Abstract
This cohort study aimed to identify the characteristics and risk factors of adult idiopathic inflammatory myopathy-associated interstitial lung disease (IIM-ILD) and further explore the prognostic factors of IIM-ILD. We extracted data regarding 539 patients with laboratory-confirmed idiopathic inflammatory myopathy (IIM) with or without interstitial lung disease (ILD) from the Second Xiangya Hospital of Central South University between January 2016 and December 2021. The regression analysis was conducted to identify the possible risk factors for ILD as well as mortality. Of 539 IIM patients, 343 (64.6%) were diagnosed with IIM-ILD. The median (IQR) baseline neutrophil-to-lymphocyte ratio (NLR), C-reactive protein to albumin ratio (CAR) and ferritin were 4.1371 (2.6994–6.8143), 0.1685 (0.0641–0.5456) and 393.6 (210.6–532.2), respectively. Risk factors associated with IIM-ILD were older age (p = 0.002), arthralgia (p = 0.014), lung infection (p = 0.027), hemoglobin (p = 0.022), high CAR (p = 0.014), anti-aminoacyl-tRNA synthetase (anti-ARS) antibody-positive (p < 0.001), and anti-MDA5 antibody-positive (p < 0.001). The IIM-ILD patients whose age at diagnosis of disease ≥ 59.5 (HR = 2.673, 95% CI 1.588–4.499, p < 0.001), NLR ≥ 6.6109 (HR = 2.004, 95% CI 1.193–3.368, p = 0.009), CAR ≥ 0.2506 (HR = 1.864, 95% CI 1.041–3.339, p = 0.036), ferritin ≥ 397.68 (HR = 2.451, 95% CI 1.245–4.827, p = 0.009) and anti-MDA5 antibody-positive (HR = 1.928, 95% CI 1.123–3.309, p = 0.017) had a higher mortality rate. High CAR and anti-MDA5 antibody-positive are more likely to be associated with a high mortality rate of IIM-ILD, which can be used as serum biomarkers, especially the CAR, a simple, objective tool to assess the prognosis of IIM.
Similar content being viewed by others
Introduction
Idiopathic inflammatory myopathy (IIM) is an umbrella term encompassing a set of disorders, most, but not all, of which are characterized by chronic muscle inflammation. The clinical presentation of IIM is varied and can affect other organ systems including the skin, lungs, and joints. According to most guidelines and expert consensus, the five major types of IIM include dermatomyositis (DM), polymyositis (PM), anti-synthetase syndrome (ASS), immune-mediated necrotizing myopathy (IMNM), inclusion body myositis (IBM)1.Interstitial lung disease (ILD) is one of the most common pulmonary manifestations of IIM that affects the interstitial space, the primary consequence of which is impaired gas exchange, thus giving rise to breathlessness, respiratory failure, and even death in IIM patients2,3,4,5. ILD has an estimated prevalence of 20–78% among the IIM population3,6,7,8, and the median survival of idiopathic inflammatory myopathy-associated interstitial lung disease (IIM-ILD) patients is usually 3–5 years9,10. To date, the survival time of IIM-ILD is longer than before, which may be related to the improving awareness and multimodality therapy of this disease. Once diagnosed with rapidly progressive ILD (RP-ILD), patients may carry a poor prognosis with a median untreated life expectancy from diagnosis of 6 months or 1 year11. The high morbidity and mortality have urged us to look for simple and convenient biomarkers that can predict the prognosis of IIM-ILD12.
Therefore, we sought to identify baseline factors associated with ILD and explore indicators that can be used as independent prognostic biomarkers in patients with IIM-ILD, which will better inform clinical decision-making for the patients.
Method
Patients
This was a retrospective cohort study. All subjects were admitted to the Second Xiangya Hospital of Central South University from January 2016 to December 2021. The eligibility criteria for IIM patients were: (1) Age at disease onset ≥ 18 years; (2) Diagnosis based on the criteria for IIM4,13,14,15. (3) The data of myositis-specific autoantibodies (MSA) and myositis-associated autoantibodies (MAA) detected using the EUROLINE Autoimmune Inflammatory Myopathies 16 Ag (IgG) commercial line blot test was complete. IIM complements other systemic autoimmune diseases such as systemic lupus erythematosus (SLE), Sjogren’s syndrome (SS), and systemic sclerosis (SSc) were excluded. The diagnosis of ILD was based on the 2013 American Thoracic Society/European Respiratory Society (ATS/ERS) criteria16.
