Abstract
The aims of this systematic review and meta-analysis were to: (1) describe the prevalence of commonly reported comorbidities in psoriatic arthritis (PsA), (2) compare the incidence and/or prevalence of comorbidities between PsA and control populations; and (3) examine the impact of comorbidities on PsA outcomes. We systematically searched Medline, PubMed, Scopus, and Web of Science using a predefined protocol in accordance with PRISMA guidelines. Studies reporting only one comorbidity, or a few closely related diseases within one organ system, were excluded. Where possible, meta-analysis was performed using random-effects models. We included 39 studies amounting to over 152 thousand PsA patients. We performed meta-analysis for the prevalence of 21 commonly reported comorbidities. The most prevalent comorbidities were hypertension (pooled prevalence 34%), metabolic syndrome (29%), obesity (27%), hyperlipidaemia (24%) and any cardiovascular diseases (19%). Eleven studies consistently showed higher prevalence of comorbidities in PsA than controls. Five studies showed that comorbid patients had more severe disease, poorer quality of life, and increased discontinuation of treatment. Comorbidities, particularly cardiometabolic disorders, were highly prevalent in PsA and more common than in healthy controls. Comorbidities were associated with adverse disease features, but more research is needed on their impact on longitudinal outcomes such as treatment response, work productivity and mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriatic arthritis (PsA) is a highly heterogeneous disease with numerous articular phenotypes and extra-articular disease features [1]. In addition, PsA patients commonly present with other coexisting medical conditions—comorbidities—or develop them after diagnosis. Comorbidities may be due to shared risk factors, consequences of reduced physical function and activity, chronic systemic inflammation and its treatment, or simply by chance. Studies in other chronic rheumatic diseases have shown that comorbidities are highly prevalent, and comorbidity burden is associated with poorer outcomes, such as quality of life, function and treatment response [2, 3]. They are also important considerations in routine clinical practice, by influencing treatment decisions (e.g. as contraindications). Others, such as cardiovascular diseases, are key drivers of mortality [4]. Despite the importance of comorbidities for clinical practice, a comprehensive approach to the study of comorbidities in PsA is lacking.
The aims of this systematic review and meta-analysis were to: (1) describe the prevalence of commonly reported comorbidities in PsA, (2) compare the incidence and/or prevalence of comorbidities between PsA and control populations; and (3) examine the impact of comorbidities on PsA outcomes.
Methods
This systematic review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [5]. The protocol for this review was pre-registered in advance (PROSPERO: CRD42020191047). We searched Medline, PubMed, Scopus, and Web of Science from inception to 24th of May 2020, using the following search term: psoriatic arthritis [MeSH] AND (multimorbid* OR comorbid* OR polymorbid* OR multi-morbid* OR co-morbid* OR poly-morbid* OR comorbidity [MeSH]).
Studies of PsA were included if they reported the prevalence or incidence of comorbidities or their impact on disease outcomes. Published abstracts were considered, but only if there was a sufficiently detailed description of study methodology and results. Studies were excluded if they focused on only one comorbidity of interest, or closely related diseases from one organ system (e.g. cardiovascular diseases only). This gives individual comorbidities context among other conditions, and distinguishes studies of comorbidity from, for example, cardiovascular risk. Furthermore, studies with risk of being non-representative of general PsA populations were excluded (e.g. males only or sample sizes < 30). Reviews, comments, and editorials were excluded. We also manually searched the bibliographies of all included papers to identify further eligible studies. Unpublished literature was not considered.
Titles and abstracts were screened by two independent reviewers (ZS and SG), who then assessed full-texts for inclusion and performed data extraction from eligible studies. Conflict at any stage was resolved through discussion moderated by a third reviewer (SSZ). We excluded psoriatic disease manifestations (skin involvement, enthesitis, dactylitis, and nail disease) and patho-mechanistically include conditions (uveitis and IBD) and inflammatory arthritides (given potential for misclassification/diagnosis) from our definition of comorbidities. Studies were assessed for risk of bias using adapted versions of the Newcastle Ottawa Scale (details in supplementary materials).
