Abstract
The present work was done with dual intentions. The primary aim was to attenuate the acid corrosion of 6061 aluminum alloy by green approach. While doing so, an attempt was made to use the expiry date pharmaceutical product Streptomycin (SEPT) injection. This unique method could provide the best possible pathway for the utility of otherwise useless, non-eco-friendly expiry date drugs. Corrosion inhibition studies were performed using electrochemical techniques. Experimental settings were augmented to attain the highest possible efficacy of SEPT by changing its concentration and temperature. Experimental values were close-fitted into proper adsorption isotherm. Surface morphology studies like SEM and AFM were performed to confirm the adsorption of inhibitor. Streptomycin emerged as an exceptionally good inhibitor with an efficacy of 77% for the addition of just 1 ppm of the inhibitor at 303 K. Outcome of the work provided a unique pathway for utility expiry date drug, thereby minimizing the environmental pollution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Materials used in industries are expected to exhibit certain qualities, of which corrosion resistance lies at the forefront [1]. Even though commercially pure aluminium has the best corrosion resistance, its machinability and weldability are not up to the mark. [2].These properties are reported to be improved by proper alloying. Major alloying elements used to achieve desirable properties to the commercial aluminium are Cu, Mn, Mg, Si, etc. [3]. The leading engineering alloy group is the 6xxx series, wherein Mg and Si are added as alloying elements. Aluminum and aluminium alloys find extensive applications in aerospace, aviation, and other industries. However, besides being all these qualities, they tend to undergo pitting corrosion in a medium containing chloride ions.
In industry, the effectiveness of organic heterocyclic compounds as inhibitors is the first line of defense against corrosion. The organic inhibitors, which are mainly heterocyclic compounds, form a protective surface film that isolates and protects the metal surface. Usage of organic corrosion inhibitors is limited due to the implementation of environmental legislation throughout the world. Therefore, there is an increasing demand for green corrosion inhibitors that protect metals and alloys at low ecological risks. There is a continuous urge to use suitable corrosion inhibitive compounds that will not significantly react with the environmental components. Off the various green corrosion inhibitors, like plant products, surfactants, ionic liquids, biopolymers, the drugs are emerging as one of the best candidates for corrosion control. Being non-toxic with insignificant harmful ecological impact, drugs have materialized as appropriate candidates to supplant the usually used noxious corrosion inhibitors [4]. They are generally of biological origin, exhibit a high inhibition performance at relatively low concentration, and also are biodegradable in nature.
Eddy et al. have detailed in their report the copious benefits of drugs over other corrosion inhibitors [5]. In the last few decades, various classes of drugs were tried and tested for their anti-corrosive properties. Drugs are large heterocyclic compounds containing a large number of nitrogen, sulphur, and phosphorous as active centers. In addition to this, many reported drugs are found to be obtained from natural compounds [6,7,8].
Antibacterial drugs are a class of green corrosion inhibitors, which are reported to be verified to attenuate corrosion of engineering materials in various mediums [9, 10]. Antibacterial drugs such as Ampicillin, Cloxacillin, Flucloxacillin, and Amoxicillin were confirmed to be highly effective in preventing acid corrosion of aluminum.
However, certain issues need to be addressed before using drugs as corrosion inhibitors. The major problem is the cost and availability. The high cost associated with fresh drugs confines their useful applications. Therefore, it is not advisable to use any pharmaceutical products, which are having great demand for the patient community. However, when a drug approaches near expiry date or expiry date, it has no medical applications and can be used as corrosion inhibitors. This will solve one of the major environmental issues. Expiry date drugs are usually put in the wastebasket or incinerated. During incineration, a large amount of harmful gases are released, which in turn will contaminate the atmosphere [11]. Rather than throwing them in the garbage, they can be utilized as effective corrosion inhibitors. Thus, the utility of expiry date drugs ensures the safe handling of waste pharmaceutical products and offers a new pathway for the conservation of material. The work is undertaken successfully by utilizing the expiry date drug Streptomycin (injection) for corrosion mitigation of 6061 aluminum alloy.
