Abstract
Introduction
Community-acquired pneumonia (CAP) and hospital-acquired pneumonia (HAP) are common complications in idiopathic inflammatory myopathy (IIM) patients, and are frequently associated with unfavorable outcome as well as prolonged antibiotic therapy. In this study, we intended to clarify whether clinical pulmonary infection score (CPIS) and multiple serum biomarkers are valuable in predicting unfavorable outcomes and prolonged antibiotic therapy in adult IIM patients complicated with CAP or HAP.
Methods
Data of IIM patients with CAP or HAP who were admitted to three tertiary centers from December 2010 to November 2019 were retrospectively collected. Cox proportional hazards regression analysis and logistic regression analysis were adopted to identify risk factors for unfavorable outcomes and prolonged antibiotic therapy in these patients. The predictive values of potential predictors were assessed using receiver operating characteristic analysis.
Results
The mortality rate was 60.6% in 109 IIM patients complicated with CAP or HAP. Myositis Disease Activity Assessment Visual Analogue Scales (MYOACT) score, CPIS and timely adjustment to antibiotics based on drug susceptibility test (DST-based antibiotic) were significantly associated with long-term outcome in these patients. With an optimal cutoff value of 6.5 and area under the curve (AUC) of 0.813, CPIS was a more satisfying predictor compared with MYOACT score. The peak C-reactive protein (CRP) level, DST-based antibiotics, and complication of interstitial lung disease (ILD) were also significantly correlated with prolonged antibiotic therapy.
Conclusions
IIM patients complicated with CAP or HAP frequently suffer from unfavorable outcomes. Compared with IIM disease activity, CPIS worked as a better predictor of outcome in these patients. Also, the peak CRP level during hospitalization might be valuable in predicting prolonged antibiotic therapy. The existence of ILD might impede early discontinuation of antibiotics. Timely adjustment to antibiotics based on drug susceptibility testing would decrease the mortality rate and reduce the incidence of prolonged antibiotic therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Community-acquired pneumonia (CAP) and hospital-acquired pneumonia (HAP) are common complications in idiopathic inflammatory myopathy (IIM) patients, and are frequently associated with unfavorable outcomes as well as prolonged antibiotic therapy. |
We intended to clarify whether clinical pulmonary infection score (CPIS) and multiple serum biomarkers were valuable in predicting unfavorable outcome and prolonged antibiotic therapy in adult IIM patients complicated with CAP or HAP. |
What was learned from the study? |
Compared with IIM disease activity, CPIS worked as a better predictor of outcome in these patients, and peak C-reactive protein level during hospitalization might be valuable in predicting prolonged antibiotic therapy. |
Timely adjustment to antibiotics based on drug susceptibility testing would decrease the mortality rate and reduce the incidence of prolonged antibiotic therapy. |
Existence of ILD might impede early discontinuation of antibiotics. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13322414.
Introduction
Idiopathic inflammatory myopathy (IIM) is a group of rare autoimmune diseases characterized primarily by skeletal muscle inflammation and muscle weakness. The diseases commonly present with extramuscular involvements such as skin rash, polyarthritis, interstitial lung disease (ILD), carcinoma, etc. [1, 2]. Dermatomyositis (DM), polymyositis (PM), and the newly recognized amyopathic dermatomyositis (ADM) are common subtypes of IIM in clinical practice [2, 3]. IIM has considerable impact on patients’ well-being and quality of life [4], and the survival of IIM patients is far from optimistic. In different studies, the 10-year survival rate for patients with DM, PM, or ADM ranged from 51 to 91% [5], and the in-hospital mortality rate was approximately 4.5% [5, 6].
Due to immune dysregulation, long-term immunosuppressant treatment, and comorbid illness, patients with connective tissue disease (CTD) suffered from greater burdens of infection [7], and infection was found to be an important risk factor for mortality in hospitalized IIM patients [5, 6, 8]. Preceding studies on IIM and infection mostly focused on prevalence of infection, risk factors for complication of infection, infectious triggers of IIM, etc. The management of infection in IIM patients and predictors of the outcome of IIM patients with infection are therefore meaningful but remain unclear. In addition, an antibiotic regimen is critical in patients complicated with infection. Inappropriate and prolonged antibiotic regimens would induce drug-resistant strains, increase medical costs, and prolong hospitalization [9]. It is thus valuable to probe into risk factors and predicting tools for unfavorable outcomes of IIM patients with infection, as well as prolonged antibiotic therapy in these patients.
