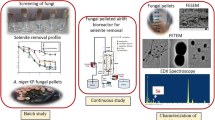
Abstract
Purpose of Review
Different selenium species released into the environment by anthropogenic activities pollute surface and ground water resources and can cause severe damage to the environment and ecosystems due to bio-accumulation. Though several physico-chemical methods are available to treat selenium oxyanion-containing wastewater, biological methods have gained significant importance due to their capability to convert selenium oxyanions to elemental selenium nanoparticles. The purpose of this review is to summarize the literature available on biological removal of selenium oxyanions and their recovery as elemental selenium nanoparticles.
Recent Findings
In recent times, the capability of several bacterial and fungal strains to reduce selenate and selenite to form elemental selenium nanoparticles has been reported. The shape, size and location of these selenium nanoparticles along with the selenium oxyanion removal efficiency depend on the operating parameters. Moreover, bioreactor configurations and operation strategies greatly influence the selenium removal and recovery efficiency.
Summary
Several conventional bioreactor systems can be used to remove selenate and selenite from wastewater and form selenium nanoparticles. However, the selenium nanoparticles are mostly entrapped in the biomass and require a secondary treatment to recover them. On the other hand, some novel bioreactors, viz. inverse fluidized bed bioreactor, rotating biological contractor, horizontal rotating packed bed bioreactor, moving bed biofilm reactor, and hybrid bioreactor, can possibly recover selenium nanoparticles following bioreduction of selenium oxyanions in a single stage system. Thus, this review will help in finding research gaps in this area and providing solutions for resource recovery from selenium oxyanion-containing wastewater.

Similar content being viewed by others
References
Sinharoy A, Baskaran D, Pakshirajan K. Process integration and artificial neural network modeling of biological sulfate reduction using a carbon monoxide fed gas lift bioreactor. Chem Eng J. 2019;123518:123518. https://doi.org/10.1016/j.cej.2019.123518.
Kanaujiya DK, Paul T, Sinharoy A, Pakshirajan K. Biological treatment processes for the removal of organic micropollutants from wastewater: a review. Curr Pollut Rep. 2019;5:112–28. https://doi.org/10.1007/s40726-019-00110-x.
Tan LC, Nancharaiah YV, van Hullebusch ED, Lens PNL. Selenium: environmental significance, pollution, and biological treatment technologies. Biotechnol Adv. 2016;34:886–907. https://doi.org/10.1016/j.biotechadv.2016.05.005.
Nancharaiah YV, Lens PNL. Selenium biomineralization for biotechnological applications. Trends Biotechnol. 2015;33:323–30. https://doi.org/10.1016/j.tibtech.2015.03.004.
Sinharoy A, Saikia S, Pakshirajan K. Biological removal of selenite from wastewater and recovery as selenium nanoparticles using inverse fluidized bed bioreactor. J Water Process Eng. 2019;32:100988. https://doi.org/10.1016/j.jwpe.2019.100988.
Torres J, Pintos V, Domínguez S, Kremer C, Kremer E. Selenite and selenate speciation in natural waters: interaction with divalent metal ions. J Solut Chem. 2010;39:1–10. https://doi.org/10.1007/s10953-009-9491-3.
Lopes G, Ávila FW, Guilherme LRG. Selenium behavior in the soil environment and its implication for human health. Cienc Agrotec. 2017;41:605–15. https://doi.org/10.1590/1413-70542017416000517.
Nancharaiah YV, Lens PNL. Ecology and biotechnology of selenium-respiring bacteria. Microbiol Mol Biol Rev. 2015;79:61–80. https://doi.org/10.1128/MMBR.00037-14.
Perkins WT. Extreme selenium and tellurium contamination in soils—an eighty year-old industrial legacy surrounding a Ni refinery in the Swansea Valley. Sci Total Environ. 2011;412:162–9. https://doi.org/10.1016/j.scitotenv.2011.09.056.
He Y, Xiang Y, Zhou Y, Yang Y, Zhang J, Huang H, et al. Selenium contamination, consequences and remediation techniques in water and soils: a review. Environ Res. 2018;164:288–301.
Jain R, Van Hullebusch ED, Lenz M, Farges F. Understanding selenium biogeochemistry in engineered ecosystems: transformation and analytical methods. In: Van Hullebusch ED, editor. Bioremediation of selenium contaminated wastewater. Springer: Cham; 2017. https://doi.org/10.1007/978-3-319-57831-6_2.
Mehdi Y, Hornick JL, Istasse L, Dufrasne I. Selenium in the environment, metabolism and involvement in body functions. Molecules. 2013;18:3292–311. https://doi.org/10.3390/molecules18033292.
