conditions were found to be 160 °C as reaction temperature, 60.6 min as reaction time and 17.6% reagent concentration where the resulting product was found to have least ash content. Present authors feel that there is a need to employ milder conditions in the alkali-acid leaching of coals. The use of HF–HNO3 treatment under milder ambient pressure conditions also leads to the higher degree of demineralisation of coals. Since HF is a costly and corrosive chemical reagent therefore, its use seems to have been made mostly on low ash coals for their demineralisation to obtain ultra-clean coal having less than 1% ash contents. Use of HF on high ash coals does not seem to be advisable and moreover, the recovery of HF is also very important from the cost of view. There is a need to use inexpensive reagents such as lime or CO2 for the chemical leaching of coals. One such process is soda-lime process and Sharma and Gihar (1991) have already reported the use of lime-acid leaching under milder ambient pressure conditions.
In a mineral carbonation process CO2 can react with minerals such as magnesium and calcium oxide (Cuéllar-Franca and Azapagic 2015). Magnesium or calcium silicates can react with HCl to give Mg (OH)2 or Ca(OH)2. The use of carbonic acid under pressure may also help in demineralisation of coals through alkali-acid leaching of coals. The role of acid may be replaced by carbonic acid under pressure. The use of the resulting coal products obtained after leaching for power generation is still farfetched because of the cost involved and environmental concerns on the use of these strong harsh chemicals which affect the nearby water bodies and also disposal of ash becomes a problem (Jeon et al. 2016). Focus should be on making these processes cost effective and recoveries of these chemicals after treatment with coal (Wang et al. 2017; Sriramoju et al. 2017; Steel and Patrick 2003). Attempts (Cuéllar-Franca and Azapagic 2015) have been made to carry out LCA studies on the chemical leaching of coals and then using the clean coal thus produced in power plants. It has been reported that it may not be economical to use such a process for power generation. However, further process intensification studies may help in reducing the economics of the chemical leaching process. Present authors feel these are value added options for using ultra clean coal obtained through chemical leaching of coals. In fact, the use of UCC in the gasification for IGCC power generation systems, may increase the availability of these plants.77 UCC may be used in carbon fuel cell having around 75%–80% efficiency. UCC may also be used for the production of carbon nanotubes, graphenes, graphite, nanofuels, nano composites, smart coke, needle coke etc. (Mathur et al. 2007; Dosodia et al. 2009). Separation engineering technique of chemical leaching of coal by using inexpensive chemicals such as lime, soda-lime, CO2 and its derivatives, HCl has potential for its application in cleaner use of coal as clean coal technology.
8 Recovery and recycling of alkalis and acids
The excessive use of chemicals for demineralising coals especially under severe conditions of high pressure at elevated temperature leave their structure altered and reduces the utility as major structural hindrances can even reduce their calorific value. Moreover, these spent chemicals need to be recycled and reused in order to make these processes economically attractive. A lot of work has been reported on the recovery of these acids and alkalis and on the recovery of useful chemicals and metals from coal using these reagents.
Steel and Patrick (2004) have already reported the recovery of HF in one of their processes. It was because to overall reduce the cost of the demineralisation process and the separated product containing valuable chemicals made this procedure even more cost effective. HF has the ability to dissolve all the major minerals i.e. silicates and aluminosilicates in coal but their inability to remove pyrite made the use of HNO3 in the process. HNO3 not only dissolved pyrite but even reacts with the fluorides in the HF leached solution. Then Al(NO3)3 was added to the spent HF leached solution. The formation of AlF 2+ and AlF 2+ ions and their high stability constants due to presence of free fluoride ions and fluoride that is bound to silica makes the separation of silica as silica gel from the solution easier. The other ions, Fe and Ti present as fluorides and cations such as Na, K, Ca, Mg can also be easily separated. Because of high affinity of fluorides to form AlF 2+ and AlF 2+ ions, other insoluble solids do not form and therefore do not solubilise. Water is collected separately through distillation followed by HNO3. HNO3 is recycled to the system and this further converts the nitrates to oxides. The solids basically containing oxides and nitrates are treated with the water stream to pyrohydrolyse them where the fluorides are converted into HF which can be recovered and collected separately. This can then be recycled and reused.
Sharma and Gihar (1991) have used NaOH and acids, HCl and H2SO4 to effectively demineralise the high ash Indian coals up to as high as 75% degree of demineralisation. These authors have proposed a scheme to recycle and reuse these chemicals. The alkali was recovered after the reaction of the leached alkali solution containing sodium silicates with lime (Ca(OH)2) which generated calcium silicates and alkali (NaOH). HCl was recovered using distillation or membrane evaporation (Tomaszewska et al. 2001) whereas H2SO4 was recovered using gypsum. Gypsum (CaSO4) on reaction with silicic acid generated H2SO4.
