Abstract
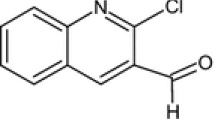
The inhibition effect of Flectofenine on corrosion of mild steel in 1M HCl solution was investigated by using traditional weight loss method and electrochemical techniques at different concentrations and temperatures. Adsorption of the inhibitor follows a Langmuir adsorption isotherm studied at the temperatures of 303–333 K. The change in free energy and change in enthalpy explain the mode of the adsorption. Activation energy values, apparent enthalpy changes and apparent entropy changes explain the corrosion process. The mechanism of inhibition was discussed.









Similar content being viewed by others
References
M.A. Dahmani, S.S. Et-Touhami, B. Al-Deyab, A. Hammouti, Bouyanzer, corrosion inhibition of C38 steel in 1M HCl: a comparative study of black pepper extract and its isolated pipe rine. Int. J. Electrochem. Sci. 5, 1060–1069 (2010). (in English)
B.M. Praveen, T.V. Venkatesha, Metol as corrosion inhibitor for steel. Int. J. Electrochem. Sci. 4, 267–275 (2009). (in English)
D. Chebabe, Z. Ait Chikh, N. Hajjaji, Corrosion inhibition of Armco iron in 1M HCl solution by alkyltriazoles. Corros. Sci. 45, 309–320 (2003). (in English)
J.M. Bastidas, J.L. Polo, E. Cano, Substitutional inhibition mechanism of mild steel hydrochloric acid corrosion by hexylamine and dodecylamine. J. Electrochem. Soc. 30, 1173–1182 (2000). (in English)
M.A. Migahed, A.M. Abdul-Raheim, A.M. Atta, W. Brostow, Synthesis and evaluation of a new water soluble corrosion inhibitor from recycled poly (ethylene) terphethalate. Mater. Chem. Phys. 195, 3590–3596 (2010). (in English)
D. Asefi, M. Arami, N.M. Mahmoodi, Electrochemical effect of cationic Gemini surfactant and halide salts on corrosion inhibition of low carbon steel in acid medium. Corros. Sci. 52, 1801–1808 (2010). (in English)
G.N. Mu, X.H. Li, Inhibition of cold steel corrosion by Tween-20 in sulfuric acid: weight loss, electrochemical and AFM approaches. J. Colloid Interf. Sci. 289, 184–192 (2005)
N.V. Likhanova, M.A. Domínguez-Aguilar, O. Olivares-Xemetl, N. Nava-Entzana, E.H. Arce, Dorantes, The effect of ionic liquids with imidazolium and pyridinium cations on the corrosion inhibition of mild steel in acidic environment. Corros. Sci. 52, 2088–2097 (2010). (in English)
H.A. Videla, L.K. Herrera, Understanding microbial inhibition of corrosion-A comprehensive overview. Int. Biodeter. Biodegr. 63, 896–900 (2010). (in English)
K.F. Khaled, Experimental and atomistic simulation studies of corrosion inhibition of copper by a new benzotriazole derivative in acid medium. Electrochim. Acta. 54, 4345–4352 (2009). (in English)
T. Arslan, F. Kandemirli, E.E. Ebenso, L. Love, H. Alemu, Quantum chemical studies on the corrosion inhibition of some sulphonamides on mild steel in acidic medium. Corros. Sci. 51, 35–47 (2009). (in English)
R. Solmaz, G. Kardas, B. Yazici, M. Erbil, Adsorption and corrosion inhibitive properties of 2-Amino-5-Mercapto-1,3,4-Thiadizole on mild steel in hydrochloric acid media. Colloid. Surf. 312, 7–17 (2008). (in English)
N. Shankaresha, T.V. Venkatesha, G. Achary, B.M. Praveen, Y. Arthoba Naik, T.V. Venkatesha, Corrosion behaviour of surface modified steel by condensation product. Bull. Electrochem. 23, 123–127 (2007). (in English)