Where results for any comorbidity (in any of the three study aims) were reported by ≥ 3 studies, a meta-analysis was performed. Pooled prevalence estimates were reported as percentages (95% confidence interval, I2 statistic), using random-effects models (DerSimonian–Laird). Heterogeneity of meta-analysis estimates was presented using the I2 statistic. Funnel plots were used to assess risk of publication bias. Meta-analyses were performed using MetaXL Version 5.3 (Sunrise Beach, Australia).
Results
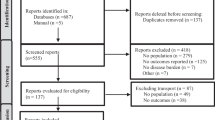
A total of 3817 publications were returned by the literature search. After exclusions and de-duplication—shown in the Fig. 1 flowchart—39 studies remained. These studies are summarised in Supplementary Table S1. Sample sizes ranged from 32 to 35,061 with the total of 158,797 PsA patients. The selected studies comprised of 10 from the USA, 7 from the UK, 6 from Spain, 4 from Italy, 3 from Canada, 2 each from Denmark, Turkey, and France, and 1 each from Belgium, Brazil, Russia, the Netherlands, Romania, Israel, Taiwan and Hong Kong. Two studies recruited participants from multiple countries [6, 7].
PsA definition varied between studies, including CASPAR criteria (n = 11), Moll and Wright criteria (n = 2), self-report (n = 1), American College of Rheumatology definition (n = 1) or by physician diagnosis either from diagnostic codes (n = 15) or medical records (n = 4). Five studies did not report PsA definition. Comorbidities were defined by self-report confirmed by healthcare professional (n = 1), physician diagnosis either from diagnostic codes (n = 16) or medical records (n = 14); ascertainment was unclear in 8 publications. Most bias scores were 4 of a potential 6 stars (Supplementary Table S2 and Figure S1) indicating moderate bias. All studies did not justify their sample size thereby losing one star.
Prevalence of comorbidities
A total of 39 studies reported prevalence of individual comorbidities with a combined sample size of 150,677 patients. The most frequently studied individual comorbidities were diabetes (n = 32 studies), hypertension (31) and hyperlipidaemia (18); all other were reported by 15 or fewer studies.
Pooled prevalence estimates of individual comorbidities (reported by ≥ 3 studies) are summarised in Fig. 2 with further details in Table 1. The top five most prevalent comorbidities were hypertension (34.2%), metabolic syndrome (28.8%), obesity (27.4%), hyperlipidaemia (24.2%), and any CVD (19.4%). There was significant heterogeneity for most meta-analyses; stratification by PsA definition or comorbidity ascertainment did not improve heterogeneity (data not shown). Forest and funnel plots of the 21 meta-analyses are provided in supplementary materials.
Comorbidities in PsA compared to controls
Eleven studies compared comorbidities between PsA and control groups: one compared incidence, while the remaining reported prevalence (odds ratios, prevalence ratios, standardised mortality ratios). Most comorbidities were cardiometabolic disease or risk factors. All except three studies matched or adjusted for potential confounders such as age and sex. Virtually all individual comorbidities had higher incidence and prevalence in PsA populations than matched controls. The comorbidities and effect estimates were too heterogenous to permit meta-analysis.
Kaine et al. reported a 20–30% higher incidence of cardiovascular comorbidities (e.g. coronary, peripheral, or cerebrovascular diseases) in PsA vs matched controls, 44–67% higher incidence of mental health comorbidities, and over two-fold higher incidence of liver disease [8]. Prevalence of cardiovascular and mental health comorbidities was also consistently higher than controls. Effect estimates adjusted for confounders were smaller and less often significant; therefore, the three studies reporting unadjusted odds ratios had limited interpretation [9,10,11] (Table 2).
Comorbidities and PsA outcomes
Five studies reported the impact of comorbidities on PsA disease outcomes (Table 3). In most studies, PsA patients with comorbidity had greater pain, functional limitation, and poorer quality of life than those without. Bavière et al. reported that the mental component score (MCS) of the Short Form 36 (SF-36) was significantly associated with the number of comorbidities [19]. For individual comorbidities, only anxiety was associated with MCS, while none were associated with the physical component score (PCS). Husted et al. found that only anxiety, depression and fibromyalgia were significantly associated with MCS [20], and only fibromyalgia and neurological disorders were associated with PCS. Only one paper reported the impact of comorbidities on treatment response. Stober et al. found that metabolic syndrome-related comorbidities were significantly associated with TNFi discontinuation (HR 2.65; p = 0.01) in multivariable models [21].
