Abstract
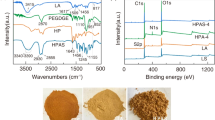
Graphene Oxide/Poly-Amidoamines Dendrimers (GO/PAMAMs) nano-composite was synthesized and activated by hydrochloride acid. It was then used for nitrate removal from aqueous solutions as a novel method. Experiments were conducted in batch mode. The prepared adsorbent was analyzed by Field Emission Scanning Electron Microscopy (FE-SEM) with Energy-dispersive X-ray spectroscopy (EDS), Fourier Transform Infrared Spectroscopy (FTIR), Thermogravimetric Analysis (TGA) and X-ray Diffraction (XRD). Additionally, adsorption isotherms and kinetic studies were investigated. Ninety percent of the nitrate was removed at pH 7.5, adsorbent 0.025 g L−1, 45 mg L−1 initial nitrate concentration and 15 min reaction time. Oxygen and chloride of activated GO/PAMAMs (A GO/PAMAMs) were approximately 19.1–21.6% and 0.4% in mass, respectively. Thermal stability of A GO/PAMAMs was more than that of Graphene Oxide (GO). Pseudo-second order kinetic model and Freundlich isotherm fitted the data well. Ion exchange between nitrate and chloride was the main mechanism of nitrate removal. The advantages of this technology include high removal efficiency, good adsorbability, good recyclability and non-toxic technology. This method can be used as a suitable method for in situ treatment of nitrate from aqueous solutions.














Similar content being viewed by others
Abbreviations
- GO:
-
Graphene oxide
- PAMAM:
-
Poly-amidoamine
- PAMAM-G2 :
-
the second generation of Poly-amidoamine dendrimer
- GO/PAMAMs:
-
Graphene oxide/poly-amidoamines dendrimers
- A GO/PAMAMs:
-
Activated Graphene oxide/poly-amidoamines dendrimers
- FE-SEM:
-
field-emission scanning electron microscopy
- EDS:
-
Energy-dispersive X-ray spectroscopy
- FTIR:
-
Fourier Transform Infrared Spectroscopy
References
Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, Hanifehpour Y, Nejati-Koshki K, Pashaei-Asl R (2014) Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett 9:247–247. https://doi.org/10.1186/1556-276X-9-247
Afkhami A, Madrakian T, Karimi Z (2007) The effect of acid treatment of carbon cloth on the adsorption of nitrite and nitrate ions. J Hazard Mater 144:427–431
Ahmadzadeh Tofighy M, Mohammadi T (2012) Nitrate removal from water using functionalized carbon nanotube sheets. Chem Eng Res Des 90:1815–1822. https://doi.org/10.1016/j.cherd.2012.04.001
Ahn SC, Oh S-Y, Cha DK (2008) Enhanced reduction of nitrate by zero-valent iron at elevated temperatures. J Hazard Mater 156:17–22
Ait Haki M, Laabd M, Chafai H, Kabli H, Ez-zahery M, Bazzaoui M, Lakhmiri R, Albourine A (2017) Comparative adsorption of nitrate ions on the polypyrrole and polyaniline from aqueous solution. J Dispers Sci Technol 38:598–603. https://doi.org/10.1080/01932691.2016.1184096
APHA, WPCF (2005) Standard method for the examination of water and wastewater, 21th edn. American Public Health Association, American Water Works Association, Water Environment Federation. American Public Health Association, Washingon, DC
Banu HT, Meenakshi S (2017) Synthesis of a novel quaternized form of melamine–formaldehyde resin for the removal of nitrate from water. J Water Process Eng 16:81–89. https://doi.org/10.1016/j.jwpe.2016.12.003
Blanco MB, Bejan I, Barnes I, Wiesen P, Teruel MA (2010) FTIR product distribution study of the Cl and OH initiated degradation of methyl acrylate at atmospheric pressure. Environ Sci Technol 44:7031–7036. https://doi.org/10.1021/es101831r
