Abstract
Background
Storage mites are frequently found in our daily environment. Nevertheless, storage mite allergy is often neglected in allergological diagnostics and possible allergies requiring therapy are thus overlooked.
Methods
Recommendations for action are made based on a literature review from March 2021 to August 2021 in PubMed, Medline, and GoogleScholar.
Results
This review article provides an overview of the species of storage mites, sensitization rates in different countries and occupations, and co-sensitization rates to house dust mite. Recommendations for diagnosis as well as therapy are given. The importance of provocation testing as well as causal therapy by allergen immunotherapy (AIT) is presented.
Conclusion
The position paper gives recommendations for the diagnosis and therapy of allergic rhinitis in case of storage mite allergy. AIT is recommended in symptomatic allergy and proven storage mite allergy. Reliable detection by provocation testing is advised in this persistent allergy. The therapy should be carried out independently of the treatment of a possible house dust mite allergy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
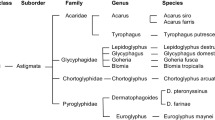
Like house dust mites, storage mites belong to the arachnid family. Here, the storage mites are classified in the families Acaridae and Glycyphagidae (Fig. 1). They are about 0.6 mm in size and are not visible to the human eye. The communication among them takes place via pheromones [1]. There are about 150 species of storage mites, of which only about 20 are of allergological importance [2]. The most important species are listed in Table 1. These include primarily
-
Plum/hay mite: Lepidoglyphus destructor,
-
Flour mite: Acarus siro,
-
House/furniture mite: Glycyphagus domesticus, and
-
Fungus mite: Tyrophagus putrescentiae.
Taxonomy of mites. (From [3])
Storage mites are among the inhalation allergens. The allergic reaction is IgE-mediated (type I allergy). The allergens of the storage mites are found in the animal body as well as in their excretions, and probably also in the eggs. The main allergens from group 2 (Lep d 2, Tyr p 2, Gly d 2) are found in the mite gut (see section “Allergens of storage mites”) [5].
Storage mites feed on protein-rich substances of animal or plant origin (cereals and forages, hay, straw, flour) [6]. Molds may also be used for feeding. Tyrophagus putrescentiae also likes to feed on protein- and fat-containing foods such as ham and cheese [5]. Optimal development conditions are an ambient temperature around 25 °C and a relative humidity of 90%. If the relative humidity falls below 60%, development is hardly possible [1]. Storage mites live shorter lives than house dust mites, but their reproductive rate is about three to seven times higher. Thus, up to 30 generations per year can develop [7]. The storage mites pass through six stages of development (egg, prelarva, larva, protonymph, tritionymph, adult) [8].
Occurrence and sensitization rates
The habitat of storage mites is oriented to the needs mentioned above. Thus, they can be found in large quantities in hay/straw and feed stores near animal husbandry facilities. However, they can also be found in private environments, especially in food storage facilities or in house dust. In a study in northern Europe, the sensitization rate was higher in men (11%) than in women (8%) [9]. Among the groups of people particularly at risk indicated in the literature are (Fig. 2):
-
Farmers and agricultural workers (13–36% awareness) [10, 11],
-
Grain worker [12],
-
Food preparation workers [13],
-
People who work with laboratory animals (35% sensitization) [14] or work in animal husbandry,
-
Workers in moisture-damaged buildings [15],
-
Workers in meat production [6],
-
Miller
In the workplaces of these groups of people, the storage mites find the optimal living and feeding conditions for reproduction. The storage mite Blomia tropicalis occurs mainly in the tropics and subtropics.
Some studies also consider bakers as a predisposed group of people to storage mite allergy, as flour stores are often located in bakeries. Studies from Denmark and Norway indicate sensitization rates of 11–20% [16, 17]. Other studies do not see a higher sensitization rate of bakers to storage mites compared to the general population. However, it has been shown that sensitization to flour is almost always accompanied by co-sensitization to storage mites [18, 19].