Coal ash is a major source of recovery of various major elements such as aluminium, titanium, Iron, calcium, magnesium and various other minor and trace elements (Sahoo et al. 2016; Dosodia et al. 2009; Shcherban 1996; Mayfield and Lewis 2013). The rare earth elements are used widely in defense, energy, and electronics industries as petroleum cracking catalysts, catalytic converters, batteries, hard discs, LEDs etc. (Taggart 2015) The use of alkali-acid leaching, solvent extraction and recrystallization has been a common practise to recover major elements from coal and coal fly ash. In one of the US Patents (Meyers 1975), Al has been recovered from Fly ash using combined techniques of magnetic separation and leaching. The Al is recovered from the leachate using evaporation and calcinations at high temperatures. Silica recovery has been mentioned by Steel and Patrick (2004) earlier where the HF recovered is separated from silica simultaneously in the form of silica gel. The silicon fluorides formed can be treated with metallic nitrates where they are converted to oxides and nitrates which are separated using water (Ahmaruzzaman 2010). Desilication of coal wastes (sodium aluminate) has been performed by Padilla and Sohn (1985) using the soda-lime process. Here, leaching of the collected sinter solution with water, caustic solutions, Na2CO3 solutions was compared with Ca(OH)2 solution under mild conditions where the sinter solution responded better to desilication with Ca(OH)2 solution. In one of the US patents, under oxidizing conditions, ash is chlorinated which selectively separates Fe from the other elements. Then under reducing conditions, the rest of the ash is chlorinated which vaporizes the chlorides of other metals apart from Si and particularly Al (70%–80%) and Ti (80%) are collected separately through condensation.
Recovery of trace elements requires much precision and depending upon the nature of the element, its physical and chemical characteristics process modification are performed (Sahoo et al. 2016). The most common method used for Vanadium recovery is a wet process where the residue is passed through a multi-stage process and then precipitated followed by oxidation and reduction processes (Sahoo et al. 2016). Because of the high commercial applicability of Germanium i.e. in fibre optics, photovoltaics, diodes, electronic devices etc., it is also extracted from coal fly ash. In one method, sublimation is used where under oxidizing conditions (Sahoo et al. 2016), the fly ash is heated to recover Ge and Ga oxides which are converted to sub-oxides under reducing conditions which are collected over the sand particles. Selenium recovery is more pH dependent where it is recovered under high pH conditions (Wang et al. 2007, 2009). Molybdenum because of high applicability in steel manufacturing industries is extracted from coal fly ash using Conc. HCl and HNO3 and then diluting the solution, extracting Mo using dithiocarbamate (Taggart 2015). Lithium recovery (Grantham and Yosim 1974) involves a series of steps where the treatment with carbon dioxide involves formation of Lithium carbonate and then to lithium bicarbonate which is then separated and recovered. Maslov et al. (2010) have reported the extraction of radioactive elements uranium and radium from coal fly ash using mixture of HNO3 and HF extraction approximately extracting 99% U and 97% Ra in the process. The other trace elements such as Cd, Cu, Pd, Zn etc. require rigorous treatments and processes. Okada et al. (2007) used acetic acid leaching on coal fly ash to recover Zn and Pb from CFA, whereas alkaline leaching using 3 M NaOH recovered 81% Pb and 35.5% Zn in the treatment.
9 Chemical leaching of coals through biochemical route
Coal desulfurization using microbes is a wet process and it also provides a cleaning step of preparation of coal slurries for combustion. It has been estimated that the 70% of the coal slurry has the same bulk calorific value as the dry coal (Wise 1992). Microbial desulfurization of coal has shown various advantages including a higher pyrite removal efficiency and lower coal wastage than with physical methods and reduced costs compared to chemical methods as microbial methods operate under ambient conditions with less consumption of reagents (Andrews and Maczuga 1982). Apart from the chemical processes, various biorefining processes have been used over the years for biodesulfurization, bio-denitrogenation, bio-deashing, bio-demetallation, bio-demineralisation etc. Certain bacteria such as Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans, Leptospirrulum ferrooxidans, Sulfolobus acidocaldarius etc. have been found to grow in coal mines and then generate sulfuric acid (chemical leachant) in the mines. This actually results in acid drainage in mines. However, these chemoautotrophic bacteria which grow on CO2 instead of using costly carbohydrates can be exploited for the bio-desulfurization and bio-demetallation of coals and lignites. Interestingly, these chemoautotrophic bacteria can also be utilized for bio sequestration of CO2. In fact, these bacteria are also utilized for the multi-million dollar operations of Cu, Au and U metallurgy world over.