B.S. Shylesha, T.V. Venkatesha, G. Harshini, B.M. Praveen, Veratraldehyde as corrosion inhibitor for mild steel in different acid medium. J. chem. Chem. Eng. 4, 1934–7375 (2010). (in English)
B.S. Shylesha, T.V. Venkatesha, B.M. Praveen, A.V. Shanbhag, Corrosion Inhibition studies of mild steel by new inhibitor in different corrosive medium. Res. J. chem. sci. 1, 46–50 (2011). (in English)
R.A. Prabhu, T.V. Venkatesha, A.V. Shanbhag, B.M. Praveen, G.M. Kulkarni, R.G. Kalkhambkar, Quinol-2-thione compounds as corrosion inhibitors for mild steel in acid solution. Mat. Chem Phy. 108, 283–289 (2008). (in English)
I.B. Obot, N.O. Obi-Egbedi, Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: experimental and theoretical investigation. Corros. Sci. 52, 198–204 (2010). (in English)
I.B. Obot, N.O. Obi-Egbedi, S.A. Umoren, Antifungal drugs as corrosion inhibitors for aluminium in 0.1M HCl. Corros. Sci. 51, 1868–1875 (2009). (in English)
J. Ishwara Bhat, D. Vijaya Alva, Meclizine hydrochloride as a potential non-toxic corrosion inhibitor for mild steel in hydrochloric acid medium. Arch. Appl. Sci. Res. 3, 343–356 (2011). (in English)
A.S. Fouda, F. Al-Sarawy, H.M.El-Abbasy Sh-Ahmed, Corrosion inhibition of aluminum 6063 using some pharmaceutical compounds. Prot. Met. Phy. Chem. Surfaces. 45, 635–643 (2009). (in English)
M.M. Saleh, A.A. Atia, Effects of structure of the ionic head of cationic surfactant on its inhibition of acid corrosion of mild steel. J. Appl. Electrochem. 36, 899–905 (2006). (in English)
H. Jafari, I. Danaee, H. Eskandari, M.R. Avei, Electrochemical and theoretical studies of adsorption and corrosion inhibition of N, N′-Bis (2-hydroxyethoxy acetophenone)-2,2-dimethyl-1,2-propanediimine on low carbon steel (API 5L Grade B) in acidic media. Ind. Eng. Chem. Res. 52, 6617–6632 (2013)
K. Benbouya, I. Forsal, M. Elbakri, T.R. Anik Touir, M. Bennajah, M. Chebab, D. Rochdi, A. Mernari, B. Ebn Touhami, Influence of pyridazine derivative on corrosion inhibition of mild steel in acidic media. Res. Chem. Intermed. https://doi.org/10.1007/s11164-013-1037. (in English)
R.T. Loto, C.A. Loto, T. Fedotova, Electrochemical studies of mild steel corrosion inhibition in sulfuric acid chloride by aniline. Res. Chem. Intermed. https://doi.org/10.1007/s11164-013-1055. (in English)
R.T. Loto, Corrosion inhibition of mild steel in acidic medium by butyl alcohol. Res. Chem. Intermed. https://doi.org/10.1007/s11164-013-1088-1. (in English)
P. Lowmunkhong, D. Ungthararak, P. Sutthivaiyakit, Tryptamine as a corrosion inhibitor of mild steel in hydrochloric acid solution. Corros. Sci. 52, 30–36 (2010). (in English)
A.K. Singh, M.A. Quraishi, Effect of Cefazolin on the corrosion of mild steel in HCl solution. Corros. Sci. 52, 152–160 (2010). (in English)
A. Popova, M. Christov, S. Raicheva, E. Sokolova, Adsorption and inhibitive properties of benzimidazole derivatives in acid mild steel corrosion. Corr. Sci. 46, 1333–1350 (2004). (in English)
C.B. Pradeep Kumar, K.N. Mohana, Adsorption and thermodynamic characteristics of plumeria rubra plant extracts on mild steel corrosion in industrial water medium. Int. Res. J. Pure Appl. Chem. 3, 330–346 (2013). (in English)
A.K. Singh, Inhibition of mild steel corrosion in hydrochloric acid solution by 3-(4-((Z)-Indolin-3-ylideneamino) phenylimino)indolin-2-one. Ind. Eng. Chem. Res. 51, 3215–3223 (2012)