For the particularly exposed group of farmers and agricultural workers, a sensitization rate of 13% [17] to 36% [10] is given. The highest density of storage mites was measured in the pigsty, in the hay and straw store, and at the grinding-mixing plant. In particular, the species Acarus siro, Lepidoglyphus destructor, Acarus farris, Tyrophagus longior, Glycyphagus domesticus, Blomia tjibodas and Thyreophagus entomophagus could be identified [20, 21]. In a Danish study of farm workers, an increase in Lepidoglyphus destructor sensitization from 6 to 13% in 12 years was seen [11]. Similarly, it was shown that an increased number of storage mites was also detected in the living quarters of the farmers, but this was less than in the working quarters [21].
Data on the sensitization rate of the general population vary from 5.3% [22] to 23.2% [3]. There are no regional differences throughout Germany [23], whereas regional differences in sensitization rates have been described for house dust mites. For certain groups of persons, such as asthmatics, strongly varying values of 7.4% [24] to 44% [25] have been reported. Studies on sensitization rates in children yielded values from 11 [26] to 25.5% [27]. Likewise, co-sensitization to grasses (45%) and early bloomers (birch 42%, hazel 31%) as well as to house dust mites (see section “Cross-reactivity”) was frequently found [28]. The occurrence of storage mites in households of the general population should not be underestimated. For example, Glycyphagus domesticus was found in 50% of house dust samples, whereas the house dust mite Dermatophagoides pteronyssinus was detected in 95% of samples [29]. In addition to Glycyphagus domesticus, Tyrophagus putrescentiae, Lepidoglyphus destructor, and Blomia tjibodas were found in urban bedrooms, living rooms, kitchens, pantries, and basements [30]. A survey of stored grain-based foods found storage mites in 21% of samples from a supermarket outlet and in 38% of samples stored for 6 weeks in private households [31].
Most of the studies performed a prick test or a test for specific IgE against storage mites to detect sensitization. The question of the presence of symptoms attributable to the storage mites remains open in most cases. It was also shown that the results of the prick test and IgE in the laboratory did not necessarily correlate [3]. In the study by Huppertz et al. a nasal provocation test was performed to detect allergy after sensitization (23.3% of the study population). Here, 17% showed a positive test for Lepidoglyphus destructor and 33% on provocation with Tyrophagus putrescentiae [3]. The sensitization rate to storage mites was higher than the actual allergy frequency. Unfortunately, most studies lack evidence of allergy via provocation testing. Furthermore, Kroidl et al. conducted a multicenter study with 90 patients (47% farmers) with bronchial asthma (grade II and III) and storage mite sensitization, in which the results of bronchial provocation were compared with prick test and laboratory results [36]. It was shown that there was no correlation between prick testing and bronchial provocation testing, whereas for the species Lepidoglyphus destructor there was a significant correlation between laboratory and positive bronchial provocation testing. In 145 bronchial provocations performed, they were positive when allergen from Acarus was used in 50%, Lepidoglyphus in 87% and Tyrophagus in 52% of tests. This suggests a high percentage of relevant allergy and the cause of bronchial asthma in storage mite sensitization. For the also tested house dust mite Dermatophagoides pteronyssinus, only 39% of the bronchial provocation tests were positive in the presence of sensitization.
Currently, no further studies with provocation tests are available in the literature, which allow a more precise statement on the clinical relevance of the presence of an allergy in case of storage mite sensitization.
Allergens of storage mites
Allergens are classified into groups based on previously identified allergens (IUIS Allergen Nomenclature Database, group 1–33 previously known, www.allergen.org) [1]. Storage mites contain mainly the protein families fatty acid binding protein and tropomyosin, but also paramyosin homologs, apolipophorin and α‑tubulin have been identified (classes 2, 5 and 7) [2]. The most significant allergens include Lep d 2 of the species Lepidoglyphus destructor, Gly d 2 of the species Glycyphagus domesticus, and Tyr p of the 2 species Tyrophagus putrescentiae. For Acarus siro, only the allergen Aca s 13 has been identified so far.
Cross reactivity of storage mites
Regarding an existing cross-reactivity of storage mites and house dust mites, there is disagreement in the literature. As listed in Fig. 2, the rates of co-sensitization with house dust mites in case of storage mite sensitization vary from 44% [9] to 85% [32] in the general population. Especially in children, very high co-sensitization rates seem to be observed from 65.9% [27] to 92% [26]. The co-sensitization rates of the individual species are given in different frequencies:
Huppertz et al. found sensitization to all three of the above-mentioned storage mites in 44% of cases of co-sensitization to house dust mites [3].
