Abstract
Introduction
Patients with rheumatoid arthritis (RA) with poor prognostic factors, such as seropositivity for anti-citrullinated protein antibodies and early erosions, may benefit from early intensive treatment. However, information to guide physicians on the best choice of therapy in these patients is limited. The objective of this study was to describe the efficacy of subcutaneous abatacept versus adalimumab over 2 years in patients with seropositive, erosive early RA in the AMPLE study.
Methods
This exploratory post hoc analysis compared clinical, functional and radiographic outcomes in two subsets of patients: patients with early RA (≤ 6 months’ disease duration) who were seropositive for rheumatoid factor and/or anti-citrullinated protein antibodies and had > 1 radiographic erosion (Cohort 1); and patients with RA and absence of ≥ 1 of these inclusion criteria (Cohort 2).
Results
Of the 646 randomized patients, Cohort 1 included 38 patients receiving abatacept and 45 receiving adalimumab, and Cohort 2 included 280 patients receiving abatacept and 283 receiving adalimumab. Baseline demographics and disease characteristics were generally similar between treatment groups in both cohorts. Over 2 years, in Cohort 1, the adjusted mean change from baseline in the Disease Activity Score in 28 joints (using C-reactive protein) was numerically greater for abatacept than for adalimumab (mean difference at day 365 was 0.9, 95% confidence interval − 1.47 to − 0.33). Similar patterns of improvement were observed for other disease activity measures and physical function, but not for radiographic outcomes. No treatment-related differences were observed in Cohort 2.
Conclusion
This analysis indicates a trend towards improved disease activity and physical function with abatacept versus adalimumab in patients with seropositive, erosive early RA.
Trial Registration
ClinicalTrials.gov NCT00929864.
Funding
Bristol-Myers Squibb.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the introduction of multiple biologic disease-modifying anti-rheumatic drugs (DMARDs) and targeted synthetic DMARDs for patients with rheumatoid arthritis (RA), it is now a reasonable undertaking to search for and identify better predictors of response to targeted, individualized therapy. The use of biomarkers, including rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA), which are highly specific for RA, along with other disease and patient characteristics, could lead to a more personalized treatment approach [1]. Combined RF/ACPA positivity is associated with increased inflammation and disease activity, as well as with increased in vitro production of proinflammatory cytokines [2]. Additionally, patients who are ACPA positive have a tendency towards severe erosive disease and more rapid disease progression [3, 4].
Current recommendations of the European League Against Rheumatism (EULAR) suggest that ACPA seropositivity and early erosions can be used as poor prognostic factors to identify patients with RA who might benefit from early aggressive treatment [5]. However, additional data to help physicians decide which biologic agent to choose for individual patients with poor prognostic factors early in the course of their disease would be valuable.
The Abatacept versus adaliMumab comParison in bioLogic-naïvE RA subjects with background methotrexate (MTX) (AMPLE) study (ClinicalTrials.gov: NCT00929864) provided the first head-to-head, investigator-blinded comparison of abatacept and the tumour necrosis factor inhibitor adalimumab over 2 years in patients with RA. In the 1- and 2-year analyses, the clinical, functional and radiographic outcomes with abatacept and adalimumab were comparable [6, 7]. A post hoc analysis of the AMPLE study results also showed that the presence of anti-cyclic citrullinated peptide-2 (anti-CCP2) antibodies was associated with improved clinical response to both agents [8] and that a high- versus low-baseline anti-CCP2 concentration was associated with increased benefit in abatacept-treated patients as compared to adalimumab-treated patients [8].
The 2015 American College of Rheumatology (ACR) treatment guidelines define early RA as RA with a duration of disease/symptoms of no more than 6 months [9]. The objective of the exploratory post hoc analysis reported here was to evaluate the efficacy of abatacept versus adalimumab in two groups of patients: (1) those with early RA with poor prognostic factors and (2) those without poor prognostic factors regardless of disease duration.