Staicu LC, van Hullebusch ED, Lens PNL. Industrial selenium pollution: wastewaters and physical–chemical treatment technologies. In: Van Hullebusch ED, editor. Bioremediation of selenium contaminated wastewater. Cham: Springer; 2017. p. 2017. https://doi.org/10.1007/978-3-319-57831-6_5.
Sun HJ, Rathinasabapathi B, Wu B, Luo J, Pu LP, Ma LQ. Arsenic and selenium toxicity and their interactive effects in humans. Environ Int. 2014;69:148–58. https://doi.org/10.1016/j.envint.2014.04.019.
Santos S, Ungureanu G, Boaventura R, Botelho C. Selenium contaminated waters: an overview of analytical methods, treatment options and recent advances in sorption methods. Sci Total Environ. 2015;521:246–60. https://doi.org/10.1016/j.scitotenv.2015.03.107.
Negi BB, Sinharoy A, Pakshirajan K. Selenite removal from wastewater using fungal pelleted airlift bioreactor. Environ Sci Pollut Res. 2019;27:1–12. https://doi.org/10.1007/s11356-019-06946-6.
Liang L, Yang W, Guan X, Li J, Xu Z, Wu J, et al. Kinetics and mechanisms of pH-dependent selenite removal by zero valent iron. Water Res. 2013;47:5846–55. https://doi.org/10.1016/j.watres.2013.07.011.
Börsig N, Scheinost AC, Shaw S, Schild D, Neumann T. Retention and multiphase transformation of selenium oxyanions during the formation of magnetite via iron (II) hydroxide and green rust. Dalton Trans. 2018;47:11002–15. https://doi.org/10.1039/C8DT01799A.
Ma B, Fernandez-Martinez A, Grangeon S, Tournassat C, Findling N, Carrero S, et al. Selenite uptake by Ca–Al LDH: a description of intercalated anion coordination geometries. Environ Sci Technol. 2018;52:1624–32. https://doi.org/10.1021/acs.est.7b04644.
Jain R, Jordan N, Tsushima S, Hübner R, Weiss S, Lens PNL. Shape change of biogenic elemental selenium nanomaterials from nanospheres to nanorods decreases their colloidal stability. Environ Sci Nano. 2017;4:1054–63. https://doi.org/10.1039/C7EN00145B.
Chen Z, Shen Y, Xie A, Zhu J, Wu Z, Huang F. L-cysteine-assisted controlled synthesis of selenium nanospheres and nanorods. Cryst Growth Des. 2009;9:1327–33. https://doi.org/10.1021/cg800398b.
Prasad KS, Vaghasiya JV, Soni SS, Patel J, Patel R, Kumari M, et al. Microbial selenium nanoparticles (SeNPs) and their application as a sensitive hydrogen peroxide biosensor. Appl Biochem Biotechnol. 2015;177:1386–93. https://doi.org/10.1007/s12010-015-1814-9.
Shakibaie M, Forootanfar H, Golkari Y, Mohammadi-Khorsand T, Shakibaie MR. Anti-biofilm activity of biogenic selenium nanoparticles and selenium dioxide against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis. J Trace Elem Med Biol. 2015;29:235–41. https://doi.org/10.1016/j.jtemb.2014.07.020.
Mal J, Nancharaiah YV, Van Hullebusch ED, Lens PNL. Metal chalcogenide quantum dots: biotechnological synthesis and applications. RSC Adv. 2016;6:41477–95. https://doi.org/10.1039/C6RA08447H.
Tugarova AV, Kamnev AA. Proteins in microbial synthesis of selenium nanoparticles. Talanta. 2017;174:539–47. https://doi.org/10.1016/j.talanta.2017.06.013.
Dessì P, Jain R, Singh S, Seder-Colomina M, van Hullebusch ED, Rene ER, et al. Effect of temperature on selenium removal from wastewater by UASB reactors. Water Res. 2016;94:146–54. https://doi.org/10.1016/j.watres.2016.02.007.
Jain R, Seder-Colomina M, Jordan N, Dessi P, Cosmidis J, van Hullebusch ED, et al. Entrapped elemental selenium nanoparticles affect physicochemical properties of selenium fed activated sludge. J Hazard Mater. 2015;295:193–200. https://doi.org/10.1016/j.jhazmat.2015.03.043.
Schiavon M, Ertani A, Parrasia S, Dalla VF. Selenium accumulation and metabolism in algae. Aquat Toxicol. 2017;189:1–8. https://doi.org/10.1016/j.aquatox.2017.05.011.