Bryan N, Loscalzo J (eds) (2011) Nitrite and nitrate in human health and disease. Springer, Humana Press, New York
Bulgariu L, Ceica A, Lazar L, Cretescu I, Balasanian I (2010) Equilibrium and kinetics study of nitrate removal from water by purolite A100 resin. Revista de Chimie 61(11):1136–1141
Cengeloglu Y, Tor A, Ersoz M, Arslan G (2006) Removal of nitrate from aqueous solution by using red mud. Sep Purif Technol 51:374–378. https://doi.org/10.1016/j.seppur.2006.02.020
Chandra V, Kim KS (2011) Highly selective adsorption of Hg2+ by a polypyrrole-reduced graphene oxide composite. Chem Commun 47:3942–3944. https://doi.org/10.1039/C1CC00005E
Chauhan K, Kaur J, Singh P, Sharma P, Sharma P, Chauhan GS (2016) An efficient and regenerable quaternary starch for removal of nitrate from aqueous solutions. Ind Eng Chem Res 55:2507–2519. https://doi.org/10.1021/acs.iecr.5b03923
Chen X, Chen B (2015) Macroscopic and spectroscopic investigations of the adsorption of nitroaromatic compounds on graphene oxide, reduced graphene oxide, and graphene nanosheets. Environ Sci Technol 49:6181–6189. https://doi.org/10.1021/es5054946
Cho DW, Chon CM, Kim YJ, Jeon BH, Schwartz FW, Lee ES, Song HC (2011) Adsorption of nitrate and Cr(VI) by cationic polymer-modified granular activated carbon. Chem Eng J 175:298–305
DeFever RS, Geitner NK, Bhattacharya P, Ding F, Ke PC, Sarupria S (2015) PAMAM dendrimers and graphene: materials for removing aromatic contaminants from water. Environ Sci Technol 49:4490–4497. https://doi.org/10.1021/es505518r
Demiral H, Gündüzoğlu G (2010) Removal of nitrate from aqueous solutions by activated carbon prepared from sugar beet bagasse. Bioresour Technol 101:1675–1680. https://doi.org/10.1016/j.biortech.2009.09.087
Dong T, Zhang Y, Su X, Chen Z, Si C (2017) Mechanism of nitrogen removal from aqueous solutions using natural scoria. Water 9(5):341. https://doi.org/10.3390/w9050341
Dykes GM (2001) Dendrimers: a review of their appeal and applications. J Chem Technol Biotechnol 76:903–918. https://doi.org/10.1002/jctb.464
EPA (2006) Nitrates and nitrites: TEACH Chemical Summary, U.S. EPA Toxicity and Exposure Assessment for Children’s Health. https://archive.epa.gov/region5/teach/web/pdf/nitrates_summary.pdf. Accessed 22 May 2007
Eroglu E, Zang W, Eggers PK, Chen X, Boulos RA, Wahid MH, Smith SM, Raston CL (2013) Nitrate uptake by p-phosphonic acid calix[8]arene stabilized graphene. Chem Commun 49:8172–8174
Esfand R, Tomalia DA (2002) Laboratory synthesis of poly(amidoamine)(PAMAM) dendrimers. In: Fréchet JMJ and Tomalia DA (eds) Dendrimers and Other Dendritic Polymers. John Wiley & Sons, Ltd, Chichester, pp 587–604. https://doi.org/10.1002/0470845821.ch25
Eslami A, Yazdanbakhsh AR, Asadi A, Ghadimi M (2014) Nitrate removal from drinking water using modified natural clays. J Water Wastewater 25:127–134
Eslami A, Yazdabakhsh A, Daraee H, Karimi FS (2015) Removal of 4-chlorophenol from aqueous solutions using graphene oxide nanoporous adsorbent. J Water Wastewater 26:95
Fan L, Luo C, Sun M, Li X, Qiu H (2013) Highly selective adsorption of lead ions by water-dispersible magnetic chitosan/graphene oxide composites. Colloids Surf B: Biointerfaces 103:523–529. https://doi.org/10.1016/j.colsurfb.2012.11.006