Methods
Study Design
The full design, methods, inclusion criteria and primary results of the AMPLE study have been described previously [6, 7]. Briefly, patients with RA (ACR 1987 classification criteria [10]) who had active disease {Disease Activity Score in 28 joints using C-reactive protein [DAS28 (CRP)] ≥ 3.2} for ≤ 5 years despite MTX treatment and were naïve to biologic therapy were randomized 1:1 to receive subcutaneous abatacept 125 mg weekly or subcutaneous adalimumab 40 mg once every 2 weeks, both administered in combination with weekly MTX (≥ 15 and ≤ 25 mg/week or ≥ 7.5 mg/week if documented intolerance of higher dose) [6, 7]. Patients were stratified by disease activity [DAS28 (CRP) > 5.1; DAS28 (CRP) ≥ 3.2 and ≤ 5.1)]. The primary endpoint was treatment non-inferiority, assessed by 20% improvement in ACR criteria at 1 year. All efficacy and safety analyses were performed using the intent-to-treat population, which included all patients who were randomized and received at least one dose of the study drug. All patients who prematurely discontinued the study after receiving the study drug, regardless of the reason, were considered to be non-responders at all scheduled visits subsequent to the point of discontinuation.
The protocol was approved by the institutional review boards and independent ethics committees at the participating sites. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration, as revised in 2013, concerning human and animal rights, and Springer’s policy concerning informed consent has been followed. Informed consent was obtained from all individual participants included in the study.
Analysis Population
This post hoc analysis compared the clinical efficacy and patient-reported outcomes with abatacept and adalimumab in two subpopulations by treatment arm. Cohort 1 included patients with multiple poor prognostic factors, including early RA (defined as disease duration ≤ 6 months), > 1 radiographic erosion and the EULAR-defined factors of poor prognosis [5, 11] RF and/or ACPA seropositivity. Cohort 2 included patients with RA in whom at least one of these poor prognostic criteria was absent (i.e. the remaining AMPLE study population).
Outcome Measures
Patient demographics and disease characteristics were analysed at baseline by treatment and cohort. Clinical, radiographic and patient-reported outcomes were assessed at baseline and at multiple intervals up to day 729 of the blinded treatment period. Outcomes included the adjusted mean change from baseline in the DAS28 (CRP), Clinical Disease Activity Index (CDAI), Simplified Disease Activity Index (SDAI), Health Assessment Questionnaire-Disability Index (HAQ-DI), pain and fatigue and ACR response rate. In addition, radiographic progression was assessed using the modified Sharp/van der Heijde scoring system [12], including the modified total Sharp score (mTSS) and the proportion of patients with radiographic non-progression (defined as a mTSS ≤ smallest detectable change, where the smallest detectable change is an estimate of the measurement error between readers of the films) [13]. Safety events were classified using the Medical Dictionary for Regulatory Activities (https://www.meddra.org).
Statistical Analysis
All randomized and treated patients were included in the analysis. Baseline patient demographics and disease characteristics were analysed descriptively by treatment for each cohort. Adjusted mean changes from baseline in disease activity measures and patient-reported outcomes were determined, with corresponding 95% confidence intervals (CIs), at each time point by treatment for each cohort. Missing values were imputed using a last observation carried forward analysis (excluding patients for whom only baseline observations were available). No formal statistical testing was performed for endpoint comparisons due to the small sample size. A mixed effect model was used to estimate the differences between abatacept and adalimumab treatment over time within cohorts. Sensitivity analyses were conducted, limiting Cohort 2 (≥ 1 of the following: disease duration ≤ 6 months; > 1 radiographic erosion and RF and/or ACPA seropositivity) to only patients with disease duration of ≤ 6 months (Cohort 3).
Results
Patient Population
In total, 646 patients were randomly assigned to receive treatment in the overall AMPLE trial (abatacept, n = 318; adalimumab, n = 328) [6], of whom 252 abatacept- and 245 adalimumab-treated patients completed year 2 [7]. In this post hoc analysis, 38 abatacept- and 45 adalimumab-treated patients were RF and/or ACPA seropositive, had disease duration of ≤ 6 months and had > 1 erosion on baseline radiographs (i.e. seropositive, erosive, early RA; Cohort 1). The remainder of the study patients, 280 abatacept- and 283 adalimumab-treated patients, did not have all of these poor prognostic factors (Cohort 2). Most demographics and disease characteristics at baseline, including anti-CCP2 titres, were similar across treatment groups in both cohorts (Table 1). In Cohort 1, at baseline, patients receiving abatacept had a lower mean body weight and received a higher mean MTX dose than did those receiving adalimumab. Also, compared to patients treated with adalimumab, a lower proportion of patients treated with abatacept had a baseline DAS28 (CRP) of > 5.1.