2 Methodology
2.1 Material, Medium, and Inhibitor Solution
The corrosion study was conducted on 6061aluminum alloy, and its composition is as presented in Table 1.
Expiry date drug, Streptomycin [SEPT] (Abbott, LeeHPL Ventures Pvt ltd), was procured from a local medical practitioner by giving a valid explanation in writing. Streptomycin is a white-colored crystalline solid. It has large number of heteroatoms like oxygen and nitrogen. The presence of these heteroatoms makes Streptomycin a good candidate for corrosion inhibition. The added advantage is that Streptomycin injection is that it is highly soluble in water. This property makes this molecule a better inhibitor to study of aqueous phase corrosion. The molecular weight of streptomycin [SEPT] is 581.5 g dm−3 Inhibitor solution of streptomycin [SEPT] was prepared in 0.025 M HCl with its concentration ranging from 0.1 to 1 mg L−1.
2.2 Electrochemical Studies
Studies were done using an electrochemical workstation (CH604 E Series, U.S. model) using a three-electrode Pyrex cell. Experimental procedures described in the literature were implemented for performing potentiodynamic polarization (PDP) and impedance spectroscopy (EIS) techniques [12]. The potentiodynamic polarization method was done immediately after electrochemical impedance readings without additional surface treatment. In both cases, an average of 3–4 trials was conducted.
The potentiodynamic polarization plot obtained is a graph of current Vs. potential. Corrosion current density (icorr), found from the Tafel plot, was used for computing corrosion rate as given in Eq. (1)
Here, 3270: constant, M: atomic mass (27), ρ: density of the material (2.7 g dm−3), and z: number of electrons involved in corrosion (3).
2.3 Surface Studies
Surface studies of the alloy were done scanning electron microscopy [JEOL JSM─6380L.] In addition, the roughness of the surface was determined by Atomic Force Microscopy (AFM) studies [1B342 Innova model].
3 Results and Discussion
3.1 Potentiodynamic Polarization (PDP) Studies
Figure 1 depicts Tafel plots for corrosion of alloy at changing concentrations of SEPT at 313 K in 0.025 M HCl. Similar types of plots were obtained for other studied temperatures.
From PDP studies, various electrochemical parameters were obtained. The inhibition efficiency was calculated using Eq. (2)
where icorr and icorr(inh) are the corrosion current densities obtained for blank and inhibited solutions. Results are tabulated in Table 2.
In Fig. 1, a plateau was detected in the anodic Tafel curve in the potential range of − 0.7 to − 0.5 V. This accounts for the passivation occurring on the alloy surface. Hence, the only cathodic curve of Tafel plots was reasoned to take corrosion current density [12]. The addition of SEPT did not alter the cathodic slope. This observation suggested that SEPT controlled the corrosion of 6061 aluminium alloy without changing overall mechanistic aspects of corrosion. It simply obstructed the energy sites on 6061 aluminium alloy and formed a physical barrier, which prevents the corrosion of the metal [13, 14].
The maximum displacement of corrosion potential after the addition of inhibitor is in the range of + 60 mV. According to reported literature [15], inhibitors can be distinctly categorized as anodic or cathodic if the variation in potential is ± 85 mV, or else it performs as a mixed type. Experimental observation suggested that SEPT behaves as a mixed type of inhibitor with an additional hold on the mechanism of the anodic reaction.
The inhibitory action of SEPT improved with a rise in its concentration and declined with an escalation in temperature. The observed variation of the efficacy of inhibitor with temperature advocated the prospect of physical adsorption of SEPT on 6061 aluminium alloy. Maximum efficacy of 78% was perceived for accumulation of 1.0 mg L−1 of SEPT at 303 K.