The study was approved by the ethics review committee of the Second Xiangya Hospital of Central South University (No. LYF2022147), and the requirement for informed consent was waived by the aforementioned research ethics board due to the retrospective nature of the study. This study was conducted in accordance with the Declaration of Helsinki.
Data collection
We extracted demographics and general information including the department of consultation, age at diagnosis, duration of disease, history of smoking, therapy before and after admission, comorbidities and symptoms. Laboratory findings, such as blood cell counts, albumin (ALB), globulin (GLO), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH), creatine kinase (CK), creatine kinase MB (CKMB), myohemoglobin (MB), Neutrophil-to-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), monocyte-lymphocyte ratio (MLR), the CRP to ALB ratio (CAR), the ESR to ALB ratio (EAR), ferritin and serologic autoantibodies were all collected. We also analyzed the imaging findings, such as irregular linear opacities, grid shadow, nodular shadow, septal thickening, ground-glass opacities, patch shadow, traction bronchodilation, honeycombing, and pleural effusion. It is worth emphasizing that an abnormal chest CT scan was defined as any ILD meeting the 2013 American Thoracic Society criteria16. High-resolution CT (HRCT) scans were reevaluated independently by two experienced chest radiologists.
Definition
NLR and PLR were calculated using hematological parameters (absolute count of neutrophil, lymphocyte and platelet) measured by automated analyzers: NLR = absolute neutrophil count (ANC)/absolute lymphocyte count (ALC) and PLR = absolute platelet count (APC)/ALC. The CAR was calculated as CAR = serum CRP level (mg/L)/serum albumin level (g/L), EAR = serum ESR level (mm/h)/serum albumin level (g/L).
Outcomes and follow-up
The primary endpoint of the IIM-ILD cohort was all-cause mortality. Survival was defined as the interval between the diagnosis of IIM-ILD and death or the latest updated data (June 30, 2022).
Statistical analysis
All data were analyzed using SPSS 25.0 software (SPSS Inc., Chicago, IL, https://www.ibm.com/cn-zh/spss) and GraphPad Prism 9.0 (GraphPad Software, https://www.graphpad-prism.cn/). Comparisons between groups of continuous variables were made using T-test or the Mann–Whitney test. Comparisons between groups of categorical variables were made using the Chi-square test or Fisher’s exact when suitable. Logistic regression analysis was used to choose candidate risk factors of ILD. The Kaplan–Meier curves with log-rank tests were employed to access predictors in survival. The optimal cut-off values of the potential biomarkers were determined using the receiver operating characteristic (ROC) analysis. A two-tailed p < 0.05 was considered statistically significant, and the odds ratio (OR) or hazard ratio (HR) and 95% confidence interval (CI) were indicated.
Result
Baseline characteristics of patients with IIM-ILD and IIM without ILD
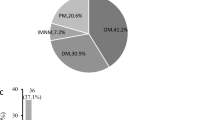
Of the 539 IIM patients enrolled in this study, 309 (57.3%) were diagnosed with DM and 107 (19.9%) were diagnosed with PM, and other patients were diagnosed with ASS or IMNM. The baseline characteristics of the IIM patients are shown in Table 1. A total of 62.5% presented to the Rheumatology and Immunology Department, and 62.3% of patients were female. Among the IIM patients, 343 (63.6%) had ILD. The mean age of IIM-ILD patients was older than that of the IIM without ILD (IIM-NILD) patients (p = 0.017). Concerning clinical symptoms, IIM-ILD patients showed more respiratory and joint manifestations (p < 0.001), and pulmonary signs (p < 0.001). Moreover, the IIM-ILD group was associated with more Gottron’s rash, mechanic’s hand, dry mouth, fever, hair loss, and weight loss. In contrast, the IIM-NILD group showed a higher percentage of dysphagia (p = 0.014). It is worth mentioning that the IIM-NILD group was more likely to combine tumors and tuberculosis, while the IIM-ILD group usually had comorbid lung infection and respiratory failure. Additionally, no statistically significant baseline differences were observed in the duration of the disease and Raynaud’s phenomenon (Table 1).
Laboratory findings in IIM-ILD and IIM-NILD
As for laboratory findings, IIM-ILD patients had higher values of PLR (p = 0.013), GLO (p < 0.001), CRP (p < 0.001), ESR (p < 0.001), CAR (p < 0.001), EAR (p < 0.001) and ferritin (p < 0.001), except for ALB levels (p < 0.001). For the myositis-associated autoantibodies (MAA), IIM-ILD patients were prone to occur anti-Ro52 antibodies positive. Unsurprisingly, there was a significant difference between these two groups in terms of MSA. The IIM-ILD group showed more anti-aminoacyl-tRNA synthetase (anti-ARS) and anti-MDA5 antibodies positive. However, the IIM-NILD group had more anti-TIF1-γ, anti-Mi-2α, anti-NXP2, and anti-SRP antibodies positive (see Supplementary Fig. S1 online).