Clinical, Patient-Reported and Radiographic Outcomes
Over 2 years, in Cohort 1, the adjusted mean improvements from baseline in the DAS28 (CRP) and HAQ-DI scores were numerically greater for the abatacept treatment group than for the adalimumab treatment group (Fig. 1a, c). There was no difference between the two treatment groups over the same period in Cohort 2 for either outcome measure (Fig. 1b, d).
Adjusted mean change from baseline in the Disease Activity Score in 28 joints using C-reactive protein [DAS28 (CRP)] (a, b) and Health Assessment Questionnaire-Disability Index (HAQ-DI) (c, d) over 2 years, by patient type. n is the number of patients with both post-baseline and baseline measurements. Panels a and c indicate patients with seropositive, erosive early rheumatoid arthritis (RA) treated with abatacept versus adalimumab, respectively. The adjusted mean change in DAS28 (CRP) from baseline at day 365 was − 2.58 [95% confidence interval (CI) − 2.99 to − 2.17] versus − 1.68 (95% CI − 2.10 to − 1.25) and that in HAQ-DI from baseline at day 365 was − 0.70 (95% CI − 0.90 to − 0.51) versus − 0.50 (95% CI − 0.71 to − 0.30). For calculation of the 95% CI within each group, normal approximation was used if n ≥ 5, otherwise the exact method was used. The dagger symbol indicates that adjustment was based on an analysis of the covariance model with treatment as a factor and baseline values and DAS28 (CRP) stratification as covariates
Regarding the other efficacy measures, similar differences between abatacept- and adalimumab-treated patients were observed in Cohort 1 but not in Cohort 2. Differences were seen in disease activity as captured by both the CDAI (Fig. 2a, b) and SDAI (Fig. 2c, d) and by the 20/50/70/90% improvement in ACR criteria (ACR20/50/70/90, respectively) composite measures (Fig. 3). Other patient-reported outcomes, including pain and fatigue scores, also captured differences between abatacept- and adalimumab-treated patients in Cohort 1 but not in Cohort 2 (pain and fatigue scores, Table 2).
Adjusted mean change from baseline in the Clinical Disease Activity Index (CDAI) (a, b) and Simplified Disease Activity Index (SDAI) (c, d) at 1 and 2 years, by patient type (Cohort 1: a, c; Cohort 2: b, d). All randomized and treated patients were included in the analysis; n is the number of patients with both post-baseline and baseline measurements. Asterisk indicates patients with seropositive, erosive early RA treated with abatacept versus adalimumab, respectively. The adjusted mean change from baseline at day 365 was − 25.68 (95% CI − 29.88 to − 21.49) versus − 19.28 (95% CI − 23.55 to − 15.02) for CDAI and − 26.71 (95% CI − 31.09 to − 22.33) versus − 20.22 (95% CI − 24.70 to − 15.74) for SDAI. For calculation of the 95% CI within each group, normal approximation was used if n ≥ 5, otherwise the exact method was used. The dagger indicates that adjustment was based on an analysis of covariance model with treatment as a factor and baseline values and DAS28 (CRP) stratification as covariates
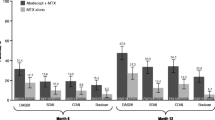
American College of Rheumatology (ACR) response rate at day 365 in Cohort 1 (a) and Cohort 2 (b). All randomized and treated patients were included in the analysis. In Cohort 1, the mean differences in ACR response rates between treatment arms were: ACR20, 29.0 (95% CI 10.1 to 47.9); ACR50, 18.6 (95% CI − 4.1 to 41.2); ACR70, 13.3 (95% CI − 6.7 to 33.4); ACR90: 5.7 (95% CI − 6.9 to 18.3). Estimates of difference and 95% CIs were based on the minimum risk weights method with randomization stratification of screening DAS28 (CRP). ACR20/50/70/90 ≥ 20/50/70/90% improvement in the ACR response criteria, SC subcutaneous
All randomized and treated patients were included in the analysis reported in Table 2. At day 365, in Cohort 1, the adjusted mean difference in pain score between the abatacept and adalimumab treatment arms was – 15.33 (95% CI – 26.30 to – 4.46), and at day 729 the adjusted mean difference in fatigue score between treatment arms was – 12.33 (95% CI – 23.83 to – 0.82).