In clinical practice, pulmonary infection is one of the most common subtypes of infection in IIM patients. Clinical pulmonary infection score (CPIS) along with serum biomarkers like procalcitonin (PCT) have been used to predict the outcome or guide discontinuation of antibiotics in ventilator-associated pneumonia, etc. [10,11,12], and they have been routinely utilized in our center to evaluate the severity of pulmonary infection. However, their predictive values in pulmonary infection among CTD patients has not been systematically explored.
To benefit future management of IIM patients with pulmonary infection, we sought to clarify the association of prognostic indices including CPIS, serum PCT, etc., and other clinical factors with outcome in these patients. Also, we intended to determine the risk factors for prolonged antibiotic therapy in the setting of pulmonary bacterial infection (PBI) in IIM cases.
Methods
Patients
After getting approval (Reference Number: 2020-104) from the Institutional Review Board of the First Affiliated Hospital, Zhejiang University School of Medicine (FAHZJU) and acquiring written informed consent (for participation and publication) from all patients involved, in accordance with the Helsinki Declaration of 1975 and its later amendments, we retrieved medical records of adult patients who were hospitalized at the Qingchun, Chengzhan, and Zhijiang divisions of FAHZJU with the diagnosis of DM, PM, or ADM from December 2010 to November 2019. The inclusion criteria of this study were: (1) age over 18 years old; (2) the diagnosis of DM, PM, or ADM that satisfied the 2017 ACR/EULAR classification criteria [13, 14]; (3) international Statistical Classification of Diseases, 10th revision (ICD-10)-coded discharge diagnosis of community-acquired pneumonia (CAP), hospital-acquired pneumonia (HAP), pulmonary fungal infection, and pulmonary infection. Exclusion criteria were: (1) overlap syndromes with other CTDs; (2) hospitalization for reasons unrelated to myositis and its complications, such as fracture, pregnancy, acquired immunodeficiency syndrome, and cataract, etc.; (3) Candida pneumonia without pathogen detection through bronchoscope-acquired specimen; (4) loss to follow-up without an outcome event within 3 months after hospitalization.
Methods
Medical records of all enrolled patients were retrospectively collected by reviewing the Electronic Medical Record (EMR) system of FAHZJU. Data including demographic information, course of disease, duration of diagnosis delay, disease activity assessment, CPIS, clinical manifestations or complications, preceding comorbidities, harmful hobbies, imaging reports, laboratory findings, immunosuppressive, antibacterial, or antifungal medications as well as outcome were acquired and analyzed. To obtain the survival of patients involved, patients were followed from hospitalization until the end of follow-up. For patients who died during hospitalization, their dates of death were clearly documented in the EMR system. For patients who were discharged, a routine return visit was arranged 2 weeks after discharge. In addition to the inpatient or outpatient visits, a concise telephone interview was performed 3 months after discharge, and at an annual frequency afterwards. The end of follow-up could be owing to death from any cause, loss to follow-up, or closure of follow-up for the purpose of this study (February 29, 2020).
On-admission disease activity was routinely assessed by the Myositis Disease Activity Assessment Visual Analogue Scales (MYOACT) within the first week of hospitalization [15]. A simplified version of CPIS was assessed for each patient after diagnosis of pulmonary infection [10, 16]. ILD and rapid progression of ILD (RP-ILD) were evaluated by experienced radiologists using high-resolution computed tomography (HRCT). A subset of patients with RP-ILD was defined as those presenting with progressive dyspnea and progressive hypoxemia or a worsening of interstitial change on the chest radiograph within 1 month after the initial visit or onset of respiratory symptoms [17,18,19,20,21]. Patients included can be subcategorized as CAP and HAP [22, 23]. HAP cases that developed in an intensive care unit (ICU) and had been mechanically ventilated for at least 48 h can also be categorized as ventilator-associated pneumonia (VAP) [24, 25]. To identify the pathogen responsible for CAP or HAP, repeated cultures of bronchoalveolar lavage fluid (BALF) or sputum were routinely adopted before intravenous use of antibiotics or anti-fungal medications. Sputum specimens counted only if > 25 squamous epithelial cells per low-power field were observed [26]. In the detection of bacterial infection, the thresholds for positivity of quantitative cultures were defined as 105 CFU/ml for sputum culture [26] and 104 CFU/ml for bronchoalveolar lavage respectively [27]. Positive findings of Pneumocystis carinii, Cryptococcus, and Aspergillus fumigatus in BALF without culture counted as well. To rule out the interference of upper respiratory tract implantation of Candida, Candida pneumonia cases with mere sputum cultures were excluded from this study.