Acidithiobacillus ferrooxidans is a chemolithoautotrophic, aerobic, mesophilic and acidophilic microorganism. It has the ability to oxidise pyritic sulphur in coal and generate FeSO4 and H2SO4 which results in coal desulfurization, metal leaching (Andrews and Maczuga 1982; Valdes et al. 2003; Gasioreck 1994). The scale up process of bacterial action on coals along with the study on different parameters need to be performed as demonstrated in one of the demonstrations (Jeon et al. 2016) using chemoautotrophic bacteria. Acidithiobacillus ferrooxidans (At. ferrooxidans) that has great ability to demineralise and desulfurize the coals e.g. leaching of coals using At. Ferrooxidans (Pathak et al. 2013). Being an autotroph, it has also been reported to possess tolerance to several heavy metals and based on these characteristic properties, At. ferrooxidans is known to solve various environmental problems caused by heavy metals in coals. Thus it possesses an ability to remove various toxic heavy metals in coal and also removes Hg. Bhatia (2007) used these for the biodesulfurisation of Assam coals, Neyveli lignite, Kutch lignite and Rajasthan lignite. This resulted in not only the biodesulfurisation of these lignites and coal but this also led to the biodemineralisation and biodemetallation of these coals. In fact, biodesulfurisation and biodemetallation of lignites and coals by using At. ferroxidans 9 k IsA takes more than 6–7 days. However, if this process is combined with oil agglomeration process, then the time of bioleaching and biodesulfurisation of coal/lignites is brought to 1 h. Thus, this combined oil agglomeration–biodesulfurisation of coal process is rendered relatively economical and saves a lot of time, energy, labour, chemicals etc. In this study, 88%–98% of the pyritic sulphur could be removed by using At. ferroxidans 9 k IsA from different coals and lignites within a time period of 7–10 days. As high as 98% and 95% pyritic sulphur could be removed from Assam coal and Rajasthan Lignite in 10 days of the biological treatment. This also resulted in the removal of 36%–49% of ash reduction from Neyveli lignite, Assam coal, Rajasthan lignite and Kutch lignite after 10 days of treatment with media containing H2SO4 without At. ferroxidans 9 k IsA action. However, this deashing increased to 46%–56% after treatment with At. ferroxidans 9 k IsA after 10 days. At. ferroxidans treatment resulted in the removal of 93%–94% pyritic sulphur from Assam coal and Rajasthan Lignite in 7 days. The action of bacteria (At. Ferroxidans) on coal along with oil agglomeration studies has also been performed in the authors laboratory. It was found that before oil agglomeration process with xylene after conditioning with diesel (diesel + xylene) led to almost 88.2% agglomerated clean coal recovery with very low sulphur content. The oil agglomeration process of the bacterially treated coal/lignite samples led to a considerable time reduction in the depyritization of coals from 7 days to 35 min. This also amounted to save in labour and energy expenditure if the whole process is commercially used. These bacteria can act on coals containing pyritic sulfur. However, there are certain bacteria and fungi which can act on nonsulfidic minerals and most of such minerals are present in coal ash. These can be used for the bioleaching of coals having nonsulfidic ash (by generating even organic acids) and this has been reviewed by Jain and Sharma (2004) and Pathak et al. (2013).
The action of Acidithiobacillus ferrooxidans (A. ferrooxidans) was also studied by Hong et al. (2013) where the biodesulfurization of coal resulted in 50.6% removal of sulfur and 69.9% removal of pyritic sulfur after 16 days of processing. Based on the analysis of the coal product through SEM, XRD, XANES, it was concluded that the different sulphur forms affect the bio-oxidation in coals. There are certain bacteria which utilize CO2 instead of carbohydrates such as chemoautotrophic bacteria which can grow on S. Chemoautotrophic bacteria such as Acidithiobacillus thiooxidans (grows on S), Leptospirrulum ferrooxidans (grows on Fe), Sulfolobus acidocaldarius (grows on inorganic as well as organic S) can also be employed for the biodesulfurisation of coals. Obligate heterotrophs such as Acidophilus cryptum (Sharma and Wadhwa 1997) and facultative acidphilic autotrophs such as T. Acidophilus (Sharma and Wadhwa 1997) have been successfully used along with T. Ferroxidans for removing inorganic sulfur from coal. Leptospirillum ferroxidans, a facultative autotroph, has also been reported to oxidize pyrite efficiently (Helle and Onken 1988; Rawlings et al. 1999). Apart from these bacteria, Acidiphilium and Acidocella species are known to carry certain metal resistant genes (Banerjee 2004) that help in the natural leaching and demetallation of sulphidic ores. These bacteria can play a major role in effective deashing and desulphurisation of coals.