Molecular biology studies of the individual allergens revealed little cross-reactivity between Der p 2, the major allergen of the house dust mite Dermatophagoides pteronyssinus, and the group 2 allergens [7, 29, 33, 34, 37, 38]. The reason for this is seen to be multiple amino acid substitutions of the allergens [37]. The authors conclude a parallel or co-sensitization by house dust mite allergens and no true cross-reactivity. In contrast, a high cross-reactivity is seen within the group 2 allergens (Lep d 2, Gly d 2, Tyr p 2) of the storage mites Lepidoglyphus destructor, Glycyphagus domesticus, and Tyrophagus putrescentiae [29, 37, 39]. The molecular basis for cross-reactivity is considered to be an amino acid sequence identity of Gly d 2 and Lep d 2 of more than 80%. In contrast, the identity of Der p 2 to Gly d 2, Lep d 2, and Tyr p 2 is only about 40%. An amino acid sequence identity of at least 60–70% is assumed for cross-reactivity. A probable cross-reactivity of Lep d 10 with Der f 10 as well as Der p 10 is also mentioned in the literature [5]. Furthermore, it could be shown that in IgE immunoblotting a Glycyphagus domesticus extract could block IgE binding to Lep d 2 in the same way as a Lepidoglyphus destructor extract [29]. In another study, an extract of Dermatophagoides pteronyssinus was able to block IgE binding to storage mites by up to 60%, whereas storage mite extracts barely blocked IgE binding to house dust mites, providing further evidence of co-sensitization [33].
Medical history for storage mite allergy
The anamnesis for suspected storage mite allergies should include all points of the general allergy anamnesis. Here, the points mentioned below should be addressed.
Symptoms
Permanent nasal obstruction, sneezing, rhinorrhea, impaired ability to smell and taste, allergic skin eczema, and allergic conjunctivitis must be queried. But also asthmoid complaints such as dry cough or recurrent dyspnea, which indicate a progression to asthma, as well as sleep disturbances belong in the anamnesis discussion [40]. Similarly, patients may report a reduction in performance at work and school due to symptoms. Studies show that nasal obstruction and hyposmia, but also atopic eczema, rhinitis or urticaria may be the main symptoms of a storage mite allergy [3]. The impairment caused by the symptoms should be recorded using a visual analog scale (0–10). This can be used as a follow-up parameter during a possible allergen immunotherapy (AIT). The evaluation of anaphylactic reactions in the past should also be performed. For storage mites in cereal flour and oilseeds these are described. The so-called “pancake syndrome” describes severe allergic symptoms, which are triggered by foods with a high mite and storage mite load and their allergens [41].
Temporal/local progression
Here it should be described since when the complaints exist and whether there is a seasonal variance in the severity of the complaints. The storage mite allergy belongs to the persistent allergies. In this context, a possible decrease in symptoms during vacations in exposed occupations or a seasonal or local variation should be investigated. A maximum of complaints is often reported in summer and autumn [3].
Occupational history
Attention should be paid to specially exposed occupational groups (see also “Occurrence and sensitization rates”), who are exposed to an increased storage mite load due to their workplace. These include farmers and agricultural workers, grain workers, food workers, people working with laboratory animals or in animal husbandry, workers in moisture-damaged buildings, workers in meat production, and millers. The occupational environment and possible storage places of grain and feed, hay, straw, flour or mold contamination of the work area should be inquired. If the occupation is not goal-directive, the domestic environment (supply-storage, mold) or hobbies (animal husbandry etc.) should be dealt with more exactly.
Medication
Of particular interest during history taking are systemic or topical antihistamines or glucocorticoids for symptomatic therapy. Information about any existing use is critical prior to any planned testing to ensure compliance with the specified withdrawal times. Furthermore, the required medication and frequency of intake provides information about the impairment caused by the allergic symptoms. However, other medications such as β‑blockers, Angiotensin-converting enzyme (ACE) inhibitors or tricyclic antidepressants can also influence diagnostic tests, so these should be inquired about.