Discussion
This meta-analysis combining data from over 150 thousand PsA patients showed that comorbidities, particularly cardiometabolic disorders, are highly prevalent, with around 1 in 3 having hypertension, 3 in 10 having metabolic syndrome, and 1 in 4 having obesity. Almost all comorbidities were more common in PsA patients than controls. The presence and number of comorbidities were associated with poorer quality of life, function, and discontinuation of TNF inhibitors.
Our results showed a clear predilection for cardiometabolic comorbidities in PsA. The top five most prevalent comorbidities—hypertension (34%), metabolic syndrome (29%), obesity (27%), hyperlipidaemia (24%) and overall cardiovascular diseases (CVD; 19%)—provide an interesting comparison against comorbidity patterns in axSpA (another member of the SpA family). In a similar review of comorbidities in axSpA [24], the commonest comorbidities were similar but differed in prevalence (hypertension (23%), infections (18%), hyperlipidaemia (17%), obesity (14%) and CVD (12%)). For example, obesity was nearly twice more common in PsA than axSpA. Although patho-mechanistically related, there are clearly different disease mechanisms at play in these two SpA phenotypes that drive comorbidity patterns. Higher prevalence of cardiometabolic disorders may partly be explained by the greater systemic inflammatory burden in peripheral joint involvement, but also the unique pathology in skin disease. Chronic psoriasis generates vascular endothelial growth factor (VEGF) and oxidative stress that directly contribute to cardiometabolic derangements [25]. Together, these findings highlight the need for better CVD risk assessment and stratification in PsA and other chronic rheumatic diseases, to prevent or reduce cardiovascular morbidity and mortality. Interestingly, despite differences in cardiometabolic risk factors, the prevalence of cardiovascular diseases was similar between PsA and axSpA: any CVD 19 vs. 12%; angina 3.6 vs. 3.6%; myocardial infarction 3.2 vs. 2.2%; heart failure 1.3 vs. 1.8%; stroke 2.8 vs. 1.8%; peripheral vascular disease 1.6 vs. 1.1%, respectively [24].
This review also showed high prevalence of pulmonary diseases and depression (each 12%), both of which are more common than in controls. Depression is a well-recognised comorbidity in psoriatic diseases that is likely underdiagnosed. Prevalence in this review is lower than reported elsewhere when using screening tools [26]. Improved screening and optimisation of mental health comorbidities are essential as they can influence patients’ experience of symptoms and treatment adherence [26]. The links between PsA and pulmonary comorbidities are receiving increasing research focus. Several observational studies have suggested a causal link between metabolic syndrome and lung disease, for example, through obesity hypoventilation and obstructive sleep apnoea [27]. Equally, they may both arise from shared risk factors such as smoking, which is causally associated with chronic obstructive pulmonary disease and PsA [28, 29].
The number of studies that assessed the impact of comorbidities on PsA outcomes was relatively scarce. Most showed cross-sectional associations between comorbidity burden and reduced quality of life, particularly the MCS (mental component score) of the SF-36. We found no studies of whether comorbidities influenced treatment response or longer-term outcomes such as work productivity or mortality. These represent urgent unmet research needs to quantify the individual and societal impact of comorbidities in PsA.