Nguyen VK, Nguyen TH, Ha MG, Kang HY. Kinetics of microbial selenite reduction by novel bacteria isolated from activated sludge. J Environ Manag. 2019;236:746–54. https://doi.org/10.1016/j.jenvman.2019.02.012.
Zhang J, Wang Y, Shao Z, Li J, Zan S, Zhou S, et al. Two selenium tolerant Lysinibacillus sp. strains are capable of reducing selenite to elemental Se efficiently under aerobic conditions. Int J Environ Sci. 2019;77:238–49. https://doi.org/10.1016/j.jes.2018.08.002.
Fischer S, Krause T, Lederer F, Merroun ML, Shevchenko A, Hübner R, et al. Bacillus safensis JG-B5T affects the fate of selenium by extracellular production of colloidally less stable selenium nanoparticles. J Hazard Mater. 2020;384:121146. https://doi.org/10.1016/j.jhazmat.2019.121146.
Wang Y, Shu X, Zhou Q, Fan T, Wang T, Chen X, et al. Selenite reduction and the biogenesis of selenium nanoparticles by Alcaligenes faecalis Se03 isolated from the gut of Monochamus Alternatus (Coleoptera: Cerambycidae). Int J Mol Sci. 2018;19:2799. https://doi.org/10.3390/ijms19092799.
Jiang S, Ho CT, Lee JH, Van Duong H, Han S, Hur HG. Mercury capture into biogenic amorphous selenium nanospheres produced by mercury resistant Shewanella putrefaciens 200. Chemosphere. 2012;87:621–4. https://doi.org/10.1016/j.chemosphere.2011.12.083.
Xia X, Wu S, Li N, Wang D, Zheng S, Wang G. Novel bacterial selenite reductase CsrF responsible for Se (IV) and Cr (VI) reduction that produces nanoparticles in Alishewanella sp. WH16-1. J Hazard Mater. 2018;342:499–509. https://doi.org/10.1016/j.jhazmat.2017.08.051.
Srivastava P, Kowshik M. Anti-neoplastic selenium nanoparticles from Idiomarina sp. PR58–8. Enzym Microb Technol. 2016;95:192–200. https://doi.org/10.1016/j.enzmictec.2016.08.002.
Kora AJ, Rastogi L. Biomimetic synthesis of selenium nanoparticles by Pseudomonas aeruginosa ATCC 27853: an approach for conversion of selenite. J Environ Manag. 2016;181:231–6. https://doi.org/10.1016/j.jenvman.2016.06.029.
Lampis S, Zonaro E, Bertolini C, Cecconi D, Monti F, Micaroni M, et al. Selenite biotransformation and detoxification by Stenotrophomonas maltophilia SeITE02: novel clues on the route to bacterial biogenesis of selenium nanoparticles. J Hazard Mater. 2017;324:3–14. https://doi.org/10.1016/j.jhazmat.2016.02.035.
Kamnev AA, Mamchenkova PV, Dyatlova YA, Tugarova AV. FTIR spectroscopic studies of selenite reduction by cells of the rhizobacterium Azospirillum brasilense Sp7 and the formation of selenium nanoparticles. J Mol Struct. 2017;1140:106–12. https://doi.org/10.1016/j.molstruc.2016.12.003.
Tugarova AV, Mamchenkova PV, Dyatlova YA, Kamnev AA. FTIR and Raman spectroscopic studies of selenium nanoparticles synthesised by the bacterium Azospirillum thiophilum. Spectrochim Acta A Mol Biomol Spectrosc. 2018;192:458–63. https://doi.org/10.1016/j.saa.2017.11.050.
Khoei NS, Lampis S, Zonaro E, Yrjälä K, Bernardi P, Vallini G. Insights into selenite reduction and biogenesis of elemental selenium nanoparticles by two environmental isolates of Burkholderia fungorum. New Biotechnol. 2017;34:1–11. https://doi.org/10.1016/j.nbt.2016.10.002.
Dhanjal S, Cameotra SS. Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil. Microb Cell Factories. 2010;9:52. https://doi.org/10.1186/1475-2859-9-52.
Bao P, Huang H, Hu ZY, Häggblom MM, Zhu YG. Impact of temperature, CO2 fixation and nitrate reduction on selenium reduction, by a paddy soil Clostridium strain. J Appl Microbiol. 2013;114:703–12. https://doi.org/10.1111/jam.12084.
Li B, Liu N, Li Y, Jing W, Fan J, Li D, et al. Reduction of selenite to red elemental selenium by Rhodopseudomonas palustris strain N. PLoS One. 2014;9:e95955. https://doi.org/10.1371/journal.pone.0095955.