10 Biosequestration of CO2
It is worth mentioning here that the chemoautotrophic bacteria such as, Acidithiobacillus ferroxoxidans, Acidithiobacillus thiooxidans, Leptospirrium ferrooxidans etc. can utilize CO2 for their growth. These do not require costly carbohydrates for their growth. Therefore, these bacteria can also be exploited for the biosequestartion of CO2 (Sharma and Sharma 2010) during biodemetallation of coals and lignites. Therefore, such bacteria can serve twin purposes of bioleaching as well as of biosequestration of CO2 in coal based power plants. These bacteria are already being commercially exploited world over in the Cu, Au, U etc. for metallurgical operations. Their potential in the use of the metallurgical operations of pyritic ores is well recognized7.
On the other hand the chemically leached mineral matter can be subjected to reaction with CO2 under pressure elevated temperatures (Cuéllar-Franca and Azapagic 2015) for the chemical sequestration of CO2. In fact, leached mineral silicates or aluminates would react with CO2 to make stabilized carbonates to CO2 can react with Mg or Ca rich silicates in coal ash or mineral matter and can form stable carbonates. The reaction takes place under high pressure at elevated temperature. Use of aggressive leaching agents helps this process. The CO2 may be used as present in the flue gas without cleaning or separation (Park and Fan 2004; Wang and Maroto-valer 2013; Wang and Maroto-valer 2011a, b; Eloneva et al. 2012; Kodama et al. 2008; Sanna et al. 2012a, b). The coal mineral matter can be extracted in the HCl as required during alkali-acid or acid chemical leaching process. The following reactions can take place here as shown (Park and Fan 2004; Wang and Maroto-valer 2013; Wang and Maroto-valer 2011a, b; Eloneva et al. 2012; Kodama et al. 2008; 2012a, b; Sanna et al. 2012b):
Similar reactions with calcium silicates or other silicates may also take place with magnesium silicates in coal mineral matter. These processes can be combined with the leaching of coals in order to make these cost effective and focus should be laid in the minimal utilization of the corrosive regents, their absolute recovery and development of novel processes for environment friendly commercial utilization of chemical leaching of coals.
11 Conclusions
The use of acids, alkalis, leachants, oxidants and various alkalis, acids combinations prove to be an attractive option to demineralise and desulfurize the coals. With as high as 80%–90% demineralisation and desulfurization being achieved even in low grade coals, however, cost of these reagents still remains a concern in order to commercially use these processes for clean coal utilization. Moreover, the recovery of these chemicals and their discard in water sources is of great concern. So work needs to be extended in this direction to minimize the use of these reagents or use physical processes with chemical methods to further reduce the consumption of these chemicals in future for coal cleaning and efficient coal utilization.
Abbreviations
- Ca(OH)2 :
-
Calcium hydroxide
- CaO:
-
Calcium oxide
- NaOH:
-
Sodium hydroxide
- Na2CO3 :
-
Sodium carbonate
- CaO:
-
Calcium oxide
- KOH:
-
Potassium hydroxide
- HCl:
-
Hydrochloric acid
- HF:
-
Hydrofluoric acid
- HNO3 :
-
Nitric acid
- H2SO4 :
-
Sulphuric acid
- HI:
-
Hydroiodic acid
- H2O2 :
-
Hydrogen peroxide
- Fe2 (SO4)3 :
-
Ferrous sulphate
- K2Cr2O7 :
-
Potassium carbonate
- NaOCl:
-
Sodium hypochlorite
- EDTA:
-
Ethylenediamminetetraacetic acid
- FeNO3 :
-
Ferric nitrate
References
Adeleke AA, Ibitoye SA, Afonja AA, Chagga MM (2011) Multistage caustic leaching de-ashing of Nigerian Lafia-Obi coal. Pet Coal 53:259–265
Ahmaruzzaman M (2010) A review on the utilization of fly ash. Prog Energy Combust Sci 36:327–363
Al-Harahsheh M, Kingman SW (2004) Microwave-assisted leaching—a review. Hydrometallurgy 73:189–203
Ali A, Srivastava SK, Haque R (1992) Chemical desulfurization of high sulfur coals. Fuel 71:835–839
Andres JM, Ferrando AC, Membrado L (1996) Chemical desulfurization of coal with hydroiodic acid. Energy Fuels 10:425–430
Andrews GF, Maczuga J (1982) Bacterial coal desulfurisation. Biotechnol Bioenergy Syst 12:337
Balaz P, LaCount RB, Kern DG, Turcaniova L (2001) Chemical treatment of coal by grinding and aqueous caustic leaching. Fuel 80:665–671