At 1 year, more abatacept- than adalimumab-treated patients in Cohort 1 achieved DAS28 (CRP) of < 2.6 (Fig. 3). This was not seen at 2 years or in Cohort 2 at either time point. At 1 year and 2 years, more abatacept- than adalimumab-treated patients in Cohort 1 achieved remission according to CDAI, SDAI and Boolean criteria, a trend not seen in Cohort 2 (Fig. 4). Longitudinal analysis showed trends towards improved disease activity in Cohort 1 [Electronic Supplementary Material (ESM) Fig. S1].
Given the difference in mean weight between the abatacept and adalimumab groups in Cohort 1 at baseline, sensitivity analyses were performed that adjusted for baseline body weight; four patients with a body weight of > 100 kg were excluded from this analysis. These analyses demonstrated that patient body weight had minimal impact on treatment efficacy (data not shown).
Sensitivity analyses, limiting Cohort 2 (≥ 1 of disease duration ≤ 6 months, > 1 radiographic erosion and RF and/or ACPA seropositivity) to only patients with disease duration of ≤ 6 months (Cohort 3), showed that there were no differences between treatments in either pain or fatigue (ESM Table S1). There was a trend towards a benefit of abatacept over adalimumab for the outcomes of DAS28 (CRP) and CDAI (ESM Fig. S2); however, no conclusions could be drawn due to the small sample size.
No between-group differences were observed in mean change from baseline in mTSS or in the proportion of patients without radiographic progression at day 365 or day 729 in either cohort (data not shown).
The safety profiles between cohorts and treatment arms were consistent with that of the overall trial (Table 3).
Discussion
The AMPLE study recorded multiple clinical, patient-reported and radiographic outcomes through 2 years of blinded head-to-head treatment, allowing explorative analyses to be performed [6, 7]. In this post hoc analysis of patients with early RA and poor prognostic factors with an inadequate response to MTX across several outcome measures (except radiographs), abatacept showed a trend towards greater efficacy compared with adalimumab. Of note, a trend towards increased efficacy for abatacept compared with adalimumab on measures of remission and DAS28 (CRP) of < 2.6 in this difficult-to-treat, biologic-naive patient population was shown at 1 year.
The limitations of this analysis include its exploratory post hoc nature and the limited sample size. The ACR 1987 criteria were used to identify patients with RA. These criteria have a limited ability to identify patients with early RA; hence, there is a possibility of bias in the patient selection. However, the post hoc nature of the analysis allowed the inclusion of the 2015 ACR criteria that included disease/symptom duration of < 6 months in the definition of early RA [11]. This analysis may also be confounded by baseline differences in body weight, MTX dose and baseline DAS28 (CRP) score between patients receiving abatacept and adalimumab in Cohort 1. The differences in body weight (lower with abatacept) and MTX dose (higher with abatacept) could be explained by the limited sample size. Our analysis attempted to minimize the weight differences between groups, but we acknowledge that such differences relevant to RA may cause concern with our interpretation of the results.
The lack of differences in radiographic progression is not surprising since there was little radiographic progression noted in either treatment arm over the 2 years of blinded therapy. If differences exist, they may require more time or the use of a more sensitive detection methodology.
Conclusion
Although the sample size was relatively small, this post hoc analysis of the AMPLE trial indicated a trend towards improved disease activity and physical function with abatacept in comparison to adalimumab in biologic-naive patients with seropositive, erosive, early RA. Our principal goal was to study disease-specific factors known to be relevant to clinical decision-making to help generate hypotheses for testing in future clinical trials in RA. It is hoped that this will lead to the generation of patient-centric, definable biomarkers that will help to achieve more effective, personalized, rapid and safe care for a greater number of patients.