Regarding antibacterial or antifungal therapies, timely adjustment to antibiotics based on drug susceptibility testing (DST-based antibiotics), application of third-line antibiotics, and intravenous antifungal medications were regarded as active and potent therapeutic regimens for pulmonary infection [28,29,30]. To be specific, third-line antibiotics referred to carbapenem, vancomycin, and linezolid [30]. Prophylactic application of sulfamethoxazole (SMZ) was taken as a preventive therapy for fungal infection during medications of steroids or other immunosuppressants. In patients with PBI, use of intravenous antibiotics would be stopped with the remission of symptoms, radiological manifestations, laboratory findings, etc., or optimized and prolonged with non-remission or aggravation. Early discontinuation of intravenous antibiotics was defined as 5–8 days use of intravenous antibiotics [31,32,33,34], and an over 8-day intravenous regimen can be viewed as prolonged antibiotic therapy.
Comparisons were made between patients with CAP and patients with HAP to clarify differences in clinical symptoms, complications, laboratory findings, radiological manifestations, etc. Survival of patients with CAP or HAP, or patients with different levels of CPIS was as well probed into. Cox proportional hazards regression analysis was performed to identify factors exerting significant influence on outcome of these patients. Logistic regression analysis was used to identify potential predicting factors for prolonged antibiotic therapy in IIM patients with PBI. The predictive values of potential predictors, serum biomarkers, and scoring systems included were assessed using receiver operating characteristic (ROC) curve analysis.
Statistical Analysis
Statistical analyses were performed using SPSS 22.0 (Chicago, IL, USA) and R 3.6.1. The normality of continuous variables was tested by the Kolmogorov–Smirnov goodness-of-fit model. Continuous variables were expressed as mean ± SD if normally distributed and median (quartiles) if skewed. Ordinal categorical variables were as well shown as median (quartiles). Unordered categorical variables were presented as numbers and percentages. In comparison between CAP patients and HAP patients, independent sample t test was used to compare normally distributed continuous variables. And Mann–Whitney U test was applied to compare skewed continuous variables or ordinal categorical variables. Chi-square test and Fisher’s exact test were adopted to compare unordered categorical variables. Survival in groups with CAP or HAP, and groups with different levels of CPIS was estimated using the Kaplan–Meier method, and differences between groups were compared using the log-rank test. Univariate and multivariate Cox proportional hazards regression analyses were used to identify factors significantly associated with the risk of death. Explanatory factors with P value < 0 0.05 in the univariate Cox proportional hazards regression analysis were entered into the multivariate analysis. Then univariate and multivariate logistic regression analyses were subsequently adopted to unveil risk factors for prolonged antibiotic therapy in IIM patients with CAP or HAP. Explanatory factors with P value < 0 0.1 in the univariate logistic regression analysis would be entered into the multivariate logistic regression analysis. P values in comparisons and univariate analyses were adjusted by false discovery rate (FDR) correction, using p.adjust function in R.3.6.1, to obtain adjusted P values (P adjusted). All tests were two-sided, and a P value < 0.05 was considered statistically significant. If there existed any positive result in serum biomarkers, MYOACT score or CPIS in multivariate analyses, a ROC curve analysis would be performed to evaluate its predictive value for outcome or prolonged antibiotic therapy.
Results
A total of 109 patients (out of 455 IIM patients) who were admitted to three divisions of FAHZJU with a diagnosis of DM, PM, or ADM between December 2010 and November 2019 satisfied the inclusion and exclusion criteria of this study and were hereby included, encompassing 63 patients complicated with CAP and 46 patients with HAP. The mean age of hospitalization was 57.5 ± 12.2 years—48 of them were males and 61 were females. In comparison to PM (35, 32.1%) and ADM (13, 11.9%), DM (61, 56.0%) was the most common IIM subtype in this study. Ninety-four patients were found to have PBI, eight patients were found to have pulmonary fungal infection, and seven suffered from both bacterial and fungal infection (Supplementary table S1). During hospitalization, all patients with pulmonary bacterial or fungal infection received timely intravenous antibiotics or antifungal medications correspondingly. In 101 patients with PBI, up to 62 of them (61.4%) received prolonged antibiotic therapy (> 8 days). The medium duration of follow-up was 5.0 months (range, 0.17–109.20 months), and the mortality rate of these patients in follow-up was 60.6%.