Banerjee PC (2004) Genetics of metal resistance in acidophilic prokaryotes of acidic mine environments. Indian J Exp Biol 42(1):9–25
Baruah BP, Khare P (2007) Desulfurization of oxidized Indian coals with solvent extraction and alkali treatment. Energy Fuels 21:2156–2164
Baruah BP, Saikia BK, Kotoky P, Rao PG (2006) Aqueous leaching on high sulfur sub-bituminous coals, in Assam, India. Energy Fuels 20:1550–1555
Beck J, Muller R, Brandenstein J, Matscheko B, Matschke J, Unterberger S, Hein KRG (2005) The behaviour of phosphorus in flue gases from coal and secondary fuel co-combustion. Fuel 84(14–15):1911–1919
Behera SK, Chakraborty S, Meikap BC (2017a) Chemical demineralization of high ash Indian coal by using alkali and acid solutions. Fuel 196:102–109
Behera SK, Chakraborty S, Meikap BC (2017b) Upgradation of low grade coal to high quality by chemical beneficiation technique. In: Proceedings of the ASME 2017 power conference joint with ICOPE-17 POWER2017-ICOPE-17 June 26–30, Charlotte, North Carolina
Bhatia S (2007) Studies on bio-refining of fossil fuels (Lignite, coal and petroleum oils). Indian Institute of Technology, New Delhi
Bolat E, Saglam S, Piskin S (1998) Chemical demineralization of a Turkish high ash bituminuous coal. Fuel Process Technol 57:93–99
Chen C, Gao J, Yan Y (1998a) Observation of the type of hydrogen bonds in coal by FTIR. Energy Fuels 12(3):446–449
Chen C, Gao J, Yan Y (1998b) Role of non-covalent bonding in swelling of coal. Energy Fuels 12(6):1328–1334
Chong C, Xingbing L, Xianyong W, Jinsheng C (1995) Study on demineralization of coal by alkali/acid treatment under mild conditions. J East China Univ Sci Technol (Natural Science Edition), 03
Choudhury S (2013) Studies on demineralisation of coal: fractional factorial design. Int J Innov Technol Res 1(1):8–15
Cuéllar-Franca RM, Azapagic A (2015) Carbon capture, storage and utilisation technologies: a critical analysis and comparison of their life cycle environmental impacts. J Util 9:82–102
Dash PS, Kumar SS, Banerjee PK, Ganguly S (2013) Chemical leaching of high ash Indian coals for production of low ash clean coal. Miner Process Extract Metall Rev 34:223–239
Dash PS, Sriramoju SK, Kargupta A, Banerjee PK, Ganguly S (2015) Characterization of chemically beneficiated Indian coals. Int J Coal Prep Util 35(5):1
Deonarine A, Kolker A, Doughten M (2015) Trace elements in coal ash. US Geological Survey
Dosodia A, Lal C, Singh BP, Mathur RB, Sharma DK (2009) Development of catalyst free carbon nanotubes from coal and waste plastics. Fuller Nanotubes Carbon Nanostruct 17(5):567–582
Duz MZ, Erdogan S, Saydut A, Merdivan M, Hamamci C (2008) Effect of molten caustic leaching on demineralization and desulfurization of asphaltite. Energy Sour Part A Recov Util Environ Effects 30(17):1637–1644
Edgar TF (1983) Ch. 11, p. 301 in coal processing and pollution control. Gulf Publishing Co., Houston
Elliot MA (1981) Chemistry of coal utilization. Wiley, New York
Eloneva S, Said A, Fogelholm KJ, Zevenhoven R (2012) Preliminary assessment of a method utilizing carbon dioxide and steel making slags to produce precipitated calcium carbonate. Appl Energy 90:329–334
Friedman SJ, Warzinski RP (1977) Chemical cleaning of coal. Trans Am Soc Mech Eng J Eng Power 99:361–364
Gasioreck J (1994) Microbial removal of sulfur dioxide from a gas stream. Fuel Process Technol 40:129–138
Grantham LRF, Yosim SJ (1974) US Patent 3857920
Gülen J (2007) Mineral matter identification in Nallıhan lignite by leaching with mineral acids. Energy Sour Part A Recov Util Environ Effects 29(3):231–237
Hacifazlioglu H (2016) The production of ultra-clean coal from Zonguldak bituminous coal by chemical leaching. Energy Sour Part A Recov Util Environ Effects 38(24):3586–3592
Helle U, Onken U (1988) Continous microbial leaching of a pyrite concentrate by Leptospirillum-like bacteria. Appl Microbiol Biotechnol 28:553–558
Hong FF, He H, Liu JY, Tao XX, Zheng L, Zhao YD (2013) Comparison analysis of coal biodesulfurization and coal’s pyrite bioleaching with Acidithiobacillus ferrooxidans. Sci World J 184964:1–9
Jain N, Sharma DK (2004) Biohydrometallurgy of nonsulfidic ores-a review. Geomicrobiol J 21(3):135–144