References
Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108.
Sokolove J, Johnson DS, Lahey LJ, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:813–21.
van der Helm-van Mil AHM, Verpoort KN, Breedveld FC, Toes RE, Huizinga TW. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R949–58.
Visser K, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. A matrix risk model for the prediction of rapid radiographic progression in patients with rheumatoid arthritis receiving different dynamic treatment strategies: post hoc analyses from the BeSt study. Ann Rheum Dis. 2010;69:1333–7.
Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–77.
Weinblatt ME, Schiff M, Valente R, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2013;65:28–38.
Schiff M, Weinblatt ME, Valente R, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis. 2014;73:86–94.
Sokolove J, Schiff M, Fleischmann R, et al. Impact of baseline anti-cyclic citrullinated peptide-2 antibody concentration on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial. Ann Rheum Dis. 2016;75:709–14.
Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1–26.
Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheumatol. 1988;31:315–24.
Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26.
van der Heijde DM, van Riel PL, Nuver-Zwart IH, Gribnau FW, vad de Putte LB. Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet. 1989;1:1036–8.
Bruynesteyn K, Boers M, Kostense P, van der Linden S, van der Heijde D. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change. Ann Rheum Dis. 2005;64:179–82.
Acknowledgements
The authors would like to thank the participants of this study. The authors are grateful to Joe Zhuo (Bristol-Myers Squibb, Princeton, NJ, USA) for helpful discussions. Under the direction of the authors, Sean Sheffler-Collins, PhD, at Caudex provided professional medical writing and editorial assistance for the development of this manuscript, funded by Bristol-Myers Squibb.
Funding
This study and the journal’s Rapid Service Fee were funded by Bristol-Myers Squibb, Princeton, NJ, USA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Roy Fleischmann, Michael Weinblatt, Harris Ahmad, Michael A. Maldonado, Evo Alemao and Michael Schiff contributed substantially to the conception or design of the study and the analysis or interpretation of the data. June Ye contributed to the acquisition and statistical analysis of the data.
Disclosures
Roy Fleischmann reports receiving grant/research support from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis and UCB; and consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Eli Lilly, Pfizer and Sanofi-Aventis. Roy Fleischmann is also the Editor-in-Chief of the journal. Michael Weinblatt reports receiving research grants from Amgen, Bristol-Myers Squibb, Crescendo Bioscience, UCB and Sanofi; and consulting fees from Amgen, Bristol-Myers Squibb, Crescendo Bioscience, UCB, AbbVie, Lilly, Pfizer and Roche. Harris Ahmad is an employee of Bristol-Myers Squibb and reports holding stock in Bristol-Myers Squibb. Michael A. Maldonado is an employee of Bristol-Myers Squibb and reports holding stock in Bristol-Myers Squibb. Evo Alemao is an employee of Bristol-Myers Squibb and reports holding stock in Bristol-Myers Squibb. June Ye is an employee of Bristol-Myers Squibb. Michael Schiff reports receiving consulting fees from AbbVie, Bristol-Myers Squibb, Eli Lilly, JNJ and UCB; and speaker fees from AbbVie and Bristol-Myers Squibb.
Compliance with Ethics Guidelines
The protocol was approved by the institutional review boards and independent ethics committees at the participating sites. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration, as revised in 2013, concerning human and animal rights, and Springer’s policy concerning informed consent has been followed. Informed consent was obtained from all individual participants included in the study.
Data Availability
The Bristol-Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to: https://doi.org/10.6084/m9.figshare.9896504.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any non-commercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fleischmann, R., Weinblatt, M., Ahmad, H. et al. Efficacy of Abatacept and Adalimumab in Patients with Early Rheumatoid Arthritis With Multiple Poor Prognostic Factors: Post Hoc Analysis of a Randomized Controlled Clinical Trial (AMPLE). Rheumatol Ther 6, 559–571 (2019). https://doi.org/10.1007/s40744-019-00174-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-019-00174-7