Abstract
Introduction
Real-world pharmacoutilization analysis of biological drugs in psoriatic arthritis (PsA) patients with the aim to evaluate biologic treatment patterns and pharmacoutilization among patients with PsA in Italy.
Methods
A retrospective study was conducted using administrative databases of Italian Entities. PsA patients were included and diagnosed by hospitalization and/or an active exemption code. Two analyses were performed: a cross-sectional for treatment patterns in patients enrolled among 2017–2020, and a longitudinal study during 2015 to investigate the pharmacoutilization, in terms of persistence and monthly maintenance dosage of biological/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs). Patients with or without b/tsDMARDs prescriptions before inclusion were defined as bioexperienced or naïve, respectively. An analysis on ixekizumab-treated patients (IXE patients) from the 2017-to study ending was performed.
Results
PsA was diagnosed in 24,786 (2017), 27,221 (2018), 28,889 (2019), and 29,292 (2020) patients. Across 2017–2020, 31.1–40.5% of PsA patients were untreated with systemic medications, and 16.4–18.8% were under biological therapies. Among b/tsDMARD-treated patients, decreasing use of TNF-inhibitors (77.6–57.1%) and increasing IL-inhibitors (19.6–33.2%) was found across 2017–2020, respectively. Persistence to TNF-inhibitors and IL inhibitors as first-line ranged, respectively, 74.9–83.0% and 73.0–84.6%; specifically, 73.1–76.9% and 73.0–83.8% among bio-naïve, 83.3–90.0%, and 87.0% among bio-experienced. Among IXE-patients (N = 178), 55.6% were bio-naïve, while 21.9% previously used secukinumab, 12.9% adalimumab, 10.1% etanercept. During a 1-year follow-up, 6.8% of IXE patients switched therapy.
Conclusions
This real-world study of PsA pharmacoutilization in Italy showed that more than one-third of patients were systemically untreated, and almost 20% were receiving biological medications. Among biological users, increasing use of IL-inhibitors and a decrease in TNF-inhibitors prescriptions over the years were found. A rather-high extent of persistency in treatment was observed. A focused analysis on IXE patients revealed over half of them to be bio-naïve, while around one-fourth were bio-experienced to IL inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With the array of biological drugs available to treat PsA and the complexity of therapeutic options, a large-scale observational study based on real-world data is needed to assess the patients' management strategies into the clinical practice. |
A retrospective analysis of administrative datasets of PsA patients was carried out to evaluate the treatment patterns and pharmacoutilization of biologic medications among PsA-diagnosed patients in Italy. |
More than one-third of patients were untreated with the systemic medications indicated for PsA, and less than 20% of patients were under biological therapies, with a relatively high extent of persistence in biologic treatment and with dosages that were comparable to the recommended labels. |
These results on routine clinical practice for PsA in Italy suggested that the therapeutic management for PsA patients should be improved to minimize the undertreatment of these patients and to select the best therapeutic option to reach remission or low disease activity. |
Introduction
Psoriatic arthritis (PsA) is a chronic multisystemic inflammatory disease characterized by joint inflammation and by heterogeneous clinical features, including psoriasis (PSO) in up to 30% of patients [1,2,3], and by several musculoskeletal and non-musculoskeletal manifestations, i.e., inflammatory bowel disease (IBD), uveitis, and cardiometabolic and mental health comorbidities [4, 5], which introduces a significant patient burden with an impact on the quality of life, and an increase of mortality [6,7,8].
The treatment of PsA is complicated by the heterogeneity in the presentation of the disease (the presence of musculoskeletal and non-musculoskeletal manifestations) and its course. The EULAR recommendations state that nonsteroidal anti-inflammatory drugs (NSAIDs) may be used to relieve musculoskeletal signs and symptoms [9]; for patients with arthritis, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) are recommended either as first-line treatment or after a short course of NSAIDs [9]. In patients with peripheral arthritis and an inappropriate response to at least one csDMARD, biological DMARDs (bDMARDs) should be initiated [9]. Multiple biologic therapies have shown efficacy for PsA treatment and have been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), and these biological therapies include tumor necrosis factor (TNF) inhibitors [adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab], an interleukin (IL)-12/23 inhibitor (ustekinumab), IL-17 inhibitors (secukinumab and ixekizumab), and more recently IL-23 inhibitors (guselkumab and risankizumab). Targeted synthetic (ts) DMARDs, such as phosphodiesterase-4 inhibitors [apremilast, and more recently the Janus kinase (JAK)-inhibitor tofacitinib], were proposed for patients in whom other drugs were inappropriate, generally when the patients have mild disease [9]. The treatment decisions also have to take into account the extra musculoskeletal disease manifestations such as IBD, uveitis, or skin involvement, which require distinct therapies: TNF inhibitors are recommended for uveitis and TNF inhibitors or IL-12/23 inhibitors for IBD in the absence of axial involvement. However, when there is relevant skin involvement, the recommendations encourage the use of IL-12/23 inhibitors or IL-17 inhibitors [9, 10].