Borghese R, Baccolini C, Francia F, Sabatino P, Turner RJ, Zannoni D. Reduction of chalcogen oxyanions and generation of nanoprecipitates by the photosynthetic bacterium Rhodobacter capsulatus. J Hazard Mater. 2014;269:24–30. https://doi.org/10.1016/j.jhazmat.2013.12.028.
Bao P, Xiao KQ, Wang HJ, Xu H, Xu PP, Jia Y, et al. Characterization and potential applications of a selenium nanoparticle producing and nitrate reducing bacterium Bacillus oryziterrae sp nov. Sci Rep. 2016;6:34054. https://doi.org/10.1038/srep34054.
Narasingarao P, Häggblom MM. Identification of anaerobic selenate-respiring bacteria from aquatic sediments. Appl Environ Microbiol. 2007;73:3519–27. https://doi.org/10.1128/AEM.02737-06.
Rosenfeld CE, Kenyon JA, James BR, Santelli CM. Selenium (IV, VI) reduction and tolerance by fungi in an oxic environment. Geobiology. 2017;1:5441–52. https://doi.org/10.1111/gbi.12224.
Liang X, Perez MAMJ, Nwoko KC, Egbers P, Feldmann J, Csetenyi L, et al. Fungal formation of selenium and tellurium nanoparticles. Appl Microbiol Biotechnol. 2019;103:7241–59. https://doi.org/10.1007/s00253-019-09995-6.
Asghari-Paskiabi F, Imani M, Razzaghi-Abyaneh M, Rafii-Tabar H. Fusariumoxysporum, a bio-factory for nano selenium compounds: synthesis and characterization. Sci Iran. 2018;25:1857–63. https://doi.org/10.24200/sci.2018.5301.1192.
Li Z, Li H, Hu H. Selenite removal and reduction by growing Aspergillus sp. J2. BioMetals. 2018;31:45–50. https://doi.org/10.1007/s10534-017-0063-5.
Urík M, Boriová K, Bujdoš M, Matúš P. Fungal selenium (VI) accumulation and biotransformation—filamentous fungi in selenate contaminated aqueous media remediation. CLEAN-Soil Air Water. 2016;44:610–4. https://doi.org/10.1002/clen.201500100.
Zare B, Babaie S, Setayesh N, Shahverdi AR. Isolation and characterization of a fungus for extracellular synthesis of small selenium nanoparticles. Nanomed J. 2013;1:13–9. https://doi.org/10.7508/NMJ.2013.01.002.
Rehan M, Alsohim AS, El-Fadly G, Tisa LS. Detoxification and reduction of selenite to elemental red selenium by Frankia. Antonie Van Leeuwenhoek. 2019;112:127–39. https://doi.org/10.1007/s10482-018-1196-4.
Fernández-Llamosas H, Castro L, Blázquez ML, Díaz E, Carmona M. Biosynthesis of selenium nanoparticles by Azoarcus sp. CIB Microb Cell Fact. 2016;15:109. https://doi.org/10.1186/s12934-016-0510-y.
Presentato A, Piacenza E, Anikovskiy M, Cappelletti M, Zannoni D, Turner RJ. Biosynthesis of selenium-nanoparticles and-nanorods as a product of selenite bioconversion by the aerobic bacterium Rhodococcus aetherivorans BCP1. New Biotechnol. 2018;41:1–8. https://doi.org/10.1016/j.nbt.2017.11.002.
Soda S, Ma W, Kuroda M, Nishikawa H, Zhang Y, Ike M. Characterization of moderately halotolerant selenate-and tellurite-reducing bacteria isolated from brackish areas in Osaka. Biosci Biotechnol Biochem. 2018;82:173–81. https://doi.org/10.1080/09168451.2017.1406794.
Che L, Xu W, Zhan J, Zhang L, Liu L, Zhou H. Complete genome sequence of Bacillus cereus CC-1, a novel marine selenate/selenite reducing bacterium producing metallic selenides nanomaterials. Curr Microbiol. 2019;76:78–85. https://doi.org/10.1007/s00284-018-1587-9.
Li B, Liu N, Li Y, Jing W, Fan J, Li D, et al. Reduction of selenite to red elemental selenium by Rhodopseudomonas palustris strain N. PLoS One. 2014;9:e95955. https://doi.org/10.1371/journal.pone.0095955.
Fernández-Llamosas H, Castro L, Blázquez ML, Díaz E, Carmona M. Speeding up bioproduction of selenium nanoparticles by using Vibrio natriegens as microbial factory. Sci Rep. 2017;7:1–9. https://doi.org/10.1038/s41598-017-16252-1.