3.2 Electrochemical Impedance Spectroscopy (EIS) Studies
Impedance spectra for 6061 aluminum alloy in 0.025 M HCl with and without the inhibitor at 313 K is illustrated in Fig. 2. The impedance plot is comprised of two loops. They are a capacitive loop in high frequency (HF) and an inductive loop on the medium (MF) frequency side. A high-frequency capacitive loop indicates resistance for charge transfer [16,17,18,19,20] dring process of corrosion and inhibition process. Greater the resistance for charge transfer grater will be the diameter of the semicircle. The diameter of the semicircle, after the addition of SEPT enlarged when compared with blank. This is further increased with the increase in concentration SEPT. This established the capability of SEPT to resist the dissolution of 6061 aluminum alloy.
The inductive loop was observed in the medium frequency (MF) region. In the absence of SEPT, an inductive loop is primarily due to the buildup of products of corrosion like Al2O3. With incremental adding up of SEPT, the inductive loop embodies the firm layer of inhibitor, which got deposited on the surface of the metal. The complex formed between SEPT and 6061 aluminium alloy gets adsorbed on the surface of the alloy, forming a tough adherent layer. This results in the development of a resilient physical obstruction amongst metal and corrosive.
An equivalent circuit with five elements was proposed using ZimpWin software 3.1. The experimental data obtained from EIS studies is fitted to an equivalent circuit model as shown in Fig. 3. Of the 5 elements, solution resistance (RS) is minimal and denotes the resistance offered by the solution. Other components being charge transfer resistance (Rct), inductive resistance (RL), inductive element (L). and constant phase element, (Q). Equations (3) and (4) were applied to compute polarization resistance (RP) as well as double-layer capacitance (Cdl). The outcome of these is presented in Table 3.
fmax denotes frequency where an imaginary component of the impedance is maximum.
From Table 3, it is evident that, after the addition of SEPT, polarization resistance became greater and double layer capacitance became lesser when compared with a blank solution. The increased polarization resistance results from resistance of the corrosive towards metal dissolution after the addition of SEPT. At the interface between metal and corrosive due to the formation of physical barrier, the thickness of the electrical double layer increases. Consequently, the capacitance of the electrical double layer decreases [21].
3.3 Temperature Effect
The activation energy (Ea) was evaluated by means of Arrhenius Eq. (5) and enthalpy of activation \(( \Delta {H}_{\mathrm{a}}\)) and entropy of activation\(( \Delta {S}_{\mathrm{a}}\)) by Transition state Eq. (6).
with B as a constant which is determined by nature of metal, R = the universal gas constant (8.314 J K−1 mol−1), h = planks constant (6.626 × 10–34 J s−1), N = Avogadro’s number (6.023 × 1023), Fig. 4a and b depicts a graphical representation of Eqs. (5) and (6) respectively. Calculated kinetic parameters for the formation of the activated complex are tabulated in Table 4.
The addition of SEPT improved activation energy (Ea). The energy of activation enhanced with further incremental addition of SEPT. An increase in energy of activation is due to a decrease in the corrosion rate. Added inhibitor expected to form an obstruction between alloy and corrosive, thereby impeding the metal dissolution. Adsorption of SEPT prevents the transfer of charge from metal and thus hinders its dissolution. Since the enthalpy of activation values was almost the same as Ea, it proves that SEPT adsorbed onto the metal surface via physisorption [22,23,24]. The endothermic nature of the corrosion and inhibition process was proved by positive values of enthalpy of activation [25]. Increased value of ∆Sa for inhibited solution suggested an increase of orderliness reactants while forming activated complex [26].
3.4 Adsorption Consideration
The degree of surface coverage (\(\theta )\) is acquired from PDP studies. These values were fixed into a number of adsorption isotherms. SEPT was found to adsorb over 6061 aluminium alloy by conforming Langmuir adsorption isotherm model. It is exemplified by Eq. (7). Graphical illustration of this is revealed in Fig. 5.
Adsorption/desorption equilibrium constant (K) was computed from the intercept of adsorption isotherm. It is related to the standard free energy of adsorption (\(\Delta {G^\circ }_{\mathrm{ads}})\) by Eq. (8) [27].