The mortality rate in 46 HAP patients (19 VAP patients included) was, nevertheless, significantly higher than those in CAP (73.9 vs. 50.8%, P = 0.015), and the duration of antibiotic therapy was as well longer in HAP patients (P = 0.020). A comparison was hereby performed within the two subgroups. Compared with CAP patients, patients in the HAP group have higher MYOACT scores (P = 0.026), CPIS (P < 0.001), peak C-reactive protein (CRP, P = 0.002), and peak PCT (P = 0.001) level during hospitalization, indicating a higher disease activity of IIM and a more severe complication of infection in HAP patients. In addition, CPIS (P-adjusted < 0.001), peak CRP (P-adjusted = 0.031), and peak PCT (P-adjusted = 0.020) remained significantly different after FDR correction (Supplementary table S2).
The 109 patients were categorized into three groups based on their CPIS: 2–4 (n = 25), 5–7 (n = 60), and 8–10 (n = 24). In Kaplan–Meier analysis, the log-rank test showed that there were significant differences in survival between the CPIS: 2–4 and the CPIS: 8–10 groups (P < 0.001), between the CPIS: 5–7 and the CPIS: 8–10 groups (P = 0.002), as well as a less significant difference between the CPIS: 2–4 and the CPIS: 5–7 groups (P = 0.065). The Kaplan–Meier curve for survival of the three groups of patients is shown in Fig. 1. Besides, the 109 patients were as well divided into the CAP group (n = 63) and the HAP group (n = 46). In Kaplan–Meier analysis, the log-rank test showed that there was significant difference (P = 0.013) in survival between the CAP and the HAP groups (Fig. 2).
Based on the records of long-term follow-up, the 109 patients were divided into the mortality group (n = 66) and the survival group (n = 43). The univariate Cox proportional hazards regression analysis revealed that MYOACT score (P = 0.002), CPIS (P < 0.001), peak CRP level (P = 0.003), application of the DST-based antibiotics (P < 0.001) and the third-line antibiotics (P = 0.001) were associated with the long-term outcome at the level of P < 0.05. After FDR correction, the significance of MYOACT score (P-adjusted = 0.025), CPIS (P-adjusted < 0.001), peak CRP level (P-adjusted = 0.032), application of the DST-based antibiotics (P-adjusted < 0.001) and the third-line antibiotics (P-adjusted = 0.016) remained. The following multivariate analysis clarified that MYOACT score (P = 0.005), CPIS (P < 0.001), and the DST-based antibiotics (P = 0.004) were significantly correlated with the long-term outcome (Table 1), and the subgroup analyses (using Kaplan–Meier method) verified the significant correlations between CPIS and outcome in CAP (P < 0.001) and HAP (P = 0.004) patients. As presented in Fig. 3, the optimal cut-off value of the MYOACT score for predicting unfavorable outcome was greater than 10.5, with a sensitivity of 45.5% and a specificity of 83.7%. The area under the curve (AUC) was 0.686. Meanwhile the optimal cut-off value of the CPIS was greater than 6.5, with a sensitivity of 62.1% and a specificity of 90.7%. The comparatively larger AUC of CPIS was 0.813.
Receiver operating characteristic curve of MYOACT score and CPIS for unfavorable outcome in IIM patients with CAP or HAP. MYOACT Myositis Disease Activity Assessment Visual Analogue Scales, CPIS clinical pulmonary infection score, IIM idiopathic inflammatory myopathies, CAP community-acquired pneumonia, HAP hospital-acquired pneumonia
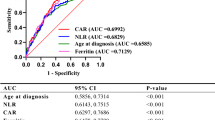
According to the duration of antibiotic therapy, the 101 patients with PBI were as well divided into two groups: ≤ 8 days (n = 39) and > 8 days (n = 62). Univariate logistic regression analysis showed that there were ten factors related to prolonged antibiotic therapy in patients with PBI at the level of P < 0.1. These factors included CPIS (P = 0.010), muscle weakness (P = 0.091), complication of ILD (P = 0.078) and RP-ILD (P = 0.023), peak CRP (P = 0.004), ferritin (P = 0.086) and PCT level (P = 0.031), anti-Jo-1 antibody (P = 0.086), application of the DST-based antibiotics (P = 0.002) and the third-line antibiotics (P = 0.009). However, the significance vanished after FDR correction. The following multivariate logistic regression analysis demonstrated that peak CRP level (P = 0.007), DST-based antibiotics (P = 0.002), and complication of ILD (P = 0.004) were significantly correlated with prolonged antibiotic therapy (> 8 days) (Table 2). As presented in Fig. 4, the best cut-off value of peak CRP level for prolonged antibiotic therapy in patients with PBI was greater than 23.4 mg/l, with a sensitivity of 72.6% and a specificity of 61.5%. The AUC was 0.710.