As a chronic condition, and without a currently available cure, PsA often require long-lasting treatment, with the primary goal reducing pain, improving other signs and symptoms of disease, and preventing disease progression [10]. With the array of biological medicines available to treat PsA and the complexity of therapeutic options, a large-scale observational study based on real-world data are needed to assess the patients' management strategies and to evaluate the persistence to medication, which is crucial to achieving positive clinical outcomes. In addition, the evaluation of treatment regimen in terms of administered dosage could be a key element to optimize biologic-based therapies, thus improving disease management to benefit patients’ health [11, 12].
Thus, in this study, a retrospective analysis of administrative datasets of PsA patients was carried out to evaluate the pharmacoutilization of biological therapies (in terms of treatment persistence and dosage) among PsA-diagnosed patients in Italy. In addition, since limited real-world data are available for the most recently approved IL-17 inhibitor, ixekizumab (IXE) (in Europe 2018), a focused analysis on PsA patients under treatment with ixekinumab in terms of the patient characteristics and pharmacoutilization variables was performed.
Methods
Data Source
This is a retrospective observational study based on the secondary data use, extracted from the administrative databases of geographically distributed Italian Entities, covering approximately 22% of the total Italian population. Administrative databases among the Italian National Health System (NHS) contain data for the reimbursement of healthcare services. In Italy, healthcare is provided to all citizens and residents by a mixed public–private system. The public system is referred to as the NHS, which is administered on a regional basis. Each region is divided into Local Health Units (LHUs) [13], which are administrative bodies to deliver health services in the broader community. These services include hospitalizations and outpatient specialist visits/diagnostic tests provided by the public hospitals (hospital centers or university hospitals) or by contracted private hospitals, reimbursed by the LHUs in which they are located. If patients moved out from the region during the study period, these patients were lost to follow-up. For the current study, the Italian Entities database was selected by their geographical distribution, by data completeness, and by the high-quality linked datasets. Within the administrative flows, the anonymous univocal numeric code assigned to each patient allowed the electronic linkage of all of the patients’ records across the databases. Specifically, data linkage was performed among the following databases: demographic database (to collect data on patients' demographic characteristics), pharmaceutical database [to collect data on prescription of drugs reimbursed by the Italian NHS, in terms of related Anatomical Therapeutic Chemical (ATC) code, and prescription date], the hospitalization database [to obtain information on discharge diagnoses at any level classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and date of diagnoses], the diagnostic tests and specialist visits database (contains the date of prescription, type, description activity of diagnostic tests, and procedure for patients in the analysis), and the payment exemption database that includes disease exemption codes and dates of exemption; the exemption code is a payment waiver code that allows avoiding the economic contribution for services/treatments in presence of a certain disease. The anonymous univocal numeric code ensured total compliance with the European General Data Protection Regulation (GDPR) (2016/679). The integration of administrative datasets allowed to represent the patient's entire clinical history and not just individual prescriptions. The analyses were conducted on exclusively anonymous data in full compliance with privacy regulations. The results are exclusively in aggregated form and are not attributable to a single institution, department, doctor, individual, or individual prescribing behaviors. The analysis was conducted in accordance with the Helsinki Declaration and in full compliance with current legislation for retrospective studies. Based on the Data Privacy Guarantor Authority (General Authorization for personal data treatment for scientific research purposes – n.9/2014), informed consent was not required, as its collection would be impossible for organizational reasons. According to Italian law on the conduction of observational analyses, the ethics committee of each participating entity was notified and approved the analysis (Table S1).
Study Design
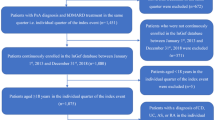
All patients with a diagnosis of PsA were enrolled and identified by the presence of at least one hospitalization with a primary or secondary discharge diagnosis of PsA (ICD-9-CM code 696.0) and/or an active exemption code for PsA (code 045.696.0). Two sets of analyses were performed on these patients: a cross-sectional study to evaluate the treatment patterns and a longitudinal analysis to investigate the b/tsDMARD pharmacoutilization parameters.