Gibbs–Helmholtz Eq. (9) was applied to obtain standard enthalpy of adsorption \((\Delta {H^\circ }_{\mathrm{ads}}\)) and standard entropy of adsorption (\(\Delta {S^\circ }_{\mathrm{ads}})\). Graphical representation of this is shown in Fig. 6. Thermodynamic parameters are tabularized in Table 5.
The spontaneity of adsorption of SEPT on alloy surface is conveyed by a large negative value of free energy of adsorption (\(\Delta G\)0ads) [28]. Standard free energy of adsorption (\(\Delta G\)0ads), which is in between − 20 and − 40 kJ mol−1 is evocative of prospect of both physical and chemical adsorption of SEPT on 6061 aluminium alloy. However negative value of enthalpy of adsorption (\(\Delta H\)0ads) is suggestive of predominate physisorption of the inhibitor. As per the repored literature [29], chemical adsorption univocally will have positive value of enthalpy of adsorption \((\Delta H\)0ads). Therefore, it can be inferred that SEPT predominantly underwent physical adsorption over the alloy surface.
3.5 Surface Morphology Studies
3.5.1 AFM Analysis
Figure 7. shows three sets three-dimensional images of 6061 aluminium alloy, one that is Freshly polished, one that is immersed in 0.025 M HCl, and the third one that is immersed in the acid medium containing the 1 ppm of inhibitor, respectively. Table 6 gives the three sets of values corresponding to the three metal coupons, namely, surface roughness (average) (Ra), root mean square roughness (Rq), and Rmax. From Table 6, it is obvious that the three values for the inhibited sample are much less compared with the uninhibited sample. Therefore, the AFM analysis confirmed the adsorption SEPT over aluminum alloy.
3.5.2 SEM Analysis
SEM picture of 6061 aluminium alloy is set in Fig. 8a. This demonstrates the smooth surface of the alloy with limited unevenness, probably arised due to the scratching effect during polishing. In contact with 0.025 M HCl, SEM image appears as shown in Fig. 8b. Here alloy has appeared to become heterogeneous. Heterogeneity and the pits are due to the interaction of the metal and the corrosive medium. Figure 8c displays the even surface of 6061 aluminium alloy after the accumulation of 1.0 mg L−1of SEPT. This is essentially due to the accumulation of SEPT on the exterior of the alloy. Inhibitor films result in the development of a physical barricade between the metal and corrosive medium. This barrier film prevents metal dissolution.
3.6 Mechanistic Perspectives
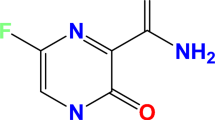
The structure of SEPT is as shown in Fig. 9. It contains heteroatoms like nitrogen and oxygen. They are high electron density active centers of the molecule.
pH at which aluminum acquires zero charges (pHZch) is 9.1, and aluminium is expected to possess a positive charge at a pH lower than this. Alloy with a positive charge at its surface draws the negatively charged chloride ions from medium and forms an electrical double layer at the boundary of two. SEPT remains protonated in the acid medium and gets attracted by positive charges at the periphery of the alloy. An electrostatic force of attraction starts operating between these. Consequently, SEPT will form an adherent, continuous layer on the surface of the alloy. This nature of shielding avoids metal from uninterrupted interaction with the destructive surroundings and diminishes material loss. Therefore SEPT physically gets adsorbs onto the alloy surface. It is illustrated in the following Fig. 10.
SEPT emerged as an effective eco-friendly corrosion inhibitor when compared with a few reported drugs. For, e.g., Cefacetrile [11] was reported to be an excellent inhibitor for corrosion control of steel with an inhibition efficiency of about 95% for the addition of 100 ppm of the inhibitor. Ampicillin, Cloxacillin, Flucloxacillin, Amoxicillin were tested as anticorrosive agents for aluminum in 2 M HCl [30]. For all the four inhibitors, an average of 80% inhibition efficiency was reported for the addition of 1000 ppm of inhibitors. However, we could get the efficacy of 78% for meager addition of 1.0 mg L−1 (1 ppm) of SEPT.