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10. https://doi.org/10.1016/j.cej.2009.09.013
Forman D, Al-Dabbagh S, Doll R (1985) Nitrates, nitrites and gastric cancer in Great Britain. Nature 313:620–625
Gao Y, Li Y, Zhang L, Huang H, Hua J, Shah SM, Su X (2012) Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J Colloid Interface Sci 368:540–546
Grassi M, Kaykioglu G, Belgiorno V, Lofrano G (2012) Removal of emerging contaminants from water and wastewater by adsorption process. In: Lofrano G (ed) Emerging compounds removal from wastewater, pp. 15–37, Springer Briefs in Green Chemistry for Sustainability. https://doi.org/10.1007/978-94-007-3916-1_2
Gupta SS, Sreeprasad TS, Maliyekkal SM, Das SK, Pradeep T (2012) Graphene from sugar and its application in water purification. ACS Appl Mater Interfaces 4:4156–4163
Hayati B, Mahmoodi N, Maleki A (2013) Dendrimer-titania nanocomposite: synthesis and dye-removal capacity. Res Chem Intermed 41(6):3743–3757
Hu X-J, Liu Y-G, Wang H, Chen A-W, Zeng G-M, Liu S-M, Guo Y-M, Hu X, Li T-T, Wang Y-Q (2013) Removal of Cu (II) ions from aqueous solution using sulfonated magnetic graphene oxide composite. Sep Purif Technol 108:189–195
Hu Q, Chen N, Feng C, Hu W (2015) Nitrate adsorption from aqueous solution using granular chitosan-Fe3+ complex. Appl Surf Sci 347:1–9. https://doi.org/10.1016/j.apsusc.2015.04.049
Huang Z-H, Zheng X, Lv W, Wang M, Yang Q-H, Kang F (2011) Adsorption of lead (II) ions from aqueous solution on low-temperature exfoliated graphene nanosheets. Langmuir 27:7558–7562
Ilaiyaraja P, Singha Deb AK, Ponraju D, Venkatraman B (2014) Removal of cobalt from aqueous solution using xanthate functionalized dendrimer. Desalin Water Treat 52(1–3):438–445
Jothinathan L, Sozhan G, Vasudevan S (2012) Recovery of hydrogen and removal of nitrate from water by electrocoagulation process. Environ Sci Pollut Res 20(4):2184–2192. https://doi.org/10.1007/s11356-012-1028-4
Karim A, Jalil A, Triwahyono S, Sidik S, Kamarudin N, Jusoh R et al (2012) Amino modified mesostructured silica nanoparticles for efficient adsorption of methylene blue. J Colloid Interface Sci 386:307–314
Katal R, Baei MS, Rahmati HT, Esfandian H (2012) Kinetic, isotherm and thermodynamic study of nitrate adsorption from aqueous solution using modified rice husk. J Ind Eng Chem 18:295–302. https://doi.org/10.1016/j.jiec.2011.11.035
Khani A, Mirzaei M (2008) Comparative study of nitrate removal from aqueous solution using powder activated carbon and carbon nanotubes. Paper presented at the 2nd international IUPAC conference on green chemistry, Russia,
Kim J, Benjamin MM (2004) Modeling a novel ion exchange process for arsenic and nitrate removal. Water Res 38:2053–2062. https://doi.org/10.1016/j.watres.2004.01.012
Kumar ASK, Rajesh N (2013) Exploring the interesting interaction between graphene oxide, Aliquat-336 (a room temperature ionic liquid) and chromium(vi) for wastewater treatment. RSC Adv 3:2697–2709. https://doi.org/10.1039/C2RA22627H
Kumar SK, Kakan SS, Rajesh N (2013) A novel amine impregnated graphene oxide adsorbent for the removal of hexavalent chromium. Chem Eng J 230:328–337
Kyzas GZ, Deliyanni EA, Matis KA (2014) Graphene oxide and its application as an adsorbent for wastewater treatment. J Chem Technol Biotechnol 89:196–205. https://doi.org/10.1002/jctb.4220