Cross-Sectional Analysis
In 2017, 2018, 2019, and 2020 (the 2020 datasets were incomplete; thus, only databases with a complete recording of 2020 were included), the treatment patterns were evaluated in the enrolled PsA patients. The prescriptions of b/tsDMARDs indicated for PsA, licensed in Italy and reimbursed by the Italian NHS during the study period, i.e., TNF-inhibitors (adalimumab, etanercept, infliximab, golimumab, certolizumab), IL-inhibitors, IL-12/23 inhibitor (ustekinumab) and IL-17 inhibitors (secukinumab, ixekizumab), and tsDMARD (apremilast) (a detailed report of ATC codes is in Table S2) and those of systemic traditional treatments, i.e., NSAIDs and csDMARDs (methotrexate, sulfasalazine, and leflunomide) (Table S2), were investigated. The index date was defined as the date of the first match with the inclusion criteria (hospitalization or exemption code) within each calendar year. Based on the presence or absence of b/tsDMARD prescriptions during the 12 months before the index date, the patients were defined as bioexperienced or bionaïve, respectively.
Longitudinal Analysis
The pharmaco-utilization analysis was carried out in patients under b/tsDMARD treatment during 2015. The index date was the date of the first prescription of b/tsDMARDs. The analysis was focused on the persistence to medication and the monthly maintenance dosage. For all PsA patients included from January 1, 2015, with a 1-year follow-up, the treatment persistence was assessed and defined as the presence of a prescription in the last trimester of the 1-year follow-up. In all PsA patients, including during 2015 and while the patients were followed-up for 3 years, the monthly maintenance dose was calculated as the overall milligrams between the end of the induction phase date and the penultimate prescription (included) for the index drug, divided by the number of months between the end of the induction phase date and the last prescription of the index drug. In the pharmacoutilization analysis, the definition of bionaïve and bioexperienced patients and the stratification of b/tsDMARD use among the lines of treatment (first or second line) were assessed after considering all the available periods before the index date.
Analysis of the Baseline Characteristics
For all patients included in the study, the baseline characteristics in terms of age and sex were evaluated at the index date. The clinical profile and manifestations related to PsA [5] were assessed considering all available periods before the index date. Both primary and secondary diagnoses for the clinical manifestations were collected in the database. If not available, the prescriptions of specific drugs were used as a proxy to determine the specific disease. The following clinical manifestations were analyzed: PSO [identified by the ICD-9-CM code: 696.1, or the exemption code 045.696.1 or by at least one prescription for antipsoriatic topical drugs (ATC code: D05AA)]; enthesopathies [identified by a hospitalization discharge diagnosis with the ICD-9-CM code: 726]; rheumatoid arthritis (RA) (as proxy of arthritis in PsA) [identified by a hospitalization discharge diagnosis with the ICD-9-CM code: 714 or the exemption code: 006]; ankylosing spondylitis (AS) [identified by a hospitalization discharge diagnosis with the ICD-9-CM code: 720 or the exemption code: 054.720.0]; cardiovascular disease (CVD) [identified by a hospitalization discharge diagnosis with the ICD-9-CM codes: 410, 411, 413, 414, 430–438, 440, 443); osteoporosis [identified by at least one prescription for anti-osteoporotic drugs (ATC codes: M05BA, M05BB, M05BX, H05AA, H05BA, G03XC) as proxy of diagnosis; depression [identified by at least one prescription for N06A, as proxy of diagnosis]; IBD [identified by a hospitalization discharge diagnosis with the ICD-9-CM codes: 555, 556 or the exemption codes: 009.555, 009.556]; Crohn’s disease (CD) only [identified by a hospitalization discharge diagnosis with the ICD-9-CM code: 555 or the exemption code: 009.555]; Ulcerative colitis (UC) only [identified by a hospitalization discharge diagnosis with the ICD-9-CM code: 556 or the exemption code: 009.556]; and diabetes [identified at least one prescription for antidiabetic (ATC code: A10, as proxy of diagnosis)].
Statistical Analysis
All analyses were descriptive, therefore no analytic adjustments for bias or confounding were necessary for the present study. Continuous variables are reported as mean ± standard deviation (SD); categorical variables are expressed as frequencies and percentages. In all analyses, the unit of the analysis was the patient. Following the ‘Opinion 05/2014 on Anonymization Techniques’ drafted by the ‘European Commission Article 29 Working Party’, the analyses involving fewer than three patients were not reported, as they were potentially traceable to single individuals. Therefore, the results referring to ≤ 3 patients were reported as NI (not issuable). All analyses were performed using STATA SE version 17.0 SE (StataCorp LLC, College Station, TX, USA).