Secondary diagnoses
The patient’s secondary diagnoses are crucial in assessing the possibility of AIT (autoimmune diseases, diabetes, hypertension, rheumatic diseases, malignant tumors, blood coagulation disorders/thromboses, infectious diseases). Particular attention should be paid to any known house dust mite allergy as well as grass and early blossom allergy [3].
Diagnosis
In the case of suspected storage mite allergy, existing preliminary findings must first be reviewed. In the diagnosis of persistent inhalation allergens, in addition to the sensitization test by prick test or serological determination of the specific IgE, a provocation test is necessary for the reliable detection of allergy. During prick and provocation testing, the necessary abstinence from medications that could influence the test (e.g., antihistamines, glucocorticoids, tricyclic antidepressants) must be ensured.
Prick test
For the diagnosis of suspected storage mite allergy, the German S2 guideline on skin testing for inhalation allergies recommends [42] in addition to testing of the two house dust mites Dermatophagoides farinae and Dermatophagoides pteronyssinus also the testing of the species Acarus siro, Lepidoglyphus destructor, and Tyrophagus putrescentiae. Currently, prick test solutions are also available only for these recommended storage mites (Table 2).
Laboratory
Serological determination of allergen-specific antibodies of type IgE is equivalent to skin testing for the detection of sensitization [43]. The specific IgE for Acarus siro, Glycophagus domesticus, Lepidoglyphus destructor, Tyrophagus putrescentiae and Blomia tropicalis can be determined in laboratories. Individual allergens are not available.
Provocation testing
For provocation testing, provocation solutions are currently available for the storage mites Acarus siro, Lepidoglyphus destructor, and Tyrophagus putrescentiae (Table 2). In case of frequent co-sensitization of house dust and storage mites, the reliable allergy detection by provocation testing is crucial for a possible AIT. Similarly, provocation testing could be used to detect local allergic rhinitis to storage mites. In addition to the nasal provocation test, the conjunctival provocation test or, in individual cases as in the case of the expert opinion, a bronchial provocation test can also be used. In this case, the respective contraindications of the provocation tests must be observed.
Therapy
In addition to the abstinence measures as well as symptomatic therapy, the patient can be offered AIT. This represents the only causal therapy for storage mite allergy [44].
Protective measures
Because storage mites are particularly common in agricultural and livestock environments, countermeasures are often difficult to implement [20]. Killing them with chemicals or acaricides, such as benzyl benzoate, or destroying their allergens with denaturing agents, such as tannic acid [43] is not feasible for ecological as well as environmentally and animal friendly farming. Occasionally, the use of acaricides in the residential sector is described [20]. The predatory mites Cheyletus eruditus are considered to be natural enemies of the storage mites, which in turn can lead to sensitization and therefore do not represent a realistic countermeasure. The wearing of a blower-supported respiratory helmet [20] for allergic farmers represents a measure for symptom reduction, but is rather impractical in daily work. More practical is the respirator (at least FFP2). This can filter the major allergens, which are bound to particles (about 20 µm in diameter) and have poor suspension properties [20].
General measures for the domestic environment are largely based on those for the house dust mite. Thus, bed mattress encasing and avoidance of textile floor coverings or curtains are recommended. To prevent the introduction of storage mites into the home, work clothes should be worn and stored away from the living area (e.g., work clothes should be stored in an airlock).
Symptomatic therapy
Symptomatic therapy with topical and/or systemic antihistamines and/or topical corticosteroids should be recommended to the patient. Here, the immediate symptom reduction and reduction of the burden of discomfort in everyday life is in the foreground. Supplementary nasal irrigation before application of the nasal spray is recommended to reduce the intranasal mite load and to cleanse the mucous membranes.
AIT indication
The indication for AIT is given in case of moderate to severe symptoms of the patient, which persist over a longer period of time (about 2 years), and allergen abstinence should also not be possible for the patient. Furthermore, a positive provocation test is necessary in addition to the laboratory or prick test for the presence of a reliable proof of allergy. The patient should have consented to the therapy after documented information about the course of treatment and possible side effects and should be compliant throughout the entire therapy period. A high degree of therapy adherence is crucial for the success of the therapy. The patient must be informed about a possible progression to asthma (bronchial asthma) in the absence of therapy. If the patient already has allergic bronchial asthma, AIT is recommended [45]. If there is severe asthma due to storage mite allergy, the use of omalizumab can be discussed.