A strength of this review is the broad inclusion of comorbidities. This approach provides wider context for the epidemiology and impact of individual diseases. However, comorbidity ascertainment may well be different in PsA than controls. People with chronic diseases are likely to have more frequent contact with healthcare providers thus opportunities to screen for and/or diagnose comorbidities. For example, hypothyroidism was more prevalent in PsA than controls in two studies—there is limited biological rationale for why there should be higher prevalence in PsA. This may explain the greater comorbidity burden across almost all comorbid conditions. However, there is also opposing evidence that suggest poorer identification and management of comorbidities in people with rheumatic diseases than without [30]. Prevalence estimates may be influenced by the fact that we did not include studies reporting individual comorbidities (e.g. depression in PsA). This was decided a priori for three reasons. First, such studies typically use more sensitive methods of ascertainment (e.g. screening questionnaires) thus may bias true estimates and introduce additional heterogeneity. Second, our objective was to review how common comorbidities were studied collectively. Focusing on depression without considering, for example, the co-existence of fibromyalgia or heart disease would be contrary to the aim of comorbidities research set to inform a holistic management approach. Third, systematically reviewing and discussing each individual condition is beyond the scope of one standalone paper. Another limitation was the high heterogeneity in meta-analysis estimates. This partly reflects the diverse case definition for PsA and comorbidities (though stratifying results did not improve heterogeneity), but also differences in study population, disease duration and treatment. Most included studies were cross-sectional in design; therefore, we were not able examine chronology of comorbidity development, though the study by Kristensen et al. suggested prevalence to be similar before and after diagnosis of PsA [9]. Future studies should investigate whether treatments contribute to (e.g. NSAIDs on CVD) or prevent (e.g. pain control and improvement in mental health) comorbidities development in PsA.
In conclusion, this systematic review showed that comorbidities are highly prevalent among patients with PsA, particularly cardiometabolic, but also mental health and pulmonary diseases. Comorbidities were more common in PsA patients than controls, and were associated with poorer quality of life, function, and discontinuation of TNF inhibitors. Research on the impact of comorbidities on longitudinal outcomes is needed, including treatment response, work productivity and mortality.
References
Bilal J, Malik SU, Riaz IB, Kurtzman DJB (2018) Psoriasis and psoriatic spectrum disease: a primer for the primary care physician. Am J Med 131:1146–1154
Dougados M (2016) Comorbidities in rheumatoid arthritis. Curr Opin Rheumatol 28:282–288
Moltó A, Nikiphorou E (2018) Comorbidities in spondyloarthritis. Front Med 12(5):62
Pincus T, Gibson KA, Block JA (2015) Premature mortality: a neglected outcome in rheumatic diseases? Arthritis Care Res 67:1043–1046
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012
Kavanaugh A, Papp K, Gottlieb AB, de Jong EMGJ, Chakravarty SD, Kafka S et al (2018) Demography, baseline disease characteristics, and treatment history of psoriasis patients with self-reported psoriatic arthritis enrolled in the PSOLAR registry. BMC Rheumatol 2:29
Favarato MH, Mease P, Gonçalves CR, Saad CG, Sampaio-Barros PD, Goldenstein-Schainberg C (2014) Hypertension and diabetes significantly enhance the risk of cardiovascular disease in patients with psoriatic arthritis. Clin Exp Rheumatol 32(2):182–187
Kaine J, Song X, Kim G, Hur P, Palmer JB (2019) Higher incidence rates of comorbidities in patients with psoriatic arthritis compared with the general population using U.S. administrative claims data. J Manag Care Spec Pharm 25:122–132
Kristensen LE, Jørgensen TS, Christensen R, Gudbergsen H, Dreyer L, Ballegaard C et al (2017) Societal costs and patients’ experience of health inequities before and after diagnosis of psoriatic arthritis: a Danish cohort study. Ann Rheum Dis 76:1495–1501
Merola JF, Han S, Xie J, Song H, Herrera V, Wei J et al (2015) Comorbidity burden and medication use among patients with psoriatic arthritis in the US. Arthritis Rheumatol 67(Suppl):10