I. Naqvi, A.R. Saleemi, S. Naveed, Cefixime: a drug as efficient corrosion inhibitor for mild steel in acidic media: electrochemical and thermodynamic studies. Int. J. Electrochem. Sci. 6, 146–161 (2011). (in English)
T. Poornima, J. Nayak, N.A. Shetty, 3,4-Dimethoxybenzaldehydethiosemicarbazone as corrosion inhibitor for aged 18 Ni 250 grade maraging steel in 0.5 M sulfuric acid. J. Appl. Electrochem. 41, 223–233 (2011). (in English)
M.K. Pavithra, T.V. Venkatesha, M.K. Punith Kumar, H.C. Tondan, Inhibition of mild steel corrosion by Rabeprazole sulfide. Corr. Sci. 60, 104–111 (2012). (in English)
H.Z. Al-Sawaad, Evaluation of the ceftriaxone as corrosion inhibitor for carbon steel alloy in 0.5 M of hydrochloric acid. Int. J. Electro. Sci. 8, 3105–3120 (2013). (in English)
I.B. Obot, N.O. Obi-Egbedi, A.O. Eseola, Anticorrosion potential of 2-Mesityl-1H-imidazo[4,5-f][1,10] phenanthroline on mild steel in sulfuric acid solution: Experimental and theoretical study. Ind. Eng. Chem. Res. 50, 2098–2110 (2011). (in English)
G.E. Badr, The role of some thiosemicarbazide derivatives as corrosion inhibitors for C-steel in acidic media. Corros. Sci. 51, 2529–2536 (2009)
M.A. Migahed, E.S.M. Azzam, A.M. Al-Sabagh, Corrosion inhibition of mild steel in 1M sulphuric acid solution using anionic surfactant. Mater. Chem. Phys. 85, 273–282 (2004)
I. Dehri, M. Ozcan, The effect of temperature on the corrosion of mild steel in acidic media in the presence of some sulphur-containing organic compounds. Mater. Chem. Phys. 98, 316–323 (2006). (in English)
N. Guan, M.L. Xueming, L. Fei, Synergistic inhibition between o-phenanthroline and chloride ion on cold rolled steel corrosion in phosphoric acid. Mater. Chem. Phys. 86, 59–68 (2004). (in English)
E. Khamis, A. Hosney, S. El-Khodary, Thermodynamics of mild steel corrosioin inhibition in phosphoric acid by ethylene trithiocarbonate. Afinidad 52, 95–106 (1995). (in English)
M.S. Kumar, S. Loganathan, A. Kumar, A. Sreekanth, Anticorrosion potential of 4-amino-3-methyl-1,2,4-triazole-5-thione derivatives (SAMTT and DBAMTT) on mild steel in hydrochloric acid solution. Ind. Eng. Chem. Res 51, 5408–5418 (2012). (in English)
D. Guzman-Lucero, O. Olivares-Xometl, R. Martínez-Palou, N.V. Likhanova, A. Domínguez-Aguilar, C. Garibay-Febles, Synthesis of selected vinylimidazolium ionic liquids and their effectiveness as corrosion inhibitors for carbon steel in aqueous sulfuric acid. Ind. Eng. Chem. Res. 50, 7129–7140 (2011). (in English)
Acknowledgments
The authors are grateful to the authorities of Srinivas School of Engineering, Mukka, Mangalore, Karnataka, India, for providing laboratory facilities. The authors also thank Department of Science and Technology, New Delhi, Government of India, under fast-track scheme for young scientist [DST: Project Sanction No. SERB/F/2231/2012-13 dated 12-07-2012] and All India council for Technical Education, New Delhi, Government of India, under MODROBS scheme [Ref. No 8024/RIFD/MOD 292 /2010-11 dated 31-03-2011 for providing instrumental facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hebbar, N., Praveen, B.M., Prasanna, B.M. et al. Anticorrosion Potential of Flectofenine on Mild Steel in Hydrochloric Acid Media: Experimental and Theoretical Study. J Fail. Anal. and Preven. 18, 371–381 (2018). https://doi.org/10.1007/s11668-018-0416-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-018-0416-6