Risk factors independently associated with IIM-ILD and IIM-NILD patients
To determine the independent risk factors of ILD in IIM patients, we employed univariate and multivariate logistic regression analyses combined with clinical applications. CAR was confirmed as a significant risk factor of ILD in multivariable analysis, increasing the risk by 55.4% (OR = 1.554, 95% CI: 1.091–2.212, p = 0.014). Other factors associated with an increased risk of mortality included the age at diagnosis (OR = 1.029, 95% CI: 1.010–1.047, p = 0.002), arthralgia (OR = 1.954, 95% CI: 1.144–3.339, p = 0.014), lung infection (OR = 1.803, 95% CI: 1.068–3.046, p = 0.027), hemoglobin (OR = 1.016, 95% CI: 1.002–1.031, p = 0.022), anti-ARS antibody positive (OR = 2.830, 95% CI: 1.673–4.787, p < 0.001) and anti-MDA5 antibody positive (OR = 7.472, 95% CI: 3.914–14.263, p < 0.001) (Table 2).
Further analysis compared the value of CAR between the IIM-ILD group and the IIM-NILD group. As expected, the value of CAR was higher in the IIM-ILD group compared with the IIM-NILD group (see Supplementary Fig. S2a online). Using receiver operating characteristic (ROC) analysis, The area under the ROC curve (AUC) was 0.6589 (95% CI: 0.6108–0.7070, p < 0.001) (see Supplementary Fig. S2b online). An optimal cutoff value for the CAR was derived from the point with the maximum Youden index. This cutoff point for CAR was 0.0680, yielding 83.7% sensitivity and 43.9% specificity.
Comparison of clinical features of survivors and decedents in IIM-ILD
IIM-ILD patients were followed up, and 38 of the 343 patients with IIM-ILD were lost during follow-up. The follow-up period for all patients ranged from 1 to 78 months, with a median follow-up of 26 months. Baseline characteristics were compared among patients between the survival and the decedent groups. Of the 72 dead patients, 68.1% (49/72) of them were diagnosed with DM, and 6.9% (5/72) were diagnosed with PM (Table 3). The average age of the patients who died was higher than that of the survivors (p < 0.001), and the decedents had a higher rate of patients presented at the onset of ILD (p = 0.003) and shorter disease duration than the survivors (p = 0.018). Concerning clinical symptoms, chest tightness, dyspnea, dry mouth and fever were significantly more prevalent in the decedents compared with the survivors, while no statistical difference was found regarding the skin, joint and muscle manifestations. As for the comorbidities, the decedents were more likely to combine with lung infection (p = 0.002) and respiratory failure (p < 0.001). Compared with the survivors, the decedents had lower values of serum hemoglobin (p < 0.001) and ALB (p < 0.001). Moreover, NLR (p < 0.001), PLR (p = 0.001), CRP (p < 0.001), ESR (p = 0.002), CAR (p < 0.001), EAR (p < 0.001) and ferritin (p < 0.001) were significantly higher in the decedents compared with the survivors. In addition, we compared antibodies between the survivors and the decedents, and no differences were found regarding anti-Ro52 antibodies. The decedents showed a higher positive rate of anti-MDA5 antibodies (66.1% vs. 44.6%, p = 0.014) and lower positive rate of anti-Jo-1 antibodies (5.6% vs.15.0%, p = 0.036) and anti-SRP antibodies (4.2% vs.13.7%, p = 0.026) (Table 3).
The HRCT was available in all 305 IIM-ILD patients. The most common abnormality was patch shadow (212/305, 69.5%), and the second common abnormality was irregular linear opacities (184/305, 60.3%). Honeycombing was the least common HRCT presentation (21/305, 6.9%). The HRCT image of the decedents had more patch shadow (p = 0.001), honeycombing (p = 0.035), and pleural effusion (p < 0.001) than the survivors. The survivors were more likely to be treated with immunosuppressants, while the decedents were treated with plasma exchange and intravenous immunoglobulin (IVIG).
To explore the potential prognostic significance of these parameters, we analyzed the relationship between these parameters and survival in combination with clinical applications.