Zhang F, Guerin A, Gauthier G, Day R, Khan Z (2011) Prevalence of autoimmune diseases and other comorbidities in patients with psoriatic arthritis in the United States. ACRARHP Sci Meet. AB1333
Cook MJ, Bellou E, Bowes J, Sergeant JC, O’Neill TW, Barton A et al (2018) The prevalence of co-morbidities and their impact on physical activity in people with inflammatory rheumatic diseases compared with the general population: results from the UK Biobank. Rheumatology 57:2172–2182
Haddad A, Ashkenazi RI, Bitterman H, Feldhamer I, Greenberg-Dotan S, Lavi I et al (2017) Endocrine comorbidities in patients with psoriatic arthritis: a population-based case-controlled study. J Rheumatol 44:786–790
Feldman SR, Zhao Y, Shi L, Tran MH, Lu J (2015) Economic and comorbidity burden among moderate-to-severe psoriasis patients with comorbid psoriatic arthritis: burden of psoriasis patients with comorbid psoriatic arthritis. Arthritis Care Res 67:708–717
Han C, Robinson DW, Hackett MV, Paramore LC, Fraeman KH, Bala MV (2006) Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 33:2167–2172
Gladman DD, Ang M, Su L, Tom BDM, Schentag CT, Farewell VT (2009) Cardiovascular morbidity in psoriatic arthritis. Ann Rheum Dis 68:1131–1135
Jafri K, Bartels CM, Shin D, Gelfand JM, Ogdie A (2017) Incidence and management of cardiovascular risk factors in psoriatic arthritis and rheumatoid arthritis: a population-based study: cardiovascular risk factors in PsA and RA. Arthritis Care Res 69:51–57
Tam L-S, Tomlinson B, Chu TT-W, Li M, Leung Y-Y, Kwok L-W et al (2008) Cardiovascular risk profile of patients with psoriatic arthritis compared to controls—the role of inflammation. Rheumatology 47:718–723
Bavière W, Deprez X, Houvenagel E, Philippe P, Deken V, Flipo R-M et al (2020) Association between comorbidities and quality of life in psoriatic arthritis: results from a multicentric cross-sectional study. J Rheumatol 47:369–376
Husted JA, Thavaneswaran A, Chandran V, Gladman DD (2013) Incremental effects of comorbidity on quality of life in patients with psoriatic arthritis. J Rheumatol 40:1349–1356
Stober C, Ye W, Guruparan T, Htut E, Clunie G, Jadon D (2018) Prevalence and predictors of tumour necrosis factor inhibitor persistence in psoriatic arthritis. Rheumatology 57:158–163
Aydin S, Bayindir M, Oksuz A, Dogru G, Kimyon E et al (2016) FRI0476 comorbidities in psoriatic arthritis: patient education counts. Ann Rheum Dis 75(Suppl 2):610–611
Fernández-Carballido C, Martín-Martínez MA, García-Gómez C, Castañeda S, González-Juanatey C, Sánchez-Alonso F et al (2020) Impact of comorbidity on physical function in patients with ankylosing spondylitis and psoriatic arthritis attending rheumatology clinics: results from a cross-sectional study. Arthritis Care Res 72:822–828
Zhao SS, Robertson S, Reich T, Harrison N, Moots RJ, Goodson NJ (2020) Prevalence and impact of comorbidities in axial spondyloarthritis: systematic review and meta-analysis. Rheumatology 59:iv47–iv57
Johnsson H, McInnes IB, Sattar N (2012) Cardiovascular and metabolic risks in psoriasis and psoriatic arthritis: pragmatic clinical management based on available evidence. Ann Rheum Dis 71:480–483
Zhao SS, Miller N, Harrison N, Duffield SJ, Dey M, Goodson NJ (2020) Systematic review of mental health comorbidities in psoriatic arthritis. Clin Rheumatol 39:217–225
Baffi CW, Wood L, Winnica D, Strollo PJ, Gladwin MT, Que LG et al (2016) Metabolic syndrome and the lung. Chest 149:1525–1534
Li X, Kong L, Li F, Chen C, Xu R, Wang H et al (2015) Association between psoriasis and chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLoS ONE 10(12):e0145221
Nguyen UDT, Zhang Y, Lu N, Louie-Gao Q, Niu J, Ogdie A et al (2018) Smoking paradox in the development of psoriatic arthritis among patients with psoriasis: a population-based study. Ann Rheum Dis 77:119–123
Baillet A, Gossec L, Carmona L, de Wit M, van Eijk-Hustings Y, Bertheussen H et al (2016) Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: a EULAR initiative. Ann Rheum Dis 75:965–973
Author information
Authors and Affiliations
Contributions
SSZ planned the review and wrote the first draft. SG and ZS performed the review, take responsibility for the data, and made substantial contributions to the manuscript. DMH performed the statistical analysis. All the authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest or funding to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gupta, S., Syrimi, Z., Hughes, D.M. et al. Comorbidities in psoriatic arthritis: a systematic review and meta-analysis. Rheumatol Int 41, 275–284 (2021). https://doi.org/10.1007/s00296-020-04775-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-020-04775-2