Results
Cross-Sectional Analysis
In the cross-sectional analysis, PsA was diagnosed in 24,786 (in 2017, mean age ± SD, 57.0 ± 12.2 years; 43.1% male), 27,221 (in 2018, mean age 57.5 ± 12.2 years; 42.7% male), 28,889 (in 2019, mean age 58.1 ± 12.2 years; 42.5% male) and 29,292 (in 2020, mean age 57.4 ± 12.2 years; 42.4% male) patients (Table 1). The analysis of the clinical characteristics of all the included patients showed that 35.2–38.5% of them had a PSO manifestations (identified by the disease-related hospitalization or an exemption code or by an antipsoriatic prescription), and 6.8–7.3% and 5.6–7.5% of the patients presented with a previous hospitalization or with an exemption code associated with RA and CVD, respectively. Additionally, 23.3–28.3% of the patients had a previous prescription of antidepressant medications, antidiabetics (11.4–13.0%), and antiosteoporotic drugs (10.7–13.0%) (Table 1). Between 2017 and 2020, considering the first-year of follow-up period (index-date included), 31.1–40.5% of the patients were untreated with systemic medications recommended for PsA, 52.4–40.7% of the patients had traditional treatments (csDMARDs/NSAIDs), and 16.4–18.8% of the patients were prescribed b/tsDMARDs (Fig. 1). Among all of the diagnosed patients, over the years 2017–2020, 18.9–22.2% and 9.7–13.4% of the patients had monotherapy with NSAIDs and csDMARDs, respectively, while 5.6–8.7% of the PsA patients took b/tsDMARDs as monotherapy. Over the years, the percent of patients taking a combination of csDMARDs and NSAIDs tended to decrease (from 17.6 to 9.8%), while the number of patients prescribed b/tsDMARDs and csDMARDs alone or plus NSAIDs remained stable (Table 2).
Treatment patterns among the PsA patients. During the first year after inclusion (index-date included), the percentage of patients untreated with systemic medication for PsA, treated with csDMARD/NSAIDs or with b/tsDMARDs for each calendar year is reported. *partial data available (analysis on databases with a complete recording of 2020)
Among all biological utilizers evaluated at the index-date, across 2017–2020, a decreasing use of TNF inhibitors (from 77.6 to 57.1%) and an increasing use of IL inhibitors (from 19.6 to 33.2%) were found; the percent of patients taking tsDMARDs ranged from 2.8 to 9.7% (Fig. 2A; Table S3). A comparable trend was observed among bioexperienced PsA patients: the proportion of patients taking TNF inhibitors varied from 82.3% (in 2017) to 57.3% (in 2020), and those taking IL inhibitors increased from 17.4% (in 2017) to 33.7% (in 2020), which are similar to the trend of patients treated with tsDMARDs (0.2% in 2017 and 9.0% in 2020) (Fig. 2B; Table S3). In the bionaïve patients, the prescriptions of TNF inhibitors ranged from 62.8% (2017) to 55.4% (2020), those of IL inhibitors fluctuated approximately 26.5% (2017) and 28.3% (2020), and the percent patients taking tsDMARD were 10.7% (in 2017) and 16.3% (in 2020) (Fig. 2C; Table S3).
Analysis of the b/tsDMARD treatment patterns in the PsA patients. At the index-date, in overall (A), bioexperienced (B), and bionaïve (C) patients, the percentage of those treated with TNF inhibitors, IL inhibitors or tsDMARD is reported per each calendar year. The number of analyzed patients (N) is also reported for each year. *partial data available (analysis on databases with a complete recording of 2020)
Longitudinal Analysis
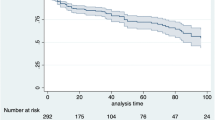
In Table 3 and in Table S4, the longitudinal analysis of first-line and second-line, respectively, b/tsDMARD treatment persistence in PsA patients during 2015 and during the 1-year follow-up was reported; however, the small-sample size of some patient subgroups should be considered. The percentage of overall PsA patients persistent to TNF inhibitors and IL inhibitors was 74.9–83.0% and 73.0–84.6%, respectively; the average rate of patients persistent to tsDMARDs was 60.6% (Table 3). Specifically, among bionaïve patients, the percentages of patients persistent to TNF inhibitors and IL inhibitors were 73.1–76.9% and 73.0–83.8%, respectively. Patients taking first-line treatment with b/tsDMARD during the follow-up, which assumed the same drug during the characterization period, were defined as bioexperienced patients. As reported in Table 3, the percentages of bioexperienced patients persistent to TNF inhibitors and IL inhibitors were 83.3–90.0% and 87.0%, respectively. In second-line treatment, the rates of PsA patients persistent to TNF inhibitors and IL inhibitors were 50.0–84.4% and 71.4–87.7%, respectively, and those persistent to tsDMARDs were 91.7% (Table S4).