Discussion
In this observational, retrospective, longitudinal cohort study, risk factors for unfavorable outcome and prolonged antibiotic therapy in IIM patients complicated with pulmonary infection were probed. To date, this is the first study to introduce CPIS into evaluation of CAP or HAP in patients with connective tissue disease, and discuss the strategies of antibiotic therapy in these patients. In follow-up, the mortality rate of IIM patients with pulmonary infection was 60.6%, which was much higher than that in IIM patients in preceding studies [5]. In particular, HAP patients seem to have more unfavorable outcomes than CAP patients, possibly due to the higher IIM disease activity and complication of more severe pulmonary infection. In the common population, the incidence of CAP ranged from 29.6 to 248 cases per 10,000 adults among different countries [35], and the incidence of HAP was approximately 21 cases per 1000 hospital admissions [36]. In this study, incidences of CAP (13.8%) and HAP (10.1%) in IIM patients were much higher than those in the common population, even taking the bias of hospitalization into consideration, which might be due to the immune dysregulation, immunosuppressant therapy, and comorbid illness.
MYOACT score was identified as a predictor for outcome of IIM patients complicated with pulmonary infection. Traditionally, various disease-activity assessing tools including the MYOACT score, multiple muscle strength assessments, and biomarkers like creatine kinase have been used to assess the disease activity and predict the short-term or long-term outcome of IIM patients [37]. In light of the heterogeneity of muscular involvement of different IIM subtypes and extramuscular organ involvement, the subjective (but more comprehensive) MYOACT score can work as a better tool compared with assessments focusing on muscular damage [38]. In addition to disease activity, multiple studies also identified infection (pulmonary infection in particular) as a risk factor for unfavorable outcome in IIM patients [5, 6, 8]. It might be valuable to incorporate assessment of pulmonary infection into the outcome-predicting tools. The CPIS, which was initially developed as a diagnostic tool for VAP, has been proven to be a satisfying predictor for unfavorable outcome in patients with VAP or CAP [10, 12, 39]. Its predictive value, nevertheless, has never been tested in patients with CTD. We hereby incorporated CPIS and several serum biomarkers into this study to figure out efficient predictors for outcome of IIM patients with infection. In this study, the non-invasive and simple CPIS was found to be a better predictor than MYOACT score in terms of outcome, which not only proved its predictive value in the setting of CTD patients receiving immunosuppressant therapy but also demonstrated the vital role of infection and appropriate anti-infectious therapy in these patients. The following subgroup analyses further verified the predictive value of CPIS in CAP and HAP patients, respectively.
There is no denying the fact that under-prescription of antibiotics may lead to deterioration into respiratory failure and sepsis in patients with pulmonary infection [40]. On the other hand, over-prescription of antibiotics may expose patients to the side effects of antibiotics, development of multidrug-resistant organisms (MDROs) as well as increase in recovery time, costs, and workload [39]. The duration of antibiotic therapy was not found to be correlated with outcome of patients with pulmonary infection, which was consistent with the conclusion of published reviews [41, 42]. However, prolonged antibiotic therapy happened in more than 60% of IIM patients suffering from PBI within this study. It is hereby valuable to identify risk factors for prolonged antibiotic therapy and optimize the regimen. Compared with the common population, the high incidence of prolonged antibiotic therapy could be partly due to the immunosuppressive state caused by glucocorticoid, disease-modifying anti-rheumatic drugs, immunoglobulin, etc. Moreover, ILD was also found to be correlated with prolonged antibiotic therapy. This was a frequent complication in IIM patients, and the existence of which might interfere our judgment of response to antibiotics based on clinical symptoms, radiological manifestations, etc., and consequently lead to a prolonged antibiotic regimen.
In addition, several studies proposed controversial use of PCT and CPIS to guide discontinuation of antibiotics in HAP/VAP patients [10, 11]. However, on-admission or peak level of CPIS and serum PCT during hospitalization were not found to be significantly associated with prolonged antibiotic therapy after adjusting for other factors. Meanwhile, elevated peak level of CRP during hospitalization was identified as a potential risk factor for prolonged antibiotic therapy in IIM patients with PBI. Patients with a peak level of CRP over 23.4 mg/l were more likely to receive prolonged antibiotic therapy during hospitalization. Previously, CRP appeared to be useful in the early prediction of VAP, evaluating severity of infection, response to antibiotics, etc. [43,44,45,46]. Our finding would help demonstrate the value of CRP in antibiotic therapy of CTD patients with pulmonary infection. The results, nevertheless, demanded further verification with the insignificance after FDR correction as well as the unsatisfying AUC.