4 Conclusions
-
The inhibition efficiency of 78% was achieved for adding 1.0 mg L−1 of SEPT at 303 K.
-
SEPT performed as a mixed inhibitor, controlling both anodic and cathodic reactions
-
SEPT adsorbed through physisorption and followed Langmuir adsorption isotherm.
-
Surface studies established the adsorption of SEPT onto the surface of the alloy.
-
Conservation of material, as well as the environment, was achieved from the study.
References
Monticelli C, Zucchi F, Brunoro G, Trabanelli G (1997) Corrosion and corrosion inhibition of alumina particulate/aluminium alloys metal matrix composites in neutral chloride solutions. J Appl Electrochem 27:325–334
Davis JR (2001) Aluminum and aluminum alloys. ASM Int. https://doi.org/10.1361/autb2001p351
Charitha BP, Rao P (2017) Carbohydrate biopolymer for corrosion control of 6061 Al-alloy and 6061Aluminum-15%(v) SiC(P) composite—green approach. Carbohydr Polym 168:337–345. https://doi.org/10.1016/j.carbpol.2017.03.098
Gece G (2011) Drugs: a review of promising novel corrosion inhibitors. Corros Sci 53:3873–3898. https://doi.org/10.1016/j.corsci.2011.08.006
Odoemalam SA, Eddy NO (2008) Norfloxacin and Sparfloxacin as corrosion inhibitors for zinc. Mater Sci 4:1–9
Ebenso EE, Eddy NO, Odiongenyi AO (2009) Corrosion inhibition and adsorption properties of methocarbamol on mild steel in acidic medium. Port Electrochim Acta 27:13–22
Eddy NO, Ebenso EE (2010) Adsorption and quantum chemical studies on cloxacillin and halides for the corrosion of mild steel in acidic medium. Int J Electrochem Sci 5:731–750
Eddy NO, Ebenso E, Ibok UJ (2010) Adsorption, synergistic inhibitive effect and quantum chemical studies of ampicillin (AMP) and halides for the corrosion of mild steel in H2SO4 Adsorption, synergistic inhibitive effect and quantum chemical studies of ampicillin (AMP) and halides fo. J Appl Electrochem 40:445–456. https://doi.org/10.1007/s10800-009-0015-z
Akpan IA, Offiong NO (2013) Inhibition of mild steel corrosion in hydrochloric acid solution by ciprofloxacin drug. Int J Corros 2013:1–6
Naqvi I, Saleemi AR, Naveed S (2011) Cefixime: a drug as efficient corrosion inhibitor for mild steel in acidic media. Electrochem Thermodyn Stud 6:146–161
Singh AK, Shukla SK, Ebenso E (2011) Cefacetrile as corrosion inhibitor for mild steel in acidic media. Int J Electrochem Sci 6:5689–5700
Charitha BP, Rao P (2018) Pullulan as a potent green inhibitor for corrosion mitigation of aluminum composite: electrochemical and surface studies. Int J Biol Macromol 112:461–472. https://doi.org/10.1016/j.ijbiomac.2018.01.218
Khadiri A, Saddik R, Bekkouche K, Aouniti A, Hammouti B, Benchat N, Bouachrine M, Solmaz R (2016) Gravimetric, electrochemical and quantum chemical studies of some pyridazine derivatives as corrosion inhibitors for mild steel in 1 M HCl solution. J Taiwan Inst Chem Eng 58:552–564. https://doi.org/10.1016/j.jtice.2015.06.031
Abd El-Maksoud SA, Fouda AS (2005) Some pyridine derivatives as corrosion inhibitors for carbon steel in acidic medium. Mater Chem Phys 93:84–90. https://doi.org/10.1016/j.matchemphys.2005.02.020
Li WH, He Q, Zhang ST, Pei CL, Hou BR (2008) Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium. J Appl Electrochem 38:289–295. https://doi.org/10.1007/s10800-007-9437-7