Lin SH, Wu CL (1996) Removal of nitrogenous compounds from aqueous solution by ozonation and ion exchange. Water Res 30:1851–1857
Loganathan P, Vigneswaran S, Kandasamy J (2013) Enhanced removal of nitrate from water using surface modification of adsorbents – a review. J Environ Manag 131:363–374. https://doi.org/10.1016/j.jenvman.2013.09.034
Mahamudur I (2008) Development of adsorption media for removal of lead and nitrate from water. Thesis to PhD degree of philosophy in chemistry. Department of chemistry national institute of technology Rourkela, India
Malinga SP, Arotiba OA, Krause RWM, Mapolie SF, Diallo MS, Mamba BB (2013) Cyclodextrin-dendrimer functionalized polysulfone membrane for the removal of humic acid in water. J Appl Polym Sci 130:4428–4439
Mena-Duran CJ, Sun Kou MR, Lopez T, Azamar-Barrios JA, Aguilar DH, Domínguez MI, Odriozola JA, Quintana P (2007) Nitrate removal using natural clays modified by acid thermoactivation. Appl Surf Sci 253:5762–5766. https://doi.org/10.1016/j.apsusc.2006.12.103
Mohseni-Bandpi A, Elliott DJ, Zazouli MA (2013) Biological nitrate removal processes from drinking water supply-a review. J Environ Health Sci Eng 11:35–35. https://doi.org/10.1186/2052-336X-11-35
Mohsenipour M, Shahid S, Ebrahimi K (2015) Nitrate adsorption on clay kaolin: batch tests. J Chem 2015:7. https://doi.org/10.1155/2015/397069
Monaco ON, Tomas SC, Kirrane MK, Balija AM (2013) Bis(benzylamine) monomers: one-pot preparation and application in dendrimer scaffolds for removing pyrene from aqueous environments. Beilstein J Org Chem 9:2320–2327
Motamedi E, Atouei MT, Kassaee MZ (2014) Comparison of nitrate removal from water via graphene oxide coated Fe, Ni and Co nanoparticles. Mater Res Bull 54:34–40
Najafi F, Rajabi M (2015) Thermal gravity analysis for the study of stability of graphene oxide–glycine nanocomposites. Int Nano Lett 5:187–190. https://doi.org/10.1007/s40089-015-0154-7
Öztürk N, Bektaş TE (2004) Nitrate removal from aqueous solution by adsorption onto various materials. J Hazard Mater 112:155–162. https://doi.org/10.1016/j.jhazmat.2004.05.001
Panda AK, Mishra BG, Mishra DK, Singh RK (2010) Effect of sulphuric acid treatment on the physico-chemical characteristics of kaolin clay. Colloids Surf A Physicochem Eng Asp 363:98–104. https://doi.org/10.1016/j.colsurfa.2010.04.022
Pirsaheb M, Khosravi T, Sharafi K, Mouradi M (2016) Comparing operational cost and performance evaluation of electrodialysis and reverse osmosis systems in nitrate removal from drinking water in Golshahr, Mashhad. Desalin Water Treat 57:5391–5397. https://doi.org/10.1080/19443994.2015.1004592
Ramesh P, Bhagyalakshmi S, Sampath S (2004) Preparation and physicochemical and electrochemical characterization of exfoliated graphite oxide. J Colloid Interface Sci 274:95–102. https://doi.org/10.1016/j.jcis.2003.11.030
Rao C, Sood A, Subrahmanyam K, Govindaraj A (2009) Graphene: the new two-dimensional nanomaterial. Angew Chem Int Ed 48:7752–7777
Sadeghi-Kiakhani M, Arami M, Gharanjig K (2013) Dye removal from colored-textile wastewater using chitosan-PPI dendrimer hybrid as a biopolymer: optimization, kinetic, and isotherm studies. J Appl Polym Sci 127:2607–2619. https://doi.org/10.1002/app.37615
Samatya S, Kabay N, Yüksel Ü, Arda M, Yüksel M (2006) Removal of nitrate from aqueous solution by nitrate selective ion exchange resins. React Funct Polym 66:1206–1214. https://doi.org/10.1016/j.reactfunctpolym.2006.03.009