Significance of parameters and cut-off determination in prognosis
Since age at diagnosis, NLR, CAR and ferritin were significantly associated with mortality in the patients with IIM-ILD, we chose the variables with p values < 0.05 to perform cut-off optimization for predicting mortality in our research. Optimized cut-offs for each variable were determined using standard ROC curve analysis. In ROC curve analyses, the AUCs for mortality of IIM-ILD were 0.6585 (p < 0.001), 0.6829 (p < 0.001), 0.6992 (p < 0.001) and 0.7129 (p < 0.001) for age at diagnosis, NLR, CAR and ferritin, respectively. Using the ROC curves, optimized cut-offs of the age at diagnosis (59.5), NLR (6.6109), CAR (0.2506) and ferritin (397.68) for IIM-ILD patients. The sensitivity and specificity were 54.2% and 75.1% for the age at diagnosis, 55.6% and 74.7% for the NLR, 73.6% and 63.9% for the CAR, and 84.7% and 50.6% for the ferritin (Fig. 1).
Survival analysis of patients in IIM-ILD
The results of multivariable Cox regression analyses of factors associated with mortality are presented in Table 4. Cox regression analyses showed that age at diagnosis ≥ 59.5 (HR = 2.673, 95% CI: 1.588–4.499, p < 0.001), NLR ≥ 6.6109 (HR = 2.004, 95% CI: 1.193–3.368, p = 0.009), CAR ≥ 0.2506 (HR = 1.864, 95% CI: 1.041–3.339, p = 0.036), ferritin ≥ 397.68 (HR = 2.451, 95% CI: 1.245–4.827, p = 0.009) and anti-MDA5 antibody (HR = 1.928, 95% CI: 1.123–3.309, p = 0.017) were significant risk factors for all-cause mortality in IIM-ILD patients (Table 4). Moreover, significantly lower cumulative survival was observed in IIM-ILD patients with higher age at diagnosis (Fig. 2a), NLR (Fig. 2b), CAR (Fig. 2c), ferritin (Fig. 2d) and anti-MDA5 antibody positive (Fig. 2e).
Survival curves for overall survival in patients with idiopathic inflammatory myopathy—associated Interstitial lung disease, stratified by age at diagnosis (a), NLR (b), CAR (c), ferritin (d) and anti-MDA5 antibody positive (e). NLR, neutrophil-to-lymphocyte ratio; CAR, C-reactive protein to albumin ratio.
Discussion
This present study reviewed the demographic, clinical, and laboratory characteristics as well as outcomes of IIM-ILD. We investigated independent risk factors of ILD and predictors of IIM-ILD patients. In our cohort, 343 (63.6%) IIM patients presented ILD, the incidence of ILD in IIM patients was close to 20% to 78%, as in previous studies6,7,8. Considering the high incidence of ILD in IIM, HRCT is essential, which has been proven as the pivotal radiologic evaluation in ILD because of its greater sensitivity compared with chest radiography, especially for early changes17. Our study identified arthralgia and lung infection as risk factors for ILD. Patients presenting with arthralgia tend to actively seek medical advice from rheumatology, which may lead to early recognition and diagnosis of ILD in IIM patients. However, the lack of specificity of arthralgia may experience delayed diagnosis and treatment of the disease due to neglect by patients and other departments. Patients may only present to respiratory departments when there is significant dyspnoea or even respiratory failure, thus missing the opportunity for early treatment. So we should strengthen the early recognition of patients’ symptoms to reduce the rate of missed diagnosis or misdiagnosis. Lung infection may cause direct damage to the lung, which in turn activates abnormal damage repair and ultimately leads to the development of pulmonary fibrosis. In addition, infection causes immune-mediated lung injury, activating immune cells such as macrophages, neutrophils, eosinophils and Th2 cells, which secrete large amounts of pro-inflammatory and pro-fibrotic factors, causing persistent lung damage and ultimately leading to pulmonary fibrosis18. Vaccination would play a key role in preventing lung infection and probably flares of ILD and mortality, we will explore the relationship between vaccines and mortality in patients with IIM-ILD in the future study. This study focuses on CAR, an indicator of the balance between CRP and ALB19. CAR is a newly discovered prognostic indicator of inflammation, which has been shown to have predictive value in neurocritical illness20, transcatheter aortic valve replacement21, neoplasms22, and sepsis23. It can be used as a predictor of disease severity in lung disease, including pneumonia24, lung cancer25, COPD26, and other diseases, but its application in ILD is rare. CRP is a marker of systemic inflammation, and ALB is frequently included as an indicator of frailty and nutrition. CRP levels increase, and ALB levels decrease due to inflammation depletion. It has been reported that IL-6 can affect both CRP and ALB27,28, IL-6 has been proven that involved in the development and progression of fibrosis, so the prognosis of IL-6 in IIM-ILD needs to be further studied. Although some studies have shown that ALB9 or CRP29 can be used as predictors of IIM, few studies have certified CAR as a predictor of IIM-ILD. CAR is superior to either CRP or ALB alone in predicting prognosis for patients with acute medical conditions30, which suggests that this biomarker may reflect parenchymal lung inflammation leading to irreversible injury. In this study, both univariate and multivariate logistic regression analyses showed that CAR was significantly associated with the occurrence of ILD in IIM. In addition, COX regression demonstrated that CAR was a strong predictor of the prognosis of IIM-ILD. Kaplan–Meier curve showed that the mortality rate in the CAR < 0.2506 group was higher than that in the CAR ≥ 0.2506 group. Although our data show relatively low sensitivity and specificity of CAR in predicting mortality in IIM-ILD patients, all these results suggest that CAR may be a suitable prognostic biomarker for patients with IIM and can even be used to assess nutritional status in patients with IIM. It is worth further studying its application value in IIM, considering its cost-practical value.