Jeon TW, Park JE, Hwang DG, Jeong MJ, Um NI, Kang YY, Shin SK, Hong SY, Lee HS, Jang MJ (2016) A study on leaching property of hazardous substances in coal ash through the column test (percolation test). J Environ Anal Toxicol 6:21–24
Jorjani E, Ghasemi CH, Tayebi KM (2011) Ultra clean coal production by microwave irradiation pretreatment and sequential leaching with HF followed by HNO3. Fuel Process Technol 92:1898–1904
Kara H, Ceylan R (1988) Removal of sulfur from four central Anatolian lignites by NaOH. Fuel 67:170–172
Karaca H, Ceylan K (1997) Chemical cleaning of Turkish lignites by leaching with aqueous hydrogen peroxide. Fuel Proc Technol 50:19–33
Karaca H, Yıldız Z (2007) Desulfurization of fuel by leaching using H2O2 and H2SO4. Pet Sci Technol 6466(June 2012):37–41
Kizgut S, Baris K, Yilmaz S (2006) Effect of chemical demineralization on thermal behaviour of bituminous coals. J Therm Anal Calorim 86(2):483–488
Kodama S, Nishimoto T, Yamamoto N, Yogo K, Yamada K (2008) Development of a new pH-swing CO2 mineralization process with are cyclable reaction solution. Energy 33:776–784
Kumar M, Shankar RH (2000) Removal of ash from Indian Assam coking coal using sodium hydroxide and acid solutions. Energy Sources 22(2):187–196
Kusakabe K, Orita M, Morooka S, Kato Y, Kusunoki K (1989) Simultaneous desulfurization and demineralization of coal. Fuel 68(3):396–399
Li W, Cho EH (2005) Coal desulfurization with sodium hypochlorite. Energy Fuels 19:499–507
Manoj B (2012) Chemical demineralization of high volatile Indian bituminous coal by carboxylic acid and characterization of the products by SEM/EDS. J Environ Res Dev 6(3A):653–659
Markuszewski R, Chung KC, Wheelock TD (1978) Coal desulfurization by leaching with alkaline solutions containing oxygen. In: Rogers SE, Lemmon AW (eds) EPA symposium on coal cleaning to achieve energy and environmental goals, Hollywood
Maslov OD, Tserenpil S, Norov N, Gustova MV, Filippov MF, Belov MG, Altangerel M, Enhbat N (2010) Uranium recovery from coal ash dumps of Mogolia. Solid Fuel Chem 44:433–438
Mathur RB, Lal C, Sharma DK (2007) Catalyst-free carbon nanotubes from coal-based material, part A: recovery, utilization, and environmental effects. Energy Sources 29:21–27
Mayfield DB, Lewis AS (2013) Environmental review of coal ash as a source for rare earth and strategic elements. In: 2013 world of coal ash (WOCA) conference, April 22–25, Lexington
Meshram P, Purohit BK, Sinha MK, Sahu SK, Pandey BD (2015) Demineralization of low grade coal—a review. Renew Sustain Energy Rev 41:745–761
Meyers RA (1975) US 3926575
Meyers RA (1977) Coal desulfurization. Marcel Dekker, Inc., New York
Mukherjee S, Borthakur PC (2003) Effect of leaching high sulfur sub bituminous coal by potassium hydroxide and acid on removal of mineral matter and sulfur. Fuel 82:783–788
Mukherjee S, Srivastava SK (2004) Kinetics and energetic of high-sulfur north eastern India coal desulfurization using acidic hydrogen peroxide. Energy Fuels 18:1764–1769
Nabeel A, Khan TA, Sharma DK (2009) Studies on the production of ultra-clean coal by alkali-acid leaching of low-grade coals. Energy Sources Part A Recov Util Environ Effects 31:594–601
Ohki A, Nakajima T, Yamashita H, Iwashita A, Takanashi H (2004) Leaching of various metals from coal into aqueous solutions containing an acid or a chelating agent. Fuel Proc Technol 85:1089–1102
Okada T, Tojo Y, Tanaka N, Matsuto N (2007) Recovery of zinc and lead from fly ash from ash-melting and gasification-melting processes of MSW-comparison and applicability of chemical leaching methods. Waste Manag 27:69–80
Padilla R, Sohn HY (1985) Sodium aluminate leaching and desilication in lime-soda sinter process for alumina from coal wastes. Metall Trans B 1:707–708
Park AHA, Fan LS (2004) CO2 mineral sequestration: physically activated dissolution of serpentine and pH swing process. Chem Eng Sci 59:5241–5247
Pathak A, Kim D, Srichandan H, Kim B (2013) Depyritization of US coal using iron-oxidizing bacteria: batch stirred reactor study, world academy of science, engineering and technology. Int J Chem Mol Nucl Mater Metall Eng 7(11):495–498
Qin Y, Yang D, Gu F, Li X, Xiong W, Zhu JY (2016) Biorefinery lignosulfonates as a dispersant for coal water slurry. Sustain Chem Process 4:5:1–8