In the case of confirmed allergy due to storage mites, AIT against storage mites should be performed separately and independently of house dust mite AIT, since for storage mites there is rather a co-sensitization to the house dust mite and no real cross-reactivity (see section “Cross-reactivity”).
Contraindications
The relative and absolute contraindications described in Table 8 of the S2k guideline on AIT for IgE-mediated diseases also apply to AIT against storage mites [44]. Before initiating subcutaneous AIT, spirometry should be performed in asthmatics to document an FEV1 above 80%.
Preparations
The Therapy Allergen Regulation (TAV) applicable in Germany regulates the approval of therapeutic allergens [46]. In this context, storage mites count as individual formulations for AIT outside the TAV (except for distribution in mixtures with TAV allergens). These individual formulations are not subject to regulatory approval and are therefore not subject to regulatory control of quality, efficacy and tolerability, nor to governmental batch testing. However, a manufacturing authorization according to Arzneimittelgesetz (Medicinal Products Act) is required here as well. Since the market share of rare allergens is below 3 [7], many manufacturers of AIT solutions are discontinuing production and distribution for economic reasons. This trend can unfortunately be observed for many of the rare allergens and leads to a shortage for the therapy preparation selection.
An overview of the preparations currently available on the market can be found in Table 3. So far, only preparations for subcutaneous injection are available. The selection of the preparation should be based on the allergen of the respective storage mite family identified in the diagnostics.
Treatment regimens and duration of therapy
AIT should always be initiated by an experienced allergist. Sufficient knowledge about the management of possible anaphylactic reactions is very important here. The injections must be administered according to the manufacturer’s technical and instructions for use. The duration of therapy must be set at a minimum of 3 years and, if necessary, extended to 5 years if symptom reduction is still insufficient.
Side effects
In addition to local reactions at the injection site, anaphylactic reactions may occur. In this case, the necessary emergency medication should be available in the practice. An observation period of at least 30 min after injection is recommended and the patient should be informed about what to do if late reactions occur.
Therapy control and treatment success
Fixed parameters for therapy follow-up do not exist yet. However, it is possible to monitor the patient clinically by means of questionnaires and visual analogue scales, which measure the severity of symptoms and the consumption of medication, and to assess developments. It is recommended to check the patient at least every 6 months. If there is no improvement in symptoms after 1 to 2 years, a change of medication may be considered. Provocation tests as well as prick and laboratory diagnostics are not suitable measuring instruments for therapy follow-up. Unfortunately, only a few studies on treatment success are available [47]. A study by Becker et al. [48] showed a significant efficacy of storage mite AIT measured by nasal provocation testing (decrease in organ-specific reactivity) as well as secondary efficacy parameters (symptom reduction and need for anti-allergic medication).
Occupational disease
Allergy to storage mites can be claimed as an occupational disease for certain occupational groups and under verifiable conditions, corresponding to “BK No. 4301”. In this context, the German Employer’s Liability Insurance Association for Agriculture, Forestry and Horticulture once again provides detailed information in the “Dust” brochure (B43) on protective measures to be taken to prevent the occupational disease.
Discussion and conclusion
Storage mite allergy often receives too little attention in allergological practice and is often forgotten in diagnosis. The prevalences shown in the cited studies vary widely. Nevertheless, sensitization is not only found in the exposed occupational groups, but also in the general population in Germany where the sensitization rate is estimated to be about 20% [3, 32]. Accordingly, the S2k guideline on skin testing for inhalation allergens [42] recommends routine testing for storage mites. This should of course be supplemented by a detailed anamnesis (including occupational anamnesis) in order to determine any occupational disease. Of high relevance in diagnostics is the proof of topicality by provocation testing. Unfortunately, in the absence of economic stimuli in the production of extracts of rare allergens, the availability of preparations and test solutions is decreasing. Thus, we can only fall back on a very limited selection of preparations. However, in case of an existing storage mite allergy, therapy should definitely be carried out. This is to be carried out independently of a house dust mite AIT, since a cross-reactivity is not to be assumed. Future studies on the frequency of allergy in case of sensitization by storage mites as well as on the effectiveness of AIT against storage mites are desirable.