In clinical practice, BALF or sputum culture and drug susceptibility test (DST) were routinely adopted for patients with PBI before initiation of antibiotic therapy. Empirical therapy of antibiotics was initially applied to patients with PBI, and timely adjustment to antibiotic regimen would take place based on a positive result of DST. However, in some patients with negative findings in BALF/sputum culture or infection of MDROs, empirical use of antibiotics would continue. In clinical practice, empirical therapy of antibiotics is recommended in patients with CAP or HAP [47,48,49]. In our study, DST-based antibiotics, nevertheless, were found to improve the outcome of patients, and significantly reduce the occurrence of prolonged antibiotic therapy. Meanwhile, the use of third-line antibiotics was not found to be significantly associated with the outcome and prolonged antibiotic therapy after adjusting for other factors. In IIM patients complicated with pulmonary infection, our study proposed a priority of timely and DST-based adjustment to antibiotic regimen compared with the continued empirical use of antibiotics. The unfavorable outcome of IIM patients with CAP or HAP might be improved if more appropriate, DST-based antibiotic regimens were more often used in these patients. Most CTD patients receive long-term immunosuppressant therapy, which not only leaves them vulnerable to infection but also worsens their response to antibiotic therapy. The consequently prolonged use of empirical antibiotics also increases the incidence of development of MDROs [39]. For the immunosuppressive CTD patients, the antibiotic regimen is a more delicate issue in comparison to that in common population. Repeated screening of responsible pathogens and timely adjustment of antibiotics based on DST might be more effective than empirical regimens and potent third-line antibiotics.
The most significant limitations of this study are the retrospective and observational nature as well as the small sample size. Furthermore, the absence of detection of myositis-associated antibodies and myositis-specific antibodies in over half of the patients also restrained us from figuring out their effect on outcome and prolonged antibiotic therapy in IIM patients complicated with pulmonary infection. Upcoming infectious biomarkers like serum amyloid A (SAA), neutrophils cell surface antigen 64(CD64) [50], heparin-binding protein (HBP), etc., were not included in this study. A large prospective cohort study is essential to confirm our findings and fill in the gaps of this study. In spite of all the limitations, we intended to shed some light on the clinical management and future study of pulmonary infection in IIM patients.
Conclusions
IIM patients were more vulnerable to CAP or HAP and frequently suffered from unfavorable outcomes. Compared with MYOACT score, CPIS could be taken as a better predictor for outcome of IIM patients with pulmonary infection. The incidence of prolonged antibiotic therapy in IIM patients with PBI was considerably high, and peak CRP level during hospitalization might be valuable in predicting prolonged antibiotic therapy. Existence of ILD might impede early discontinuation of antibiotics. The antibiotic regimen in the immunosuppressive IIM patients demands delicate management. Timely adjustment to antibiotics based on drug susceptibility test would decrease the mortality rate and reduce the incidence of prolonged antibiotic therapy.
References
Li L, D’Silva KM, Lu N, et al. Mortality trends in polymyositis and dermatomyositis: a general population-based study. Semin Arthritis Rheum. 2020;50:834–9.
Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EKL. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1–19.
Nelson WW, Philbin MJ, Gallagher JR, Heap K, Carroll S, Wan GJ. A retrospective medical record review of utilization patterns and medical resource use associated with repository corticotropin injection among patients with rheumatologic diseases in the United States. Rheumatol Ther. 2017;4:465–74.
Regardt M, Welin Henriksson E, Alexanderson H, Lundberg IE. Patients with polymyositis or dermatomyositis have reduced grip force and health-related quality of life in comparison with reference values: an observational study. Rheumatology (Oxford). 2011;50:578–85.
Murray SG, Schmajuk G, Trupin L, et al. A Population-based study of infection-related hospital mortality in patients with dermatomyositis/polymyositis. Arthritis Care Res. 2015;67:673–80.
Wu CY, Wang Q, He LR, Yang EH, Zeng XF. Hospitalization mortality and associated risk factors in patients with polymyositis and dermatomyositis: a retrospective case–control study. PLoS ONE. 2018;13:e0192491.
Genovese MC, Greenwald MW, Gutierrez-Urena SR, et al. Two-year safety and effectiveness of peficitinib in moderate-to-severe rheumatoid arthritis: a phase IIb, open-label extension study. Rheumatol Ther. 2019;6:503–20.