Rahman M, Pudasainee D, Gupta R (2017) Review on chemical upgrading of coal: production processes, potential applications and recent developments. Fuel Proc Technol 158:35–56
Rawlings DE, Coram NJ, Gardener MN, Deane SM (1999) Thiobacillus caldus and Leptospirillum ferroxidans are widely distributed in continuous flow biooxidation tanks used to treat a variety of metal containing ores and concentrates. In: Amils R, Ballester A (eds) Biohydrometallurgy and the environment towards the mining of the 21st century, process metallurgy, vol 9. Elsevier, Amsterdam, pp 777–786
Rodriguez RA, Jul CC, Gomez-Limon D (1996) The influence of process parameters on coal desulfurization by nitric leaching. Fuel 75:606–612
Rubieira F, Arenillas A, Pevida C, Garcia R, Pis JJ, Steel KM, Patrick JW (2002) Coal structure and reactivity changes induced by chemical demineralization. Fuel Proc Technol 79:273–279
Sahoo PK, Kim K, Powell MA, Equeenuddin SM (2016) Recovery of metals and other beneficial products from coal flyash: a sustainable approach for fly ash management. Int J Coal Sci Technol 3(3):267–283
Saikia BK, Sarmah M, Khare P, Baruah BP (2013) Investigation on inorganic constituents in Indian coal and emission characteristics of the particulates (PM2.5 and PM10). Energy Explor Exploit 31(2):287–315
Sanna A, Dri M, Hall MR, Maroto-Valer MM (2012a) Micro-silica for high-end application from carbon capture and storage by mineralisation. Key Eng Mater 517:737–744
Sanna A, Dri M, Hall MR, Maroto-Valer MM (2012b) Waste materials as a potential resource for carbon capture and storage by mineralisation (CCSM) in the UK context. Appl Energy 99:545–554
Saydut A, Duz MZ, Erdogan S, Tonbul Y, Hamam C (2011) Chemical leaching on sulfur and mineral matter removal from asphaltite (Harbul, SE Anatolia, Turkey). Energy Sources Part A 33:383–391
Shakirullah M, Ahmad I, Rehman H, Ishaq M, Khan U, Ullah H (2006) Effective chemical leaching and ash depletion of low rank coal with EDTA and citric acid. J Chem Soc Pak 28:56–61
Sharma DK, Gihar S (1991) Chemical cleaning of low grade coals through alkali-acid leaching employing mild conditions under ambient pressure. Fuel 70(5):663–665
Sharma DK, Giri CC (2016) CO2 gasification reactivity and kinetics studies of raw coal, super clean coal and residual coals obtained after organo-refining (solvent extraction). J Power Technol 96(3):157–169
Sharma R, Sharma DK (2010) Emerging trends in the sequestration of CO2—role of geomicrobiology, biosequestration, knowledge management and industrial approachs. J Appl Geochem 4:520–534
Sharma DK, Singh SK (1995) Advanced process for the production of clean coal by chemical leaching technique. Energy Sources 17:485–493
Sharma DK, Wadhwa G (1997) Demineralization of coal by stepwise bioleaching: a comparative study of three Indian coals by Fourier Transform Infra Red and X-ray diffraction techniques. World J Micro Biotechol 13:29–36
Shcherban S (1996) Ash utilization with Skica and metals recovery. The International Association of Sciences Inc, New York
Speight JG (2012) Chemistry and technology of coal, 3rd edn. CRC Press, Boca Raton
Sriramoju SK, Suresh A, Lingam RK, Dash PS (2017) Mechanism of a coal chemical-leaching process and recovery of spent chemicals: a pilot-scale study. Int J Coal Prep Util 37(6):293–302
Srirmajou SK, Suresh A, Dash PS, Banerjee PK (2014) Parameter estimation of kinetic model equations for chemical leaching of coal. Chem Prod Process Model 9(2):15
Srirmajou SK, Suresh A, Lingam RK, Ray T, Dash PS, Banerjee PK (2018) Optimization of process conditions for leaching of middling coal. Int J Coal Prep Util 38(2):88–97
Stambaugh EP (1977a) Hydrothermal coal process. In: Wheelock TD (ed) Coal desulfurization: chemical and physical methods. American Chemical Society symposium series, Washington, DC, pp 198–205
Stambaugh EP (1977b) Extracting sulfur and ash. US Patent 4,055,400
Stambaugh EP, Miller JF, Tam SS, Chauhan SP, Feldman HE, Carlton HE (1975) Hydrothermal process produces clean fuel. Hydrocarb Process 54(7):115–116
Steel KM, Patrick JW (2001) The production of ultra-clean coal by chemical demineralization. Fuel 80:2019–2023
Steel KM, Patrick JW (2003) The production of ultra-clean coal by sequential leaching with HF followed by HNO3. Fuel 82:1917–1920