References
Raulf M, Bergmann KC, Kull S, Sander I, Hilger Ch, Brüning T, et al. Mites and other indoor allergens—from exposure to sensitization and treatment. Allergo J Int. 2015;24:68–80.
Fernández-Caldas E, Puerta L, Caraballo L. Mites and allergy. History of allergy. Chem Immunol Allergy. 2014;100:234–42.
Huppertz T, Schmidtmann I, Becker S, Haxel B. Characterization of a cohort of storage mite sensitized subjects. Allergo J Int. 2020;29:1–8.
Klimek L, Brehler R, Bergmann K‑C. Allergen-specific immunotherapy with storage mites. Allergo J Int. 2018;27:15–9.
Hilger C, Kühn A, Raulf M, Jakob T. Allergien auf Schaben, Zecken, Vorratsmilben und andere Gliederfüßer: molekulare Aspekte. In: Kleine-Tebbe J, Jakob T, editors. Molekulare Allergiediagnostik. Berlin, Heidelberg: Springer; 2015. pp. 315–27.
Tafuro F, Ridolo E, Goldoni M, Montagni M, Mutti A, Corradi M. Work-related allergies to storage mites in Parma (Italy) ham workers. BMJ Open. 2015. https://doi.org/10.1136/bmjopen-2014-007502
Klimek L, Brehler R, Bergmann K‑C. Allergenspezifische Immuntherapie mit Vorratsmilben – eine vernachlässigte Indikation. Allergo J. 2018;27:32–6.
Bergmann K, Müsken H. Milben sind nicht gleich Milben: Artenvielfalt im Hausstaub. AL. 2015;38(2):47–54.
Jõgi NO, Olsen RK, Svanes C, Gislason D, Gislason T, Schlünssen V, et al. Prevalence of allergic sensitization to storage mites in Northern Europe. Clin Exp Allergy. 2020;50:372–82.
Müsken H, Franz J, Wahl R, Paap A, Cromwell O, Masuch G, et al. Sensitization to different mite species in German farmers: in vitro analyses. J Investig Allergol Clin Immunol. 2003;13:26–35.
Elholm G, Schlünssen V, Doekes G, Basinas I, Omland Ø, Grønager PM, et al. Adult farming exposure does not protect against sensitization to the storage mite Lepidoglyphus destructor. Allergy. 2018;73:2234–7.
Blainey AD, Topping MD, Ollier S, Davies RJ. Allergic respiratory disease in grain workers: the role of storage mites. J Allergy Clin Immunol. 1989;84:296–303.
Armentia A. Occupational asthma from storage mites contaminating foods. J Investig Allergol Clin Immunol. 1997;7:407–8.
Ruoppi P, Koistinen T, Pennanen S. Sensitisation to mites in laboratory animal workers with rhinitis. Occupational and environmental medicine. BMJ. 2005;62:612–5.
Roponen M, Kiviranta J, Seuri M, Tukiainen H, Myllykangas-Luosujärvi R, Hirvonen M‑R. Inflammatory mediators in nasal lavage, induced sputum and serum of employees with rheumatic and respiratory disorders. Eur Respir J. 2001;18:542–8.
Skjold T, Nielsen SC, Adolf K, Hoffmann HJ, Dahl R, Sigsgaard T. Allergy in bakers’ apprentices and factors associated to non-participation in a cohort study of allergic sensitization. Int Arch Occup Environ Health. 2007;80:458–64.
Storaas T, Steinsvåg SK, Florvaag E, Irgens Å, Aasen TB. Occupational rhinitis: diagnostic criteria, relation to lower airway symptoms and IgE sensitization in bakery workers. Acta Otolaryngol. 2005;125:1211–7.
Revsbech P, Dueholm M. Storage mite allergy among bakers. Allergy. 1990;45:204–8.