As for MSA, It is well known that the incidence of ILD is closely related to the anti-ARS and anti-MDA5 antibodies. Anti-ARS antibodies are reported be detected in 20–40% of patients with IIM31,32 and the incidence of ILD in anti-ARS antibody-positive patients is 79–95%33,34,35. In the current study, 145 (26.9%) patients were tested for the anti-ARS antibody, and 76% (110/145) of the patients showed ILD, which is in line with previous estimates. Our analysis also proved that it is a risk factor for ILD. The anti-MDA5 antibody has been regarded as a vital serum predictive marker for IIM, often indicative of a poor prognosis of IIM36,37,38,39. Early diagnosis and more effective treatment are essential, so we should pay more attention to the patients with anti-MDA5 antibody positive and give them aggressive treatment.
About MAA, Gui et al.40showed that the anti-Ro52 antibody could predict the prognosis of IIM-ILD, but this study did not verify its role as a predictor of IIM-ILD. Given that the anti-Ro52 antibody often combines with other MSA antibodies positive, it was difficult to determine the 'target antibody' in duo-positive or multi-positive patients. A real-world Australian study41did not support a prognostic role for the anti-RO52 antibody in IIM-ILD. Its prophetic role in IIM-ILD is still controversial42,43. Currently, no clear guideline or consensus exists, and more extensive prospective studies are needed for validation.
The older the age at diagnosis, the worse the prognosis, which may be related to the fact that elderly patients are prone to immune disorders, and their lung function becomes worse with age. This study also collected data related to pulmonary function tests (PFT). However, given that most patients were treated in the rheumatology and dermatology department, there were too much missing data on PFT, which was not included in the discussion. In the subsequent study, we will further supplement the data related to PFT. NLR has been recognized as a practical and valuable prognostic biomarker. It can be used as a prognostic indicator for COVID-1944, systemic sclerosis45, spinal arthritis46, malignant neoplasms47, idiopathic pulmonary fibrosis48 and other diseases. Regarding the use of NLR in IIM, a retrospective study in South Korea has demonstrated the predictive role of NLR in IIM-ILD, with higher values associated with a worse prognosis49. The optimal cut-off value for this study was 4.775, while our study demonstrated that the optimal cut-off was 6.6109. Studies have suggested that ferritin is associated with the prognosis of IIM-ILD patients50,51. Nevertheless, our study with a larger sample size showed that serum ferritin was a risk factor for poor prognosis, thus providing more parameters for evaluating the prognosis of IIM-ILD patients. However, different researches have different cut-off values of ferritin for prognosis. Ferritin showed a lower value in our study than in others. This difference may be due to the different populations and the testing methods.
This study had some limitations. Firstly, as this study is retrospective, there will inevitably be information and recall bias. Secondly, our follow-up method, mainly through telephone follow-up, could not obtain the treatment method for the whole follow-up period, so the impact of treatment on prognosis could not be evaluated. Finally, there were some truncated data in the survival analysis, so we need to further track the survival status of these patients in the subsequent time.