Elahian F, Reiisi S, Shahidi A, Mirzaei SA. High-throughput bioaccumulation, biotransformation, and production of silver and selenium nanoparticles using genetically engineered Pichia pastoris. Nanomed Nanotechnol. 2017;13:853–61. https://doi.org/10.1016/j.nano.2016.10.009.
Kora AJ. Bacillus cereus, selenite-reducing bacterium from contaminated lake of an industrial area: a renewable nanofactory for the synthesis of selenium nanoparticles. Bioresour Bioprocess. 2018;5:1–12. https://doi.org/10.1186/s40643-018-0217-5.
Espinosa-Ortiz EJ, Gonzalez-Gil G, Saikaly PE, Van Hullebusch ED, Lens PNL. Effects of selenium oxyanions on the white-rot fungus Phanerochaete chrysosporium. Appl Microbiol Biotechnol. 2015;99:2405–18. https://doi.org/10.1007/s00253-014-6127-3.
Espinosa-Ortiz EJ, Rene ER, Pakshirajan K, van Hullebusch ED, Lens PNL. Fungal pelleted reactors in wastewater treatment: applications and perspectives. Chem Eng J. 2016;283:553–71. https://doi.org/10.1016/j.cej.2015.07.068.
Espinosa-Ortiz EJ, Rene ER, van Hullebusch ED, Lens PNL. Removal of selenite from wastewater in a Phanerochaete chrysosporium pellet based fungal bioreactor. Int Biodeterior Biodegradation. 2015;102:361–9. https://doi.org/10.1016/j.ibiod.2015.04.014.
Espinosa-Ortiz EJ, Pechaud Y, Lauchnor E, Rene ER, Gerlach R, Peyton BM, et al. Effect of selenite on the morphology and respiratory activity of Phanerochaete chrysosporium biofilms. Bioresour Technol. 2016;210:138–45. https://doi.org/10.1016/j.biortech.2016.02.074.
Rasouli M. Biosynthesis of selenium nanoparticles using yeast Nematospora coryli and examination of their anti-candida and anti-oxidant activities. IET Nanobiotechnol. 2018;13:214–8. https://doi.org/10.1049/iet-nbt.2018.5187.
Ruocco MH, Chan CS, Hanson TE, Church TM. Characterization and distribution of selenite reduction products in cultures of the marine yeast Rhodotorula mucilaginosa-13B. Geomicrobiol J. 2014;31:769–78. https://doi.org/10.1080/01490451.2014.888909.
Lazard M, Dauplais M, Plateau P. Contribution of the yeast Saccharomyces cerevisiae model to understand the mechanisms of selenium toxicity. In: Michalke B, editor. Selenium. Springer: Cham; 2018. https://doi.org/10.1007/978-3-319-95390-8_4.
Sinharoy A, Baskaran D, Pakshirajan K. A novel carbon monoxide fed moving bed biofilm reactor for sulfate rich wastewater treatment. J Environ Manag. 2019;249:109402. https://doi.org/10.1016/j.jenvman.2019.109402.
Tan LC, Mal J, Lens PNL. Selenium remediation using granular and biofilm systems. In: Nancharaiah YV, Venugopalan VP, editors. Microbial Biofilms in Bioremediation and Wastewater Treatment: CRC Press; 2019. https://doi.org/10.1201/b22046-6.
Jain R, Dominic D, Jordan N, Rene ER, Weiss S, van Hullebusch ED, et al. Preferential adsorption of Cu in a multi-metal mixture onto biogenic elemental selenium nanoparticles. Chem Eng J. 2016;284:917–25. https://doi.org/10.1016/j.cej.2015.08.144.
Tan LC, Espinosa-Ortiz EJ, Nancharaiah YV, van Hullebusch E, Gerlach R, Lens PNL. Biological treatment of selenium-laden wastewater containing nitrate and sulfate in an upflow anaerobic sludge bed reactor at pH 5.0. Chemosphere. 2018;211:684–93. https://doi.org/10.1016/j.chemosphere.2018.07.079.
Soda S, Kashiwa M, Kagami T, Kuroda M, Yamashita M, Ike M. Laboratory-scale bioreactors for soluble selenium removal from selenium refinery wastewater using anaerobic sludge. Desalination. 2011;279:433–8. https://doi.org/10.1016/j.desal.2011.06.031.
Zhang Y, Zahir ZA, Frankenberger WT. Factors affecting reduction of selenate to elemental selenium in agricultural drainage water by Enterobacter taylorae. J Agric Food Chem. 2003;51:7073–8. https://doi.org/10.1021/jf0304019.
Mishra RR, Prajapati S, Das J, Dangar TK, Das N, Thatoi H. Reduction of selenite to red elemental selenium by moderately halotolerant Bacillus megaterium strains isolated from Bhitarkanika mangrove soil and characterization of reduced product. Chemosphere. 2011;84:1231–7. https://doi.org/10.1016/j.chemosphere.2011.05.025.