Droste J, Myny K, Van Sprundel M, Kusters E, Bulat P, Braeckman L, et al. Allergic sensitization, symptoms, and lung function among bakery workers as compared with a nonexposed work population. J Occup Environ Med. 2003;45:648–55.
Müsken H. Vorratsmilben: aktuelles Wissen und Karenzmöglichkeiten. Allergo J. 2004;13:452–9.
Franz J‑T, Masuch G, Müsken H, Bergmann K‑C. Mite fauna of German farms. Allergy. 1997;52:1233–7.
Yadav A, Elder BL, Morgan MS, Vyszenski-Moher DL, Arlian LG. Prevalence of serum IgE to storage mites in a southwestern Ohio population. Ann Allergy Asthma Immunol. 2006;96:356–62.
Milbenatlas Deutschland, data on file Firma LETI, personal communication Dr. med. Christoph Langer, HNO-Praxis Füssen
Iversen M, Dahl R. Allergy to storage mites in asthmatic patients and its relation to damp housing conditions. Allergy. 1990;45:81–5.
Hardel P, de Lajudie J, Portal B, Ville G, Guilloux L, D’Athis P. Allergy to Thyrophagus putrescentiae and Lepidoglyphus destructor in a population of young asthmatic adults. Allerg Immunol. 1987;19:399–404.
Gaig P, Botey J, Pena M, Marín A, Eseverri J. Study of the sensitization to storage mites in a pediatric population in Barcelona. J Investig Allergol Clin Immunol. 1993;3:151–5.
Akdemir C, Soyucen E. Sensitization of children to storage mites in Kutahya, Turkey. Korean J Parasitol. 2009;47:387–91.
Schumacher B, Klimek L. AR-Patienten oft gegen Vorratsmilben sensibilisiert. Allergo J. 2020;29:12–3.
Arias-Irigoyen J, Lombardero M, Arteaga C, Carpizo JA, Barber D. Limited IgE cross-reactivity between Dermatophagoides pteronyssinus and Glycyphagus domesticus in patients naturally exposed to both mite species. J Allergy Clin Immunol. 2007;120:98–104.
Franz J, Masuch G, Müsken H, Bergmann K. Erweiterte Untersuchungen zur Domestic Mite Fauna im rein städtischen Wohnmilieu. Allergo J. 1997. https://doi.org/10.1111/j.1398-9995.1997.tb02529.x.
Thind BB, Clarke PG. The occurrence of mites in cereal-based foods destined for human consumption and possible consequences of infestation. Exp Appl Acarol. 2001;25:203–15.
Müsken H, Fernández-Caldas E, Marañón F, Franz J, Masuch T, Bergmann K. In vivo and in vitro sensitization to domestic mites in German urban and rural allergic patients. J Investig Allergol Clin Immunol. 2002;12(3):177–81.
van der Heide S, Niemeijer NR, Hovenga H, de Monchy JGR, Dubois AEJ, Kauffman HF. Prevalence of sensitization to the storage mites Acarus siro, Tyrophagus putrescentiae, and Lepidoglyphus destructor in allergic patients with different degrees of sensitization to the house-dust mite Dermatophagoides pteronyssinus. Allergy. 1998;53:426–30.
Luczynska CM, Griffin P, Davies RJ, Topping MD. Prevalence of specific IgE to storage mites (A. siro, L. destructor and T. longior) in an urban population and crossreactivity with the house dust mite (D. pteronyssinus). Clin Exp Allergy. 1990;20:403–6.
Gislason D, Gislason T. IgE-mediated allergy to Lepidoglyphus destructor in an urban population—an epidemiologic study. Allergy. 1999;54:878–83.
Kroidl R, Schwichtenberg U, Frank E. Asthma bronchiale bei Allergie gegen Vorratsmilben. Pneumologie. 2007;61:525–30.
Smith AM, Benjamin DC, Hozic N, Derewenda U, Smith W‑A, Thomas WR, et al. The molecular basis of antigenic cross-reactivity between the group 2 mite allergens. J Allergy Clin Immunol. 2001;107:977–84.
van Hage-Hamsten M, Johansson SGO. Storage mites. Exp Appl Acarol. 1992;16:117–28.