Conclusion
IIM with elevated CAR and the anti-MDA5 antibody positive are more likely to occur ILD and have an increased risk of death. In clinical application, CAR is a readily accessible measurement that can be incorporated into the risk assessment of patients considering IIM. For future studies, our study may help provide longitudinal information on prognosis, survival, and mortality in patients with IIM.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Selva-O’Callaghan, A. et al. Classification and management of adult inflammatory myopathies. Lancet Neurol. 17, 816–828. https://doi.org/10.1016/s1474-4422(18)30254-0 (2018).
Hallowell, R. W. & Danoff, S. K. Interstitial lung disease associated with the idiopathic inflammatory myopathies and the antisynthetase syndrome: Recent advances. Curr. Opin. Rheumatol. 26, 684–689. https://doi.org/10.1097/bor.0000000000000104 (2014).
Fathi, M., Dastmalchi, M., Rasmussen, E., Lundberg, I. E. & Tornling, G. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann. Rheum. Dis. 63, 297–301. https://doi.org/10.1136/ard.2003.006122 (2004).
Solomon, J., Swigris, J. J. & Brown, K. K. Myositis-related interstitial lung disease and antisynthetase syndrome. J. Bras. Pneumol. 37, 100–109. https://doi.org/10.1590/s1806-37132011000100015 (2011).
Wijsenbeek, M., Suzuki, A. & Maher, T. M. Interstitial lung diseases. Lancet 400, 769–786. https://doi.org/10.1016/s0140-6736(22)01052-2 (2022).
Li, S. et al. Prognosis of adult idiopathic inflammatory myopathy-associated interstitial lung disease: A retrospective study of 679 adult cases. Rheumatol. (Oxf.) 60, 1195–1204. https://doi.org/10.1093/rheumatology/keaa372 (2021).
Lega, J. C. et al. Idiopathic inflammatory myopathies and the lung. Eur. Respir. Rev. 24, 216–238. https://doi.org/10.1183/16000617.00002015 (2015).
Fathi, M. et al. Interstitial lung disease in polymyositis and dermatomyositis: Longitudinal evaluation by pulmonary function and radiology. Arthritis Rheum. 59, 677–685. https://doi.org/10.1002/art.23571 (2008).
Bai, Z., Shen, G. & Dong, L. Analysis of risk factors of interstitial lung disease and mortality rates in Chinese patients with idiopathic inflammatory myopathy. Int. J. Rheum. Dis. 24, 815–827. https://doi.org/10.1111/1756-185x.14128 (2021).
Rivière, A. et al. Lung transplantation for interstitial lung disease in idiopathic inflammatory myositis: A cohort study. Am. J. Transplant. https://doi.org/10.1111/ajt.17177 (2022).
Zuo, Y. et al. Different multivariable risk factors for rapid progressive interstitial lung disease in anti-MDA5 positive dermatomyositis and anti-synthetase syndrome. Front. Immunol. 13, 845988. https://doi.org/10.3389/fimmu.2022.845988 (2022).
So, J. et al. Predictors of rapidly progressive- interstitial lung disease and mortality in patients with autoantibodies against melanoma differentiation-associated protein 5 dermatomyositis. Rheumatol. (Oxf. Engl.) https://doi.org/10.1093/rheumatology/keac094 (2022).
Bohan, A. & Peter, J. B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 292, 344–347. https://doi.org/10.1056/nejm197502132920706 (1975).
Bohan, A. & Peter, J. B. Polymyositis and dermatomyositis (second of two parts). N. Engl. J. Med. 292, 403–407. https://doi.org/10.1056/nejm197502202920807 (1975).
Lundberg, I. E. et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthrit. Rheumatol. (Hobok. N. J.) 69, 2271–2282. https://doi.org/10.1002/art.40320 (2017).
Travis, W. D. et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 188, 733–748. https://doi.org/10.1164/rccm.201308-1483ST (2013).
Collins, C. D. et al. Observer variation in pattern type and extent of disease in fibrosing alveolitis on thin section computed tomography and chest radiography. Clin. Radiol. 49, 236–240. https://doi.org/10.1016/s0009-9260(05)81847-1 (1994).
Huang, W. J. & Tang, X. X. Virus infection induced pulmonary fibrosis. J. Transl. Med. 19, 496. https://doi.org/10.1186/s12967-021-03159-9 (2021).
Wang, W. et al. Prognostic efficacy of high-sensitivity C-reactive protein to albumin ratio in patients with acute coronary syndrome. Biomark. Med. 13, 811–820. https://doi.org/10.2217/bmm-2018-0346 (2019).
Bai, M. et al. Prognostic value of C-reactive protein/albumin ratio in neurocritically ill patients. Miner. Anestesiol. 85, 1299–1307. https://doi.org/10.23736/s0375-9393.19.13625-5 (2019).