As shown in Table 4, in bionaïve (N = 470) and bioexperienced patients (N = 1131) included during 2015 and during the 3-year follow-up, the prescribed monthly dosages of b/tsDMARDs during the maintenance phase were within the ranges of the label-recommended doses (in view of the small-sample size of some patient subgroups). For the IL inhibitor secukinumab, the recommended monthly dosage ranges from 150 to 300 mg; in our PsA population, both dosages were prescribed (Table 4).
Analysis on Ixekizumab (IXE)-Treated Patients
A focused analysis was carried out, including all IXE patients, from 2017 to all data available. As shown in Table 5, 178 IXE patients were identified (mean age 52.1 ± 12.0 years, 56.2% male). During the characterization period, 83.7% of the patients had a prescription of antipsoriatic medications or a PSO-related hospitalization or exemption code, 34.8% were previously treated with antidepressants, 16.9% with antidiabetic drugs, 6.7% with antiosteoporotic medications, and 5.6% of IXE patients had previous hospitalizations associated with CVD (Table 5). Among the IXE patients, considering the 1-year period before inclusion, 55.6% (N = 99) were bionaïve for b/tsDMARDs, 22.5% (N = 40) were previously treated with one b/tsDMARD, and 9.6% (N = 17) and 12.4% (N = 22) were treated with two and three (or more) b/tsDMARDs, respectively (not shown). During the year prior to IXE initiation, 30.9% of the IXE patients took IL inhibitors (21.9% secukinumab and 9.0% ustekinumab), 7.9% previously took tsDMARDs, and 12.9% and 10.1% were taking TNF inhibitors, adalimumab, and etanercept, respectively. During the 1-year follow-up, 6.8% (N = 12) of IXE patients had switched therapy, with 2.2% (N = 4) of IXE patients starting adalimumab treatment (not shown). No patient switched to certolizumab and etanercept; the number of switches towards apremilast, golimumab, infliximab, secukinumab and ustekinumab were not reported since data were referred to N < 3 patients.
Discussion
This is an Italian real-world study focusing on the systemic medication exposure of PsA patients across the years 2017–2020 (a cross-sectional analysis) and the pharmacoutilization analysis of b/tsDMARDs in patients enrolled during 2015 (a longitudinal study). The demographic and clinical characteristics of the population analyzed were comparable to those observed in previous real-world studies conducted on PsA patients in Italy [14,15,16,17]. During the characterization period, around 35% of PsA patients had a diagnosis for PSO; this results is in line with that reported in the literature, i.e., that PSO represent a clinical features in 30% of PsA patients [2]. In addition, almost 7% and 2% of patients presented RA and AS, respectively, as previous manifestations [18, 19].
The results of the cross-sectional analysis have shown that across the years, the percentage of patients who remained untreated with systemic medications tended to increase (from almost 31% in 2017 to 40% to 2020), there was a decreasing trend in the percent of patients taking conventional therapies (from almost 52% in 2017 to 41% in 2020), and less than 20% of patients over the years were prescribed b/tsDMARDs.
Nontreatment and undertreatment of patients with PsA are significant problems since the worsening of clinical conditions augments the burden associated with the disease [20]. The undertreatment issue among PsA patients was explored both in administrative claims-based analyses and in surveys [17, 20,21,22]. A previous real-world analysis among the southern Italian PsA population found that almost 15% of patients were not receiving any drugs [17]. In contrast, the results of survey-based studies carried out both in the United States and Europe have found that over half of the patients with PsA (58.0-64.0%) self-reported that they were receiving no treatment or were receiving topical therapy only, which leaved their joint disease untreated [21, 22]. In the present analysis, although data on the confirmation of the PsA diagnosis, by following established diagnostic criteria and the grading of disease severity, were not retrievable from the claims database, the high percentages of systemically untreated patients suggest a potential undertreatment that may require further investigation.
Based on the recommendations, biological therapies represent the first-line treatment usually reserved for patients who have failed or have contraindications to csDMARDs [9]. However, it has been reported that in patients with active PsA and with a higher risk of CVD, bDMARDs may be prescribed at an earlier stage since these drugs may reduce the inflammatory burden and are associated with a safer profile than csDMARDs in high-risk CV patients [23]. In our study, we found that less than 20% of PsA patients were prescribed b/tsDMARDs. This result was in line with a previous retrospective database study carried out on PsA patients among the German population, showing that almost 19% of PsA patients were treated with bDMARDs between 2014 and 2017 [24]. In addition, a real-world trend in biologic therapy was described for US patients; systemic therapy was used in 20.9% (18.1–24.0%) of patients, most commonly with biologics [8.0% (6.2–10.3%)] [25]. However, a higher frequency of biological use among PsA patients (32.0%) was found in a real-world study in southern Italy [17], and the MAPP survey among the North American and European populations reported an approximately 31% biological use [21]. The main reasons for not initiating biological therapies could be related to concerns about the long-term safety, tolerability, efficacy, and costs. In addition, the physician responses indicated that the most burdensome aspects of biologic therapy were related to the time requirements for patient education and management [26].