Taborda AL, Azevedo P, Isenberg DA. Retrospective analysis of the outcome of patients with idiopathic inflammatory myopathy: a long-term follow-up study. Clin Exp Rheumatol. 2014;32:188–93.
Smith TT, Tamma PD, Do TB, et al. Prolonged linezolid use is associated with the development of linezolid-resistant Enterococcus faecium. Diagn Microbiol Infect Dis. 2018;91:161–3.
Wongsurakiat P, Tulatamakit S. Clinical pulmonary infection score and a spot serum procalcitonin level to guide discontinuation of antibiotics in ventilator-associated pneumonia: a study in a single institution with high prevalence of nonfermentative Gram-negative bacilli infection. Ther Adv Respir Dis. 2018;12:1753466618760134.
Akagi T, Nagata N, Wakamatsu K, et al. Procalcitonin-guided antibiotic discontinuation might shorten the duration of antibiotic treatment without increasing pneumonia recurrence. Am J Med Sci. 2019;358:33–44.
Wang YH, Zhang S, Li L, Xie J. The usefulness of serum procalcitonin, C-reactive protein, soluble triggering receptor expressed on myeloid cells 1 and Clinical Pulmonary Infection Score for evaluation of severity and prognosis of community-acquired pneumonia in elderly patients. Arch Gerontol Geriatr. 2019;80:53–7.
Lundberg IE, Tjarnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017;76:1955–64.
Barsotti S, Dastmalchi M, Notarnicola A, et al. Performance of the new EULAR/ACR classification criteria for idiopathic inflammatory myopathies (IIM) in a large monocentric IIM cohort. Semin Arthritis Rheum. 2020;50:492–7.
Isenberg DA, Allen E, Farewell V, et al. International consensus outcome measures for patients with idiopathic inflammatory myopathies. Development and initial validation of myositis activity and damage indices in patients with adult-onset disease. Rheumatology (Oxford). 2004;43:49–54.
Luna CM, Blanzaco D, Niederman MS, et al. Resolution of ventilator-associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit Care Med. 2003;31:676–82.
Hozumi H, Fujisawa T, Nakashima R, et al. Comprehensive assessment of myositis-specific autoantibodies in polymyositis/dermatomyositis-associated interstitial lung disease. Respir Med. 2016;121:91–9.
Horiike Y, Suzuki Y, Fujisawa T, et al. Successful classification of macrophage-mannose receptor CD206 in severity of anti-MDA5 antibody positive dermatomyositis associated ILD. Rheumatology (Oxford). 2019;58:2143–52.
Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kD polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571–6.
Abe Y, Matsushita M, Tada K, Yamaji K, Takasaki Y, Tamura N. Clinical characteristics and change in the antibody titres of patients with anti-MDA5 antibody-positive inflammatory myositis. Rheumatology (Oxford). 2017;56:1492–7.
Nandy A, Gaini S, Sore P. Rapidly progressive interstitial lung disease in a patient with anti-MDA5-positive amyopathic dermatomyositis. Scand J Rheumatol. 2018;47:334–5.
Trapani D, Bonzi M. GrAm. Antibiotic treatment strategies for community-acquired pneumonia in adults. Intern Emerg Med. 2015;10:861–3.
American Thoracic S, Infectious Diseases Society of A. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J. 2017;50:1700582.
Spalding MC, Cripps MW, Minshall CT. Ventilator-associated pneumonia: new definitions. Crit Care Clin. 2017;33:277–92.
Joyce SM. Sputum analysis and culture. Ann Emerg Med. 1986;15:325–8.
Chastre J, Fagon JY, Bornet-Lecso M, et al. Evaluation of bronchoscopic techniques for the diagnosis of nosocomial pneumonia. Am J Respir Crit Care Med. 1995;152:231–40.
Pulcini C, Tebano G, Mutters NT, et al. Selective reporting of antibiotic susceptibility test results in European countries: an ESCMID cross-sectional survey. Int J Antimicrob Agents. 2017;49:162–6.
Rae N, Kenny C, Muldoon EG. Can intravenous antifungal therapy be safely used in the outpatient parenteral antimicrobial therapy (OPAT) setting? Mycoses. 2019;62:196–203.
Vasudevan A, Mukhopadhyay A, Goh EY, Li J, Tambyah PA. Risk factors for infection/colonization caused by resistant Gram-negative bacilli in critically ill patients (an observational study of 1633 critically ill patients). Prev Med. 2013;57(Suppl):S70–3.