Zhang Y, Kuroda M, Nakatani Y, Soda S, Ike M. Removal of selenite from artificial wastewater with high salinity by activated sludge in aerobic sequencing batch reactors. J Biosci Bioeng. 2019;127:618–24. https://doi.org/10.1016/j.jbiosc.2018.11.002.
Jain R, Matassa S, Singh S, van Hullebusch ED, Esposito G, Lens PNL. Reduction of selenite to elemental selenium nanoparticles by activated sludge. Environ Sci Pollut Res. 2016;23:1193–202. https://doi.org/10.1007/s11356-015-5138-7.
Mal J, Nancharaiah YV, van Hullebusch ED, Lens PNL. Biological removal of selenate and ammonium by activated sludge in a sequencing batch reactor. Bioresour Technol. 2017;229:11–9. https://doi.org/10.1016/j.biortech.2016.12.112.
Nancharaiah YV, Sarvajith M, Lens PNL. Selenite reduction and ammoniacal nitrogen removal in an aerobic granular sludge sequencing batch reactor. Water Res. 2018;131:131–41. https://doi.org/10.1016/j.watres.2017.12.028.
Zhang Y, Kuroda M, Nakatani Y, Soda S, Ike M. Removal of selenite from artificial wastewater with high salinity by activated sludge in aerobic sequencing batch reactors. J Biosci Bioeng. 2019;127:618–24. https://doi.org/10.1016/j.jbiosc.2018.11.002.
Zeng T, Rene ER, Hu Q, Lens PNL. Continuous biological removal of selenate in the presence of cadmium and zinc in UASB reactors at psychrophilic and mesophilic conditions. Biochem Eng J. 2019;141:102–11. https://doi.org/10.1016/j.bej.2018.10.013.
Wadgaonkar SL, Mal J, Nancharaiah YV, Maheshwari NO, Esposito G, Lens PNL. Formation of Se (0), Te (0), and Se (0)–Te (0) nanostructures during simultaneous bioreduction of selenite and tellurite in a UASB reactor. Appl Microbiol Biotechnol. 2018;102:2899–911. https://doi.org/10.1007/s00253-018-8781-3.
Tan LC, Papirio S, Luongo V, Nancharaiah YV, Cennamo P, Esposito G, et al. Comparative performance of anaerobic attached biofilm and granular sludge reactors for the treatment of model mine drainage wastewater containing selenate, sulfate and nickel. Chem Eng J. 2018;345:545–55. https://doi.org/10.1016/j.cej.2018.03.177.
Eregowda T, Rene ER, Lens PNL. Bioreduction of selenate in an anaerobic biotrickling filter using methanol as electron donor. Chemosphere. 2019;225:406–13. https://doi.org/10.1016/j.chemosphere.2019.02.158.
Lai CY, Yang X, Tang Y, Rittmann BE, Zhao HP. Nitrate shaped the selenate-reducing microbial community in a hydrogen-based biofilm reactor. Environ Sci Technol. 2014;48:3395–402. https://doi.org/10.1021/es4053939.
Xia S, Xu X, Zhou L. Insights into selenate removal mechanism of hydrogen-based membrane biofilm reactor for nitrate-polluted groundwater treatment based on anaerobic biofilm analysis. Ecotox Environ Safe. 2019;178:123–9. https://doi.org/10.1016/j.ecoenv.2019.04.005.
Luo JH, Chen H, Hu S, Cai C, Yuan Z, Guo J. Microbial selenate reduction driven by a denitrifying anaerobic methane oxidation biofilm. Environ Sci Technol. 2018;52:4006–12. https://doi.org/10.1021/acs.est.7b05046.
Lai CY, Wen LL, Shi LD, Zhao KK, Wang YQ, Yang X, et al. Selenate and nitrate bioreductions using methane as the electron donor in a membrane biofilm reactor. Environ Sci Technol. 2016;50:10179–86. https://doi.org/10.1021/acs.est.6b02807.
Van Ginkel SW, Zhou C, Lien M, Rittmann BE. Hydrogen-based nitrate and selenate bioreductions in flue-gas desulfurization brine. J Environ Eng. 2011;137:63–8. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000288.
Werkneh AA, Rene ER, Lens PNL. Simultaneous removal of selenite and phenol from wastewater in an upflow fungal pellet bioreactor. J Chem Technol Biotechnol. 2018;93:1003–11. https://doi.org/10.1002/jctb.5452.