Brett CMA (1990) The application of electrochemical impedance techniques to aluminium corrosion in acidic chloride solution. J Appl Electrochem 20:1000–1003. https://doi.org/10.1007/BF01019579
Metikos-Hukovic M, Babic R, Grubac Z (1998) Corrosion protection of aluminium in acidic chloride solutions with nontoxic inhibitors. J Appl Electrochem 28:433–439. https://doi.org/10.1023/A:1003200808093
Frers SE, Stefenel MM, Mayer C, Chierchie T (1990) AC-impedance measurements on aluminium in chloride containing solutions and below the pitting potential. J Appl Electrochem 20:996–999. https://doi.org/10.1007/BF01019578
Brett CMA (1992) On the electrochemical behaviour of aluminium in acidic chloride solution. Corros Sci 33:203–210. https://doi.org/10.1016/0010-938X(92)90145-S
Umoren SA, Obot IB, Ebenso EE, Okafor PC, Ogbobe O, Oguzie EE (2006) Gum arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics. Anti-Corros Methods Mater 53:277–282. https://doi.org/10.1108/00035590610692554
Khaled KF (2008) Guanidine derivative as a new corrosion inhibitor for copper in 3% NaCl solution. Mater Chem Phys 112:104–111. https://doi.org/10.1016/j.matchemphys.2008.05.052
Singh AK, Quraishi MA (2010) Effect of Cefazolin on the corrosion of mild steel in HCl solution. Corros Sci 52:152–160. https://doi.org/10.1016/j.corsci.2009.08.050
Mansfeld F, Lin S, Kim K, Shih H (1987) Pitting and surface modification of SIC/Al. Corros Sci 27:997–1000. https://doi.org/10.1016/0010-938X(87)90065-5
Ashassi-Sorkhabi H, Shaabani B, Seifzadeh D (2005) Corrosion inhibition of mild steel by some schiff base compounds in hydrochloric acid. Appl Surf Sci 239:154–164. https://doi.org/10.1016/j.apsusc.2004.05.143
Wang HL, Bin LR, Xin J (2004) Inhibiting effects of some mercapto-triazole derivatives on the corrosion of mild steel in 1.0 M HC1 medium. Corros Sci 46:2455–2466. https://doi.org/10.1016/j.corsci.2004.01.023
Sahin M, Bilgic S, Yilmaz H (2002) The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl mediums. Appl Surf Sci 195:1–7. https://doi.org/10.1016/S0169-4332(01)00783-8
Biswas A, Pal S, Udayabhanu G (2015) Experimental and theoretical studies of xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in 15% HCl. Appl Surf Sci 353:173–183. https://doi.org/10.1016/j.apsusc.2015.06.128
Hosseini M, Mertens SFL, Arshadi MR (2003) Synergism and antagonism in mild steel corrosion inhibition by sodium dodecylbenzenesulphonate and hexamethylenetetramine. Corros Sci 45:1473–1489. https://doi.org/10.1016/S0010-938X(02)00246-9
Belkhaouda M, Bammou L, Zarrouk A, Salghi R, Ebenso EE, Zarrok H, Hammouti B, Bazzi L, Warad I (2013) Inhibition of c-steel corrosion in hydrochloric solution with chenopodium ambrorsioides extract. Int J Electrochem Sci 8:7425–7436
Abdallah M (2004) Antibacterial drugs as corrosion inhibitors for corrosion of aluminium in hydrochloric solution. Corros Sci 46:1981–1996. https://doi.org/10.1016/j.corsci.2003.09.031
Acknowledgements
Authors acknowledge lab facilities provided by the Department of Chemistry, MIT, MAHE, Manipal, and also CIF MIT, MAHE Manipal for giving the instrumental facilities for surface morphology studies.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fathima, H., Pais, M. & Rao, P. The Use of Green Inhibitors in Evaluating the Safe Expiry Dates of Therapeutics. J Bio Tribo Corros 7, 108 (2021). https://doi.org/10.1007/s40735-021-00544-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-021-00544-1