Seoudy, H. et al. C-reactive protein to albumin ratio in patients undergoing transcatheter aortic valve replacement. Mayo Clin. Proc. 97, 931–940. https://doi.org/10.1016/j.mayocp.2021.11.022 (2022).
Zhou, J. et al. Prognostic value of C-reactive protein, glasgow prognostic score, and C-reactive protein-to-albumin ratio in colorectal cancer. Front. Cell Dev. Biol. 9, 637650. https://doi.org/10.3389/fcell.2021.637650 (2021).
Li, T. et al. Predictive value of C-reactive protein-to-albumin ratio for neonatal sepsis. J. Inflamm. Res. 14, 3207–3215. https://doi.org/10.2147/jir.S321074 (2021).
Kunutsor, S. K. & Laukkanen, J. A. Serum C-reactive protein-to-albumin ratio is a potential risk indicator for pneumonia: Findings from a prospective cohort study. Respir. Med. 199, 106894. https://doi.org/10.1016/j.rmed.2022.106894 (2022).
Frey, A. et al. C-reactive protein to albumin ratio as prognostic marker in locally advanced non-small cell lung cancer treated with chemoradiotherapy. Biomedicines 10, 598. https://doi.org/10.3390/biomedicines10030598 (2022).
Li, H. et al. C-reactive protein to serum albumin ratio as a novel biomarker to predict prognosis in patients with chronic obstructive pulmonary disease. Clin. Lab. 67, 14. https://doi.org/10.7754/Clin.Lab.2020.200630 (2021).
Rhodes, B., Fürnrohr, B. G. & Vyse, T. J. C-reactive protein in rheumatology: Biology and genetics. Nat. Rev. Rheumatol. 7, 282–289. https://doi.org/10.1038/nrrheum.2011.37 (2011).
Mihara, M., Hashizume, M., Yoshida, H., Suzuki, M. & Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. (Lond. Engl. 1979) 122, 143–159. https://doi.org/10.1042/cs20110340 (2012).
Sato, S. et al. Initial predictors of poor survival in myositis-associated interstitial lung disease: A multicentre cohort of 497 patients. Rheumatol. (Oxf.) 57, 1212–1221. https://doi.org/10.1093/rheumatology/key060 (2018).
Fairclough, E., Cairns, E., Hamilton, J. & Kelly, C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin. Med. (Lond.) 9, 30–33. https://doi.org/10.7861/clinmedicine.9-1-30 (2009).
Lee, L. W., Narang, N. S., Postolova, A., Seminara, N. & Kantor, M. A. Anti-MDA5-positive dermatomyositis presenting as fever of unknown origin. J. Gen. Intern. Med. 31, 1530–1536. https://doi.org/10.1007/s11606-016-3769-0 (2016).
Nakashima, R. Clinical significance of myositis-specific autoantibodies. Immunol. Med. 41, 103–112. https://doi.org/10.1080/25785826.2018.1531188 (2018).
Zhang, Y. et al. Clinical features and outcomes of the patients with anti-glycyl tRNA synthetase syndrome. Clin. Rheumatol. 39, 2417–2424. https://doi.org/10.1007/s10067-020-04979-8 (2020).
Hamaguchi, Y. et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: Heterogeneity within the syndrome. PLoS ONE 8, e60442. https://doi.org/10.1371/journal.pone.0060442 (2013).
Cavagna, L. et al. Influence of antisynthetase antibodies specificities on antisynthetase syndrome clinical spectrum time course. J. Clin. Med. 8, 2013. https://doi.org/10.3390/jcm8112013 (2019).
Li, Y. et al. Predictors of poor outcome of Anti-MDA5-associated rapidly progressive interstitial lung disease in a chinese cohort with dermatomyositis. J. Immunol. Res. 2020, 2024869. https://doi.org/10.1155/2020/2024869 (2020).
Gono, T. & Kuwana, M. Inflammatory myopathies: Choosing the right biomarkers to predict ILD in myositis. Nat. Rev. Rheumatol. 12, 504–506. https://doi.org/10.1038/nrrheum.2016.120 (2016).
Gan, Y. Z. et al. Risk factors of interstitial lung diseases in clinically amyopathic dermatomyositis. Chin. Med. J. 133, 644–649. https://doi.org/10.1097/cm9.0000000000000691 (2020).
Moghadam-Kia, S., Oddis, C. V., Sato, S., Kuwana, M. & Aggarwal, R. Anti-Melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res. 68, 689–694. https://doi.org/10.1002/acr.22728 (2016).