Sharma SK, Sobti RC (2012) Nitrate removal from groundwater: a review. J Chem 9:1667–1675
Sun L, Yu H, Fugetsu B (2012) Graphene oxide adsorption enhanced by in situ reduction with sodium hydrosulfite to remove acridine orange from aqueous solution. J Hazard Mater 203:101–110
Vadahanambi S, Lee S-H, Kim W-J, Oh I-K (2013) Arsenic removal from contaminated water using three-dimensional graphene-carbon nanotube-iron oxide nanostructures. Environ Sci Technol 47:10510–10517. https://doi.org/10.1021/es401389g
Wang K, Wu Z, Yang L, Zhang Q, Sun J, Shi Y, Xia L, Kaetsu I (2007) Grafting copolymerization of N,N-dimethylaminoethyl methacrylate (DMAEMA) onto preirradiated polypropylene films. Radiat Phys Chem 76(8-9):1367–1370. https://doi.org/10.1016/j.radphyschem.2007.02.034
Wang J, Chen Z, Chen B (2014) Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ Sci Technol 48:4817–4825. https://doi.org/10.1021/es405227u
Weber WJ, Morris JC (1963) Kinetics of adsorption carbon from solutions.American Society of Civil Engineers. J Sanit Eng Div Proc 89:31–60
WHO (2011) Guidelines for drinking-water quality, vol 38. WHO chronicle, 4 ed. World Health Organization, Geneva
Wu Y, Wang Y, Wang J, Xu S, Yu L, Philippe C, Wintgens T (2016) Nitrate removal from water by new polymeric adsorbent modified with amino and quaternary ammonium groups: Batch and column adsorption study. J Taiwan Inst Chem Eng 66:191–199. https://doi.org/10.1016/j.jtice.2016.06.019
Xu J, Pu Y, Qi W-K, Yang XJ, Tang Y, Wan P, Fisher A (2017) Chemical removal of nitrate from water by aluminum-iron alloys. Chemosphere 166:197–202. https://doi.org/10.1016/j.chemosphere.2016.09.102
Yang S-T, Chen S, Chang Y, Cao A, Liu Y, Wanga H (2011) Removal of methylene blue from aqueous solution by graphene oxide. J Colloid Interface Sci 359:24–29
Yu B, Xu J, Liu J-H, Yang S-T, Luo J, Zhou Q, Wana J, Liao R, Wang H, Liu Y (2013) Adsorption behavior of copper ions on graphene oxide–chitosan aerogel. J Environ Chem Eng 1(4):1044–1050. https://doi.org/10.1016/j.jece.2013.08.018
Zhao G, Li J, Ren X, Chen C, Wang X (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45:10454–10462. https://doi.org/10.1021/es203439v
Zhao J, Wang Z, White JC, Xing B (2014) Graphene in the aquatic environment: adsorption, dispersion, toxicity and transformation. Environ Sci Technol 48:9995–10009. https://doi.org/10.1021/es5022679
Zhou T, Chen F, Liu K, Deng H, Zhang Q, Feng J, Fu Q (2011) A simple and efficient method to prepare graphene by reduction of graphite oxide with sodium hydrosulfite. Nanotechnology 22:045704. https://doi.org/10.1088/0957-4484/22/4/045704
Acknowledgements
This article is extracted from Ph.D. thesis supported by the National Elites Foundation that is implemented in the Faculty of Civil, Water and Environmental Engineering of Shahid Beheshti University.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors.
Corresponding author
Rights and permissions
About this article
Cite this article
Alighardashi, A., Kashitarash Esfahani, Z., Najafi, F. et al. Development and Application of Graphene Oxide/Poly-Amidoamines Dendrimers (GO/PAMAMs) Nano-Composite for Nitrate Removal from Aqueous Solutions. Environ. Process. 5, 41–64 (2018). https://doi.org/10.1007/s40710-017-0279-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-017-0279-y