Abstract
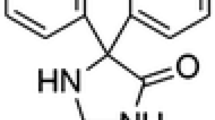
Seroquel drug was studied for corrosion inhibition of low carbon steel in 1 M HCl solution by using experimentally with optimized concentrations at an elevated temperature range of 303–333 K. The inhibition efficiency was studied in the absence and presence of Seroquel by weight loss, electrochemical impedance spectroscopy and potentiodynamic polarization measurements. The inhibition effect of the inhibitor increases with the increasing concentration of Seroquel. Tafel polarization studies revealed that the inhibitor acts as mixed type inhibitor. The inhibition effect was attributed due to the adsorption process on low carbon steel and it obeys Temkin adsorption isotherm. The effect of temperature with the inhibition effect of inhibitor from the solution was examined by activation and thermodynamic parameters. Surface morphology of the corroded specimens were studied by SEM technique and IR spectrum of the corrosion product shows the influence of the Seroquel for corrosion inhibition.










Similar content being viewed by others
References
Thirumalairaj B, Jaganathan M (2016) Corrosion protection of mild steel by a new binary inhibitor system in hydrochloric acid solution. Egypt J Pet 25:423–432
Kolo AM, Sani UM, Kutama IU, Usman U (2016) Adsorption and inhibitive properties of Januvia for the corrosion of zinc in 0.1 M HCl. Pharm Chem J 3:109–119
Aziz RJ (2016) Study of some drugs as corrosion inhibitors for mild steel in 1 M H2SO4 solution. Int J Curr Pharm Sci 3:1–7
Fouda AAS, Rashwan SM, Abd El-Aal NF, Ramadan NH (2016) Unused augmentin drug as save corrosion inhibitor for α-brass in nitric acid solution. Zastita Mater 57:326–338
Andreani S, Znini M, Paolini J, Majidi L, Hammouti B, Costa J, Muselli A (2016) Study of corrosion inhibition for mild steel in hydrochloric acid solution by Limbarda crithmoides (L.) essential oil of Corsica. J Mater Environ Sci 7:187–195
Pavithra MK, Venkatesha TV, Punith Kumar MK, Anantha NS. Electrochemical, gravimetric and quantum chemical analysis of mild steel corrosion inhibition by colchicines in 1 M HCl medium. Res Chem Intermed. https://doi.org/10.1007/s11164-015-2158-3
Al-Shafey HI, Abdel Hameed RS, Ali FA, Aboul-Magd AE-AS, Salah M (2014) Effect of expired drugs as corrosion inhibitors for carbon steel in 1 M HCl solution. Int J Pharm Sci Rev Res 27:146–152
Wang J, Xu S-A (2016) The inhibition effect of a novel Mannich base on the corrosion of A3 mild steel in 1.0 M hydrochloric acid solution. Int J Electrochem Sci 1:2621–2637
Narayana Hebbar, Praveen BM, Prasanna BM, Sachin HP (2018) Anticorrosion potential of flectofenine on mild steel in hydrochloric acid media: experimental and theoretical study. J Fail Anal Prev 18:371–381
Shylesha BS, Venkatesha TV, Praveen BM (2011) Ziprasidone as a corrosion inhibitor for zinc in different acid medium. J Chem Pharm Res 3:501–507
Nataraja SE, Venkatesha TV, Tandon HC, Shylesha BS (2011) Quantum chemical and experimental characterization of the effect of ziprasidone on the corrosion inhibition of steel in acid media. Corros Sci 53:4109–4117
Lgaz H, Salghi R, Jodeh S, Hammouti B (2016) Effect of clozapine on inhibition of mild steel corrosion in 1.0 M HCl medium. J Mol Liq. https://doi.org/10.1016/j.molliq.2016.11.039
Ameh PO, Sani UM (2016) Cefuroxime axetil: a commercially available drug as corrosion inhibitor for aluminium in hydrochloric acid solution. Electrochim Acta 34:131–141
Fouda AS, Rashwan SM, Abd El-Aal NF, Ramadan NH (2016) Corrosion protection and adsorption properties of amoxicillin as save corrosion inhibitor for α- brass in nitric acid solution. J Chem Pharm Res 8:705–715
Fouda AS, El-Ewady G, Ali AH. Modazar as promising corrosion inhibitor of carbon steel in hydrochloric acid solution. Green Chem Lett Rev. https://doi.org/10.1080/17518253.2017.1299228
Prasanna BM, Praveen BM, Narayana Hebbar, Venkatesha TV, Tandon HC, Abd Hamid SB (2017) Electrochemical study on inhibition effect of Aspirin on mild steel in 1 M hydrochloric acid. J Assoc Arab Univ Basic Appl Sci 22:62–69
Prasanna BM, Praveen BM, Narayana Hebbar, Venkatesha TV (2016) Experimental and theoretical studies of hydralazine hydrochloride as corrosion inhibitor for mild steel. Anticorros Mater Methods 63:47–55
Praveen BM, Prasanna BM, Narayana Hebbar, Shivakeshava Kumar P, Jagadeesh MR (2018) Experimental and theoretical studies on inhibition effect of the Praziquantel on mild steel corrosion in 1 M HCl. J Bio Tribo-Corros 4:21
Narayana Hebbar, Prasanna BM, Venkatesha TV (2015) Corrosion inhibition behavior of ketosulfone for zinc in acidic medium. J Fundam Appl Sci 7:271–289
Naqvi I, Saleemi AR, Naveed S (2011) Cefixime: a drug as efficient corrosion inhibitor for mild steel in acidic media. Electrochemical and thermodynamic studies. Int J Electrochem Sci 6:146–161