Chakraborty S, Rene ER, Lens PNL. Reduction of selenite to elemental Se (0) with simultaneous degradation of phenol by co-cultures of Phanerochaete chrysosporium and Delftia lacustris. J Microbiol. 2019;57:738–47. https://doi.org/10.1007/s12275-019-9042-6.
Sinharoy A, Pakshirajan K. A novel application of biologically synthesized nanoparticles for enhanced biohydrogen production and carbon monoxide bioconversion. Renew Energy. 2020;147:864–73. https://doi.org/10.1016/j.renene.2019.09.027.
Lenz M, Janzen N, Lens PNL. Selenium oxyanion inhibition of hydrogenotrophic and acetoclastic methanogenesis. Chemosphere. 2008;73:383–8. https://doi.org/10.1016/j.chemosphere.2008.05.059.
Mal J, Nancharaiah YV, Van Hullebusch ED, Lens PNL. Effect of heavy metal co-contaminants on selenite bioreduction by anaerobic granular sludge. Bioresour Technol. 2016;206:1–8. https://doi.org/10.1016/j.biortech.2016.01.064.
Zeng T, Rene ER, Zhang S, Lens PNL. Removal of selenate and cadmium by anaerobic granular sludge: EPS characterization and microbial community analysis. Process Saf Environ. 2019;126:150–9. https://doi.org/10.1016/j.psep.2019.03.039.
Zeng T, Rene ER, Hu Q, Lens PNL. Continuous biological removal of selenate in the presence of cadmium and zinc in UASB reactors at psychrophilic and mesophilic conditions. Biochem Eng J. 2019;141:102–11. https://doi.org/10.1016/j.bej.2018.10.013.
Mal J, Nancharaiah YV, Bera S, Maheshwari N, van Hullebusch ED, Lens PNL. Biosynthesis of CdSe nanoparticles by anaerobic granular sludge. Environ Sci Nano. 2017;4:824–33. https://doi.org/10.1039/C6EN00623J.
Ayano H, Miyake M, Terasawa K, Kuroda M, Soda S, Sakaguchi T, et al. Isolation of a selenite-reducing and cadmium-resistant bacterium Pseudomonas sp. strain RB for microbial synthesis of CdSe nanoparticles. J Biosci Bioeng. 2014;117:576–81. https://doi.org/10.1016/j.jbiosc.2013.10.010.
Tan LC, Espinosa-Ortiz EJ, Nancharaiah YV, van Hullebusch ED, Gerlach R, Lens PNL. Selenate removal in biofilm systems: effect of nitrate and sulfate on selenium removal efficiency, biofilm structure and microbial community. J Chem Technol Biotechnol. 2018;93:2380–9. https://doi.org/10.1002/jctb.5586.
Zhou L, Xu X, Xia S. Effects of sulfate on simultaneous nitrate and selenate removal in a hydrogen-based membrane biofilm reactor for groundwater treatment: performance and biofilm microbial ecology. Chemosphere. 2018;211:254–60. https://doi.org/10.1016/j.chemosphere.2018.07.092.
Besha AT, Gebreyohannes AY, Tufa RA, Bekele DN, Curcio E, Giorno L. Removal of emerging micropollutants by activated sludge process and membrane bioreactors and the effects of micropollutants on membrane fouling: a review. J Environ Chem Eng. 2017;5:2395–414. https://doi.org/10.1016/j.jece.2017.04.027.
Dutta A, Davies C, Ikumi DS. Performance of upflow anaerobic sludge blanket (UASB) reactor and other anaerobic reactor configurations for wastewater treatment: a comparative review and critical updates. J Water Supply Res T. 2018;67:858–84. https://doi.org/10.2166/aqua.2018.090.
Sonstegard J, Harwood J, Pickett T. Full scale implementation of GE ABMet biological technology for the removal of selenium from FGD wastewaters. In: Proceedings of 68th International Water Conference (Vol. 2, p. 580). Engineer’s Society of Western Pennsylvania: Pittsburgh, PA; 2007.
Singh SP, Prerna P. Review of recent advances in anaerobic packed-bed biogas reactors. Renew Sust Energ Rev. 2009;13:1569–75. https://doi.org/10.1016/j.rser.2008.08.006.
Kumar M, Sinharoy A, Pakshirajan K. Process integration for biological sulfate reduction in a carbon monoxide fed packed bed reactor. J Environ Manag. 2018;219:294–303. https://doi.org/10.1016/j.jenvman.2018.04.033.
Goswami L, Kumar RV, Borah SN, Manikandan NA, Pakshirajan K, Pugazhenthi G. Membrane bioreactor and integrated membrane bioreactor systems for micropollutant removal from wastewater: a review. J Water Process Eng. 2018;26:314–28. https://doi.org/10.1016/j.jwpe.2018.10.024.