The distribution of the use of a different class of biologics (TNF inhibitors, IL inhibitors, and tsDMARDs) among the PsA population is in concordance with what was found previously [15,16,17], with TNF inhibitors being prescribed the most, followed by IL inhibitors. A trend towards an increase in IL inhibitor prescriptions was observed across the years from 2017 to 2020, with a decreasing trend for prescribing TNF inhibitors that was more pronounced among bioexperienced patients, and this was probably due to the recommendations and the use of biosimilars among biologically naïve PsA patients [27].
Several studies have examined biological persistence in PsA patients, most of which focused on TNF inhibitor therapies. More recently, some works have evaluated persistence in IL-12/23 and IL-17 inhibitors in PsA patients among the US population [28,29,30], and only one study was performed among European patients [31]. In the present study, we investigated the persistence of TNF- and IL-inhibitors among the Italian population. We found that the persistence averaged almost 75–80% in both the first- and second-line treatments. These data align with previous reports among the Italian population [14, 32] and among other studies carried out in British patients (almost 75% persistence) [33].
To date, the treatment strategy adopted with biologics prescription in chronic immunological disease such as PsA, is finalized to maintain the disease control, and standard dosages are still administered when patients reach remission or a low disease activity [12]. Thus, evidence on dosing patterns from the real-life practice may provide important information on drug prescription regimens. In the present study, the monthly dosage of b/tsDMARDs prescribed to PsA-enrolled patients during the maintenance phase was comparable to the label-recommended doses. A mixed prescription of 150 and 300 mg was found for secukinumab, probably due to the fact that 35% of the PsA patients also manifested as PSO or as anti-TNF inadequate responders in whom the 300 mg monthly dosage is recommended [34].
Although there are substantial real-world data on the treatment pattern of older biologics, IXE was approved for the treatment of PsA in 2018, and there are limited published data on IXE users. Thus, in the present study, a focused analysis on IXE patients was performed. The patients' characteristics were comparable to those of another real-world study on the use of IXE in PsA patients among the US population [35]. In our research, almost 90% of the patients taking IXE had a PSO manifestation, which is in line with findings reported by Murage et al. [35] from a study carried out in IXE-treated PsA patients. Our data, despite being derived from a small-sample population of patients, could be explained by the fact that patients were included during 2017, which is before the approval of IXE for the treatment of PsA in Europe. Thus, the high percentage of IXE-treated PsA patients could be referred to those who started IXE treatment after being prescribed by a dermatologist with the intent of treating the PSO manifestation [35]. In addition, these data are in line with the recommendations that encourage the use of IL-12/23 inhibitors or IL-17 inhibitors in patients with relevant skin involvement [9]. Over 50% of IXE patients were bionaïve, and almost 30% took IL inhibitors before the initiation of ixekizumab therapy. This result is in contrast with the previous finding that found that nearly 90% of IXE patients were bioexperienced [35], but further research on patients taking IXE should be performed to confirm these data.
The limitations of the present study were related to its retrospective observational nature and the use of anonymized data derived from administrative databases. Region/LHUs administrative databases have progressively improved the quality of the collected data. Nevertheless, some information may be missing; if the necessary information was missing for a given patient, that patient was excluded from the analysis. In addition, there was a lack or limited clinical information on comorbidities, the severity of PsA, disease duration, and other potential confounders that could have influenced the present results. Since the comorbidities herein analyzed were addressed based on any available data before inclusion (using proxy of diagnosis), there might be incomplete capture of these variables among patients. Data on pharmacological treatments were captured from medical prescription and dispensing information; thus, the reason for non-persistence was not retrievable from the dataset. Primary care data could not be collected. Therefore, the limitations are related to the limited follow-up period for the analysis of persistence to treatment and the small sample size of some patient subgroups, especially regarding the persistence to medication, the monthly dose data, and the partial data available for 2020 for the cross-sectional study. Despite these limitations and the fact that administrative claims data are not collected with the purpose of supporting research, analysis of real-world data from large datasets can deliver vital information about patient management in clinical practice [36].