Fouda AS, Mahmoud WM, Abdul Mageed HA (2016) Evaluation of an expired non toxic amoldipine besylate drug as a corrosion inhibitor for low carbon steel in hydrochloric acid solutions. J Bio Tribo Corros 7:2–11
Abdel Hameed RS, Ismail EA, Abu Nawwas AH, Al-Shafey HI (2015) Expired Voltaren drugs as corrosion inhibitor for aluminium in hydrochloric acid. Int J Electrochem Sci 10:2098–2109
Abdel Hameed RS (2011) Rantidine drugs as non toxic corrosion inhibitors for mild steel in hydrochloric acid medium. Port Electrochim Acta 29:273–285
Al-Amiery AA, Binti Kassim FA, Kadhum AA, Mohamad AB. Synthesis and characterization of a novel eco-friendly corrosion inhibition for mild steel in 1 M hydrochloric acid. Sci Rep. https://doi.org/10.1038/srep19890
Fouda AS, El Morsi MA, El Mogy T (2017) Studies on the inhibition of carbon steel corrosion in hydrochloric acid solution by expired Carvedilol drug. Green Chem Lett Rev 10:336–345
Zhao Q, Tang T, Tang P, Zhang Z, Wang F (2017) The corrosion inhibition effect of triazinedithiol inhibitors for aluminium alloy in a 1 M HCl. Solut Met 7:44
Prasanna BM, Praveen BM, Narayana Hebbar, Venkatesha TV (2015) Corrosion inhibitory action of mild steel in 1 M HCl by Chlorophenicol. Moroc J Chem 3:824–837
Karthi G, Sundaravadivelu M (2016) Studies on the inhibition of mild steel corrosion in hydrochloric acid solution by atenolol drug. Egypt J Pet 25:183–191
Obi-Egbedi NO, Obot IB (2011) Inhibitive properties, thermodynamic and quantum chemical studies of alloxazine on mild steel corrosion in H2SO4. Corros Sci 53:263–275
Obi-Egbedi NO, Essien KE, Obot IB (2011) Computational simulation and corrosion inhibitive potential of alloxazine for mild steel in 1 M HCl. J Comput Methods Mol Des 1:26–43
Singh AK, Quraishi MA (2010) The effect of some bis-thiadiazole derivatives on the corrosion of mild steel in hydrochloric acid. Corros Sci 52:1373–1385
Safak S, Duran B, Yurt A, Turkoglu G (2012) Schiff bases as corrosion inhibitor for aluminium in HCl solution. Corros Sci 54:251–259
Rodriguez-Torres A, Olivares-Xometl O, Valladares-Cisneros MG, Gonzalez-Rodriguez JG (2018) Effect of green corrosion inhibition by Prunus persica on AISI 1018 carbon steel in 0.5 M H2SO4. Int J Electrochem Sci 13:3023–3049
Wang K, Lai C, Tan B, Xie B, Zhu S, Zhu H, Liu K, Wei J (2018) Corrosion inhibition of mild steel by S-benzyl-O,O′-dialkyldithiophosphates in HCl solution. Int J Electrochem Sci 13:2627–2640
Abd El-Lateef HM (2015) Experimental and computational investigation on the corrosion inhibition characteristics of mild steel by some novel synthesized imines in hydrochloric acid solutions. Corros Sci 92:104–117
Manimegalai S, Manjula P (2015) Thermodynamic and adsorption studies for corrosion inhibition of mild steel in aqueous media by Sargasam swartzii (brown algae). J Mater Environ Sci 6:1629–1637
Narayana Hebbar, Praveen BM, Prasanna BM, Venkatesha TV (2015) The corrosion inhibition effect of Hydralazine·HCl on the zinc in acidic media. Moroc J Chem 3:496–506
Prasanna BM, Praveen BM, Narayana Hebbar, Venkatesha TV, Tandon HC (2014) Ketosulfone drug as a green corrosion inhibitor for mild steel in acidic medium. Ind Eng Chem Res 53:8436–8444
Idouhli R, N’Ait Ousidi A, Koumya Y, Abouelfida A, Benyaich A, Auhmani A, Itto MYA. Electrochemical studies of monoterpenic thiosemicarbazones as corrosion inhibitor for steel in 1 M HCl. Hindwai Int J Corros. https://doi.org/10.1155/2018/9212705
Aiad I, Shaban SM, Elged AH, Aljoboury OH. Cationic surfactant based on alignate as green corrosion inhibitors for the mild steel in 1.0 M HCl. https://doi.org/10.1016/j.ejpe.2018.01.003
Zhou L, Lv Y-L, Hu Y-X, Zhao J-H, Xia X, Li X (2018) Experimental and theoretical investigations of 1,3,5-tris(4-aminophenoxy) benzene as an effective corrosion inhibitor for mild steel in 1 M HCl. J Mol Liq 249:179–187
El Hafi M, Ezzanad A, Boulhaoua M, El Ouasif L, Saadouni M, El Aoufir Y, Ramli Y, Zarrouk A, Oudda H, Essassi EM (2018) Corrosion inhibition effect of novel pyrazolo [3, 4-d] pyramidine derivative on mild steel in 1 M HCl medium. Experimental and theoretical approach. J Mater Environ Sci 9:1234–1246
Prasanna BM, Praveen BM, Narayana Hebbar, Venkatesha TV, Tandon HC (2016) Inhibition study of mild steel corrosion in 1 M hydrochloric acid solution by 2-chloro 3-formyl quinolone. Int J Ind Chem 7:9–19
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Swetha, G.A., Sachin, H.P., Guruprasad, A.M. et al. Use of Seroquel as an Effective Corrosion Inhibitor for Low Carbon Steel in 1 M HCl. J Bio Tribo Corros 4, 57 (2018). https://doi.org/10.1007/s40735-018-0173-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-018-0173-9