Gui, X. et al. Anti-Ro52 antibodies are associated with the prognosis of adult idiopathic inflammatory myopathy-associated interstitial lung disease. Rheumatol. (Oxf. Engl.) https://doi.org/10.1093/rheumatology/keac090 (2022).
He, J., Wei, X. & Sturgess, A. Concordance between myositis autoantibodies and anti-nuclear antibody patterns in a real-world, Australian cohort. Rheumatol. (Oxf. Engl.) https://doi.org/10.1093/rheumatology/keac039 (2022).
Chen, F. et al. Clinical characteristics of dermatomyositis patients with isolated anti-Ro-52 antibody associated rapid progressive interstitial lung disease: Data from the largest single Chinese center. Respir. Med. 155, 127–132. https://doi.org/10.1016/j.rmed.2019.07.020 (2019).
Bauhammer, J. et al. Rituximab in the treatment of Jo1 antibody-associated antisynthetase syndrome: Anti-Ro52 positivity as a marker for severity and treatment response. J. Rheumatol. 43, 1566–1574. https://doi.org/10.3899/jrheum.150844 (2016).
Varim, C. et al. Neutrophil count to albumin ratio as a new predictor of mortality in patients with COVID-19 ınfection. Rev. Assoc. Med. Brasil. 66(2), 77–81. https://doi.org/10.1590/1806-9282.66.S2.77 (2020).
Wareing, N. et al. Blood neutrophil count and neutrophil-to-lymphocyte ratio predict disease progression and mortality in two independent systemic sclerosis cohorts. Arthritis Care Res. https://doi.org/10.1002/acr.24880 (2022).
Slouma, M. et al. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, C-reactive Protein to albumin ratio, and albumin to fibrinogen ratio in axial spondyloarthritis: A monocentric study. Curr. Rheumatol. Rev. 17, 312–317. https://doi.org/10.2174/1573397116666201224105359 (2021).
Chen, F. et al. Prognostic significance of neutrophil-to-lymphocyte ratio and C-reactive protein/albumin ratio in luminal breast cancers with HER2-negativity. Front. Oncol. 12, 845935. https://doi.org/10.3389/fonc.2022.845935 (2022).
Achaiah, A. et al. Neutrophil lymphocyte ratio as an indicator for disease progression in Idiopathic Pulmonary Fibrosis. BMJ Open Respir. Res. 9, 1202. https://doi.org/10.1136/bmjresp-2022-001202 (2022).
Ha, Y. J. et al. Baseline peripheral blood neutrophil-to-lymphocyte ratio could predict survival in patients with adult polymyositis and dermatomyositis: A retrospective observational study. PLoS ONE 13, e0190411. https://doi.org/10.1371/journal.pone.0190411 (2018).
González-Moreno, J. et al. Rapidly progressive interstitial lung disease due to anti-MDA5 antibodies without skin involvement: A case report and literature review. Rheumatol. Int. 38, 1293–1296. https://doi.org/10.1007/s00296-018-3991-7 (2018).
Lian, X. et al. Mortality risk prediction in amyopathic dermatomyositis associated with interstitial lung disease: The FLAIR model. Chest 158, 1535–1545. https://doi.org/10.1016/j.chest.2020.04.057 (2020).
Funding
This work was supported by the National Natural Science Foundation of China [No. 81670062], the Natural Science Foundation of Hunan Province [No. 2020JJ8070], Changsha City Natural Science Foundation [No. kq2208305], Xiangya Medical Big Data of Central South University and the National Key Clinical Specialty Construction Projects of China.
Author information
Authors and Affiliations
Contributions
P.Z. contributed to statistical analysis and interpretation of the data and drafting of the manuscript. Q.S., S.Z., X.O., T.G., M.S., W.G., Y.Z. contributed to acquisition, analysis and interpretation of data and revised the manuscript. H.P. contributed to conception and design of the study, analysis and interpretation of data and drafting of the manuscript. All coauthors critically revised the article and gave final approval of this version to be published. The manuscript has been read and approved by all the authors, the requirements for authorship as stated earlier in this document have been met, and each author believes that the manuscript represents honest work, the information is not provided in another form.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, P., Shen, Q., Zhou, S. et al. The prognostic role of C-reactive protein to albumin ratio and anti-MDA5 antibody-positive in idiopathic inflammatory myopathy: a retrospective study. Sci Rep 13, 3863 (2023). https://doi.org/10.1038/s41598-023-30595-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30595-y
- Springer Nature Limited