Griffin P, Ford AW, Alterman L, Thompson J, Parkinson C, Blainey AD, et al. Allergenic and antigenic relationship between three species of storage mite and the house dust mite, Dermatophagoides pteronyssinus. J Allergy Clin Immunol. 1989;84:108–17.
Klimek L, Gröger M, Becker S. Milbenallergie im HNO-Bereich: Bedeutung, Diagnostik und Therapieoptionen. Laryngorhinootologie. 2018;97:56–69.
Sánchez-Borges M, Fernandez-Caldas E. Hidden allergens and oral mite anaphylaxis: the pancake syndrome revisited. Curr Opin Allergy Clin Immunol. 2015;15:337–43.
Rueff F, Bergmann K‑C, Brockow K, Grübl A, Jung K, Klimek L, et al. Hauttests zur Diagnostik von allergischen Soforttyp-Reaktionen. Leitlinie der Deutschen Gesellschaft für Allergologie und klinischen Immunologie (DGAKI). Pneumologie. 2011;65:484–495. https://doi.org/10.1055/s-0030-1256476.
Funes P, Brehler R, Bergmann C, Klimek L. Milbenallergie: Auch an Vorratsmilben denken! HNO Nachr. 2018;48:32–5.
Pfaar O, Bachert C, Bufe A, Buhl R, Ebner C, Eng P, et al. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases. Allergo J Int. 2014;23:282–319.
Nationale Versorgungsleitlinie Asthma 4. Auflage, Version 1. AWMF-Register-Nr.: nvl-002 Sep 7, 2020
Bundesministerium für Justiz und Verbraucherschutz. Verordnung über die Ausdehnung der Vorschriften über die Zulassung der Arzneimittel auf Therapieallergene, die für einzelne Personen auf Grund einer Rezeptur hergestellt werden, sowie über Verfahrensregelungen der staatlichen Chargenprüfung (Therapieallergene-Verordnung). 2008
Armentia-Medina A, Tapias JA, Martín JF, Ventas P, Fernández A. Immunotherapy with the storage mite lepidoglyphus destructor. Allergol Immunopathol. 1995;23:211–23.
Becker K, Sperl A, Bardenhewer C, Spielhaupter M, Hörmann K, Pfaar O, et al. (Allergen‑)spezifische Immuntherapie bei Rhinitis allergica auf Vorratsmilben. Allergologie. 2017;40(5):185–95. https://doi.org/10.5414/ALX1925.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Cuevas reports personal fees from Novartis and Sanovi-Aventis, Allergopharma, HAL Allergie, Leti Pharma, and personal fees and nonfinancial support from AstraZeneca, GalaxoSmithKline, ALK, Bencard Allergie, Stallergenes, Roxall, as well as conducting studies for Lofarma, outside of the submitted work; and membership in the following organizations: AeDA, DGHNO. H. Wrede reports grants and/or honoraria from Allergopharma, MEDA/Mylan, Allergy Therapeutics/Bencard, HAL Allergy, LETI Pharma, Novartis, Stallergenes, Lofarma, GSK, Sanofi outside the submitted work; and membership in the following organizations: AeDA. L. Klimek reports grants and/or honoraria from Allergopharma, MEDA/Mylan, Allergy Therapeutics/Bencard, AstraZeneca, ASIT Biotech, HAL Allergie, ALK Abelló, LETI Pharma, Novartis, Stallergenes, Quintiles, Lofarma, GSK, Inmunotek, Sanofi, Thermofisher, outside the submitted work; and membership in the following organizations: AeDA, DGHNO, German Academy of Allergology and Clinical Immunology, GPA, EAACI. L. Klimek is the editor in chief of the Allergo Journal and Allergo Journal International in which this manuscript will be published. M.-L. Polk, S. Becker, T. Huppertz, J. Hagemann, C. Bergmann, W. Schlenter, B. Haxel and K.-C. Bergmann declare that they have no competing interests.
Additional information
The authors M. Cuevas and M.-L. Polk contributed equally to the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cuevas, M., Polk, ML., Becker, S. et al. Rhinitis allergica in storage mite allergy. Allergo J Int 31, 59–68 (2022). https://doi.org/10.1007/s40629-022-00205-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-022-00205-w