Conclusions
In conclusion, this real-world study of PsA pharmacoutilization in Italy showed that from 2017 to 2020, a considerable percentage of patients remained untreated with the systemic medications that are indicated for PsA, and less than 20% of patients were under biological therapies. Patients took b/tsDMARDs with a relatively high extent of persistence in treatment and with maintenance dosages that were comparable to the recommended label dosages, and this suggests that there was an adequate response to biological therapies among PsA patients. The focused analysis on a small sample of patients taking IXE revealed that over half of these patients were bionaïve, while approximately 30% of these patients were bioexperienced with IL inhibitors. Altogether, these results on routine clinical practice for PsA in Italy suggested that the therapeutic management for PsA patients should be improved to minimize the undertreatment of these patients and to select the best therapeutic option to reach remission or low disease activity.
References
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376:957–70.
FitzGerald O, Ogdie A, Chandran V, et al. Psoriatic arthritis. Nat Rev Dis Primers. 2021;7:59.
Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915–23.
Paiva ES, Macaluso DC, Edwards A, Rosenbaum JT. Characterisation of uveitis in patients with psoriatic arthritis. Ann Rheum Dis. 2000;59(1):67–70.
Gupta S, Syrimi Z, Hughes DM, Zhao SS. Comorbidities in psoriatic arthritis: a systematic review and meta-analysis. Rheumatol Int. 2021;41(2):275–84.
Michelsen B, Fiane R, Diamantopoulos AP, et al. A comparison of disease burden in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis. PLoS ONE. 2015;10(4): e0123582.
Kotsis K, Voulgari PV, Tsifetaki N, et al. Anxiety and depressive symptoms and illness perceptions in psoriatic arthritis and associations with physical health-related quality of life. Arthritis Care Res (Hoboken). 2012;64:1593–601.
Tillett W, Shaddick G, Askari A, et al. Factors influencing work disability in psoriatic arthritis: first results from a large UK multicentre study. Rheumatology (Oxford). 2015;54:157–62.
Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700–12.
Ogdie A, Coates LC, Gladman DD. Treatment guidelines in psoriatic arthritis. Rheumatology (Oxford). 2020;59(Suppl 1):i37–46.
Maniadakis N, Toth E, Schiff M, et al. Targeted literature review examining biologic therapy compliance and persistence in chronic inflammatory diseases to identify the associated unmet needs, driving factors, and consequences. Adv Ther. 2018;35(9):1333–55.
Uhrenholt L, Schlemmer A, Hauge EM, et al. Dosage reduction and discontinuation of biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis: protocol for a pragmatic, randomised controlled trial (the BIOlogical Dose OPTimisation (BIODOPT) trial). BMJ Open. 2019;9(7): e028517.
Lo Scalzo A, Donatini A, Orzella L, et al. Italy: health system review. Health Syst Transit. 2009;11:1–216.
Degli Esposti L, Perrone V, Sangiorgi D, et al. Analysis of drug utilization and health care resource consumption in patients with psoriasis and psoriatic arthritis before and after treatment with biological therapies. Biologics. 2018;12:151–8.
Degli Esposti L, Perrone V, Sangiorgi D, et al. Therapeutic strategies utilization and resource consumption in patients treated for psoriatic arthritis: findings from a real-world analysis in an Italian setting. Patient Pref Adher. 2019;13:187–94.
Perrone V, Losi S, Filippi E, Sangiorgi D, Degli EL. Pattern of drug use in patients with psoriatic arthritis in Italy: study in a real-world setting. Expert Rev Pharmacoecon Outcomes Res. 2021;21:721–7.
Ingrasciotta Y, Isgrò V, Ientile V, Tari M, Trifirò G, Guarneri C. Are patients with psoriasis and psoriatic arthritis undertreated? A population-based study from southern Italy. J Clin Med. 2021;10:3431.
Merola JF, et al. Distinguishing rheumatoid arthritis from psoriatic arthritis. RMD Open. 2018;4(2): e000656.
Feld J, et al. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nat Rev Rheumatol. 2018;14(6):363–71.
Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013;149:1180–5.
Kavanaugh A, Helliwell P, Ritchlin CT. Psoriatic arthritis and burden of disease: patient perspectives from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Rheumatol Ther. 2016;3:91–102.
Gottlieb A, Gratacos J, Dikranian A, et al. Treatment patterns, unmet need, and impact on patient-reported outcomes of psoriatic arthritis in the United States and Europe. Rheumatol Int. 2019;39:121–30.
Puig L. Cardiometabolic comorbidities in psoriasis and psoriatic arthritis. Int J Mol Sci. 2017;19:58.
Sewerin P, Borchert K, Meise D, Schneider M, Mahlich J. Real-world treatment persistence with biologic disease-modifying antirheumatic drugs among German patients with psoriatic arthritis-a retrospective database study. Rheumatol Ther. 2021;8:483–97.