Steel KM, Patrick JW (2004) Re-generation of hydrofluoric acid and selective separation of Si(IV) in a process for producing ultra-clean coal. Fuel Process Technol 86:179–190
Steel KM, Besida J, O’Donnell TA, Wood DG (2001a) Production of ultra clean coal: part I. Dissolution behaviour. Fuel Proc Technol 70:171–192
Steel KM, Besida J, O’Donnell TA, Wood DG (2001b) Production of ultra clean coal—part II—ionic equilibria in solution when mineral matter from black coal is treated with aqueous hydrofluoric acid. Fuel Proc Technol 70:193–219
Suresh A, Lingam RK, Sriramoju SK, Bodewar A, Ray T, Dash PS (2015) Pilot scale demineralization study on coal flotation tailings and optimization of the operational parameters with modelling. Int J Miner Process 145:23–31
Taggart R (2015) Recovering rare earth metals from coal fly ash. Department of Civil and Environmental Engineering, 2015, World of Coal Ash (WOCA) Conference
Tomaszewska M, Gryta M, Morawski AW (2001) Recovery of hydrochloric acid from metal pickling solutions by membrane distillation. Sep Purif Technol 22–23:591–600
Valdes J, Velso F, Jedlecki E, Holmes D (2003) Metabolicreconstruction of sulfur assimilation in the extremophile Acidithiobacillus ferroxidans based on genome analysis. BMC Genom 4(51):533
Vasilakos NP, Clinton CS (1984) Chemical beneficiation of coal with aqueous hydrogen peroxide/sulfuric acid solutions. Fuel 63(11):1561–1563
Wang X, Maroto-Valer MM (2011a) Dissolution of serpentine using recyclable ammonium salts for CO2 mineral carbonation. Fuel 90:1229–1237
Wang X, Maroto-Valer MM (2011b) Dissolution of serpentine using recyclable ammonium salts for CO2 mineral carbonation. Fuel 90(3):1229–1237
Wang X, Maroto-Valer MM (2013) Optimization of carbon dioxide capture and storage with mineralisation using recyclable ammonium salts. Energy 51:431–438
Wang J, Tomita A (1997) Hydrothermal reaction of Ca(OH)2 with quartz inconnection with coal demineralization. Ind Eng Chem Res 36(5):1464–1469
Wang J, Zhang ZG, Kobayashi Y, Tomita A (1996) Chemistry of Ca(OH)2 leaching on mineral matter removal from coal. Energy Fuels 10:386–391
Wang T, Wang J, Burken JG, Ban H, Ladwig K (2007) The leaching characteristics of selenium from coal fly ashes. J Environ Qual 36:1784–1792
Wang T, Wang J, Tang Y, Honglan S, Ladwig K (2009) Leaching characteristics of arsenic and selenium from coal fly ash: role of calcium. Energy Fuels 23(6):2959–2966
Wang K, Zhang Y, Huang J, Liu T, Wang J (2017) Recovery of sulfuric acid from a stone coal acid leaching solution by diffusion dialysis. Hydrometallurgy 173:9–14
Waugh AB, Bouling KG (1984) Removal of mineral matter from bituminous coals by aqueous chemical leaching. Fuel Process Technol 9:217–233
Wijaya N, Zhang L (2011) A Critical review of coal demineralization and its implication on understanding the speciation of organically bound metals and submicrometer mineral grains in coal. Energy Fuels 25:1–16
Wijaya N, Choo TK, Zhang L (2011) Generation of ultra-clean coal from Victorian brown coal—sequential and single leaching at room temperature to elucidate the elution of individual inorganic elements. Fuel Proc Technol 92:2127–2137
Wijaya N, Choo TK, Zhang L (2012) Generation of ultra-clean coal from Victorian Brown coal: effect of hydrothermal treatment and particle size on coal demineralization and the extraction kinetic of individual metals. Energy Fuel 26:5028–5035
Wise DL (1992) Bioprocessing and biotreatment of coal. Marker and Decker Inc, New York
Wu Z, Steel KM (2007) Demineralization of a UK bituminous coal using HF and ferric ions. Fuel 86:2194–2200
Yang RT, Das SK, Tsai BMC (1985) Coal demineralization using sodium hydroxide and acid solutions. Fuel 64:735–742
Zheng Q, Bian X, Wu WX (2017) An environmental friendly Coal-Ca(OH)2–NaOH roasting decomposition strategy for Bayan Obo tailings. Metall Res Technol 114(2):201–206
Acknowledgements
Authors would like to thank Dr. S. Bhatia for the useful discussions on biodesulfurisation of Indian coals and lignites.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dhawan, H., Sharma, D.K. Advances in the chemical leaching (inorgano-leaching), bio-leaching and desulphurisation of coals. Int J Coal Sci Technol 6, 169–183 (2019). https://doi.org/10.1007/s40789-019-0253-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-019-0253-6