Le Clech L, Talarmin JP, Couturier MA, et al. Early discontinuation of empirical antibacterial therapy in febrile neutropenia: the ANTIBIOSTOP study. Infect Dis (Lond). 2018;50:539–49.
Bougle A, Foucrier A, Dupont H, et al. Impact of the duration of antibiotics on clinical events in patients with Pseudomonas aeruginosa ventilator-associated pneumonia: study protocol for a randomized controlled study. Trials. 2017;18:37.
Foolad F, Huang AM, Nguyen CT, et al. A multicentre stewardship initiative to decrease excessive duration of antibiotic therapy for the treatment of community-acquired pneumonia. J Antimicrob Chemother. 2018;73:1402–7.
Van de Wyngaert Z, Berthon C, Debarri H, et al. Discontinuation of antimicrobial therapy in adult neutropenic haematology patients: a prospective cohort. Int J Antimicrob Agents. 2019;53:781–8.
Wunderink RG, Waterer G. Advances in the causes and management of community acquired pneumonia in adults. BMJ. 2017;358:j2471.
Giuliano KK, Baker D, Quinn B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am J Infect Control. 2018;46:322–7.
Rider LG, Aggarwal R, Machado PM, et al. Update on outcome assessment in myositis. Nat Rev Rheumatol. 2018;14:303–18.
Liang J, Xu D, Sun C, Chen W, Cao H, Lin J. Hemophagocytic lymphohistiocytosis: prevalence, risk factors, outcome, and outcome-related factors in adult idiopathic inflammatory myopathies. J Rheumatol. 2020;47:1532–40.
Karakioulaki M, Stolz D. Biomarkers in pneumonia-beyond procalcitonin. Int J Mol Sci. 2019;20:2004.
Meehan TP, Fine MJ, Krumholz HM, et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278:2080–4.
Pugh R, Grant C, Cooke RP, Dempsey G. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev. 2015;8:CD007577.
Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, Grammatikos AP, Athanassa Z, Falagas ME. Short- versus long-course antibacterial therapy for community-acquired pneumonia—a meta-analysis. Drugs. 2008;68:1841–54.
Xia JJ, Zhang DC, Xu YP, Gong ML, Zhou Y, Fang XQ. A retrospective analysis of carbapenem-resistant Acinetobacter baumannii-mediated nosocomial pneumonia and the in vitro therapeutic benefit of cefoperazone/sulbactam. Int J Infect Dis. 2014;23:90–3.
Povoa P, Coelho L, Almeida E, et al. Early identification of intensive care unit-acquired infections with daily monitoring of C-reactive protein: a prospective observational study. Crit Care. 2006;10:R63.
Povoa P, Coelho L, Almeida E, et al. C-reactive protein as a marker of ventilator-associated pneumonia resolution: a pilot study. Eur Respir J. 2005;25:804–12.
Povoa P, Martin-Loeches I, Ramirez P, et al. Biomarker kinetics in the prediction of VAP diagnosis: results from the BioVAP study. Ann Intensive Care. 2016;6:32.
Ottosen J, Evans H. Pneumonia challenges in the definition, diagnosis, and management of disease. Surg Clin N Am. 2014;94:1305–17.
Torres A, Chalmers JD, Dela Cruz CS, et al. Challenges in severe community-acquired pneumonia: a point-of-view review. Intensive Care Med. 2019;45:159–71.
Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:575–82.
Mangalam AK, Yadav R. Utility of CD64 expression on neutrophils as a marker to differentiate infectious versus noninfectious disease flares in autoimmune disorders. Indian J Rheumatol. 2019;14:9–11.
Acknowledgements
We express our gratitude to all the participants in this study.
Funding
This study and the Journal’s Rapid Service Fee was funded by National Natural Science Foundation of China (81701602).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Junyu Liang, Chuanyin Sun, Liqin Xu, Guanhua Xu, Heng Cao, and Jin Lin have nothing to disclose.
Compliance with Ethics Guidelines
The approval (Reference Number: 2020-104) was obtained from the Institutional Review Board of the First Affiliated Hospital, Zhejiang University School of Medicine (FAHZJU) prior to initiation of the study. Written informed consent (for participation and publication) was acquired from all patients involved. The study was performed in accordance with the Helsinki Declaration of 1975 and its later amendments.
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Liang, J., Sun, C., Xu, L. et al. Community-Acquired Pneumonia and Hospital-Acquired Pneumonia in Adult Patients with Idiopathic Inflammatory Myopathy: Outcome and Antibiotic Therapy. Rheumatol Ther 8, 255–272 (2021). https://doi.org/10.1007/s40744-020-00268-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-020-00268-7