Singh P, Silverberg JI. Real-world trends in biologic, oral systemic, and phototherapy in US patients with psoriasis or psoriatic arthritis. J Am Acad Dermatol. 2020;83:256–7.
van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002–10.
Marchesoni A, Olivieri I, Salvarani C, et al. Recommendations for the use of biologics and other novel drugs in the treatment of psoriatic arthritis: 2017 update from the Italian Society of Rheumatology. Clin Exp Rheumatol. 2017;35:991–1010.
Sauer BC, Teng C-C, He T, et al. Treatment patterns and annual biologic costs in US veterans with rheumatic conditions or psoriasis. J Med Econ. 2016;19:34–43.
Oelke KR, Chambenoit O, Majjhoo AQ, Gray S, Higgins K, Hur P. Persistence and adherence of biologics in US patients with psoriatic arthritis: analyses from a claims database. J Comp Effect Res. 2019;8:607–21.
Walsh JA, Adejoro O, Chastek B, Palmer JB, Hur P. Treatment patterns among patients with psoriatic arthritis treated with a biologic in the United States: descriptive analyses from an administrative claims database. J Manag Care Spec Pharm. 2018;24:623–31.
Geale K, Lindberg I, Paulsson EC, et al. Persistence of biologic treatments in psoriatic arthritis: a population-based study in Sweden. Rheumatol Adv Pract. 2020;4: rkaa070.
Fabbroni M, Cantarini L, Caso F, et al. Drug retention rates and treatment discontinuation among anti-TNF-α agents in psoriatic arthritis and ankylosing spondylitis in clinical practice. Mediat Inflamm. 2014;2014:862969.
Saad AA, Ashcroft DM, Watson KD, Hyrich KL, Noyce PR, Symmons DP, British Society for Rheumatology Biologics Register. Persistence with anti-tumour necrosis factor therapies in patients with psoriatic arthritis: observational study from the British Society of Rheumatology Biologics Register. Arthritis Res Ther. 2009;11:52.
European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/product-information/cosentyx-epar-product-information_en. Accessed Jan 2022.
Murage MJ, Princic N, Park J, et al. Real-world treatment patterns and healthcare costs in patients with psoriatic arthritis treated with ixekizumab: a retrospective study. ACR Open Rheumatol. 2021;3:879–87.
Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, LaVange L, Marinac-Dabic D, Marks PW, Robb MA, Shuren J, Temple R, Woodcock J, Yue LQ, Califf RM. Real-world evidence: what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–7.
Acknowledgements
Funding
Eli Lilly purchased the study report, which is the basis for this manuscript. This manuscript was developed with Eli Lilly and CliCon S.r.l. Società Benefit. The agreement signed by CliCon S.r.l. Società Benefit and Eli Lilly & Company does not create any entity, joint venture or any similar relationship between the parties. CliCon S.r.l. Società Benefit is an independent company. Neither CliCon S.r.l. Società Benefit nor any of their representatives are employees of Eli Lilly & Company for any purpose. Eli Lilly funded the journal’s Rapid Service Fee.
Editorial Assistance
The manuscript was edited for English language, grammar, punctuation, spelling, and overall style by Dr. Cassy J. of American Journal Experts. Eli Lilly provided funding for the assistance.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: VP and LDE; data curation: VP and LDE; funding acquisition: LDE; methodology: DS; supervision: LDE; visualization: LDE; writing—original draft: VP and MD; writing—review and editing: VP, MD, SL, EF, MM. All authors have read and approved the final version of the manuscript.
Disclosures
Valentina Perrone, Melania Dovizio, Diego Sangiorgi, and Luca Degli Esposti have nothing to disclose. Serena Losi, Erica Filippi, and Maurizio Mezzetti are employees of Eli Lilly Italy S.p.A., Italy.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Helsinki Declaration and in full compliance with current legislation for retrospective studies. Based on the Data Privacy Guarantor Authority (General Authorization for personal data treatment for scientific research purposes – n.9/2014), informed consent was not required, as its collection would be impossible for organizational reasons. According to Italian law on the conduction of observational analyses, the ethics committee of each participating entity was notified and approved the analysis (Supplementary Material).
Data Availability
All data used for the current study are available upon reasonable request to CliCon S.r.l. Società Benefit, which is the body entitled to data treatment and analysis by Local Health Units.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Perrone, V., Losi, S., Filippi, E. et al. Analysis of the Pharmacoutilization of Biological Drugs in Psoriatic Arthritis Patients: A Real-World Retrospective Study Among an Italian Population. Rheumatol Ther 9, 875–890 (2022). https://doi.org/10.1007/s40744-022-00440-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00440-1