Chung J, Rittmann BE, Wright WF, Bowman RH. Simultaneous bio-reduction of nitrate, perchlorate, selenate, chromate, arsenate, and dibromochloropropane using a hydrogen-based membrane biofilm reactor. Biodegradation. 2007;18:199–209. https://doi.org/10.1007/s10532-006-9055-9.
Staicu LC, Van Hullebusch ED, Lens PNL. Production, recovery and reuse of biogenic elemental selenium. Environ Chem Lett. 2015;13:89–96. https://doi.org/10.1007/s10311-015-0492-8.
Villa-Gomez D, Ababneh H, Papirio S, Rousseau DPL, Lens PNL. Effect of sulfide concentration on the location of the metal precipitates in inversed fluidized bed reactors. J Hazard Mater. 2011;192:200–7. https://doi.org/10.1016/j.jhazmat.2011.05.002.
Haribabu K, Sivasubramanian V. Biodegradation of organic content in wastewater in fluidized bed bioreactor using low-density biosupport. Desalin Water Treat. 2016;57:4322–7. https://doi.org/10.1080/19443994.2014.992978.
Reyes-Alvarado LC, Camarillo-Gamboa Á, Rustrian E, Rene ER, Esposito G, Lens PNL, et al. Lignocellulosic biowastes as carrier material and slow release electron donor for sulphidogenesis of wastewater in an inverse fluidized bed bioreactor. Environ Sci Pollut R. 2018;25:5115–28. https://doi.org/10.1007/s11356-017-9334-5.
Arun N, Razack AA, Sivasubramanian V. Recent progress in hydrodynamics of inverse fluidized bed reactors: a review. Chem Eng Comm. 2013;200:1260–77. https://doi.org/10.1080/00986445.2012.744747.
Kiran MG, Pakshirajan K, Das G. A new application of anaerobic rotating biological contactor reactor for heavy metal removal under sulfate reducing condition. Chem Eng J. 2017;321:67–75. https://doi.org/10.1016/j.cej.2017.03.080.
Kiran MG, Pakshirajan K, Das G. Metallic wastewater treatment by sulfate reduction using anaerobic rotating biological contactor reactor under high metal loading conditions. Front Environ Sci Eng. 2018;12:12. https://doi.org/10.1007/s11783-018-1073-4.
Shen Y, Brown RC, Wen Z. Syngas fermentation by Clostridium carboxidivorans P7 in a horizontal rotating packed bed biofilm reactor with enhanced ethanol production. Appl Energy. 2017;187:585–94. https://doi.org/10.1016/j.apenergy.2016.11.084.
Chen YS, Lin CC, Liu HS. Mass transfer in a rotating packed bed with viscous Newtonian and non-Newtonian fluids. Ind Eng Chem Res. 2005;44:1043–51. https://doi.org/10.1021/ie0499409.
Sinharoy A, Baskaran D, Pakshirajan K. A novel carbon monoxide fed moving bed biofilm reactor for sulfate rich wastewater treatment. J Environ Manag. 2019b;249:109402. https://doi.org/10.1016/j.jenvman.2019.109402.
Zhang Y, Ma Y, Quan X, Jing Y, Dai S. Rapid startup of a hybrid UASB-AFF reactor using bi-circulation. Chem Eng J. 2009;155:266–71. https://doi.org/10.1016/j.cej.2009.08.005.
Xu Q, Tian Y, Wang S, Ko JH. A comparative study of leachate quality and biogas generation in simulated anaerobic and hybrid bioreactors. Waste Manag. 2015;41:94–100. https://doi.org/10.1016/j.wasman.2015.03.023.
Neshat SA, Mohammadi M, Najafpour GD. Photosynthesis assisted anaerobic digestion of cattle manure leachate in a hybrid bioreactor: an integrated system for enhanced wastewater treatment and methane production. Chem Eng J. 2017;330:616–24. https://doi.org/10.1016/j.cej.2017.08.001.
Acknowledgements
The authors acknowledge the funding provided by the Science Foundation Ireland (SFI) Research Professorship Programme “Innovative Energy Technologies for Biofuels, Bioenergy and a Sustainable Irish Bioeconomy” (IETSBIO3; award no. 15/RP/2763).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Water Pollution
Rights and permissions
About this article
Cite this article
Sinharoy, A., Lens, P.N.L. Biological Removal of Selenate and Selenite from Wastewater: Options for Selenium Recovery as Nanoparticles. Curr Pollution Rep 6, 230–249 (2020). https://doi.org/10.1007/s40726-020-00146-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-020-00146-4