Abstract
Diabetic kidney disease (DKD) affects approximately one-third of patients with diabetes and taking into consideration the high cardiovascular risk burden associated to this condition a multifactorial therapeutic approach is traditionally recommended, in which glucose and blood pressure control play a central role. The inhibition of renin–angiotensin–aldosterone RAAS system represent traditionally the cornerstone of DKD. Clinical outcome trials have demonstrated clinical significant benefit in slowing nephropathy progression mainly in the presence of albuminuria. Thus, international guidelines mandate their use in such patients. Given the central role of RAAS activity in the pathogenesis and progression of renal and cardiovascular damage, a more profound inhibition of the system by the use of multiple agents has been proposed in the past, especially in the presence of proteinuria, however clinical trials have failed to confirm the usefulness of this therapeutic approach. Furthermore, whether strict blood pressure control and pharmacologic RAAS inhibition entails a favorable renal outcome in non-albuminuric patients is at present unclear. This aspect is becoming an important issue in the management of DKD since nonalbuminuric DKD is currently the prevailing presenting phenotype. For these reasons it would be advisable that blood pressure management should be tailored in each subject on the basis of the renal phenotype as well as related comorbidities. This article reviews the current literature and discusses potentials and limitation of targeting the RAAS in order to provide the greatest renal protection in DKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout their lifetime, approximately one-third of patients with diabetes will eventually develop diabetic kidney disease (DKD) [1]. Furthermore, DKD is currently the leading cause of chronic kidney disease as well as of end-stage kidney disease (ESKD) worldwide [2]. In patients with type 2 diabetes, DKD entails an unfavorable impact on cardiovascular (CV) risk burden [3, 4]. As a matter of fact, subjects with diabetes and renal impairment are more likely to die from CV disease than to survive long enough and face the need of renal replacement therapy [5].
In the context of this rather complex and clinically challenging scenario a multifactorial therapeutic approach has traditionally been recommended. As a matter of fact, more than a decade ago, in the Steno-2 study an intensified multifactorial intervention with tight glucose control, the use of renin–angiotensin-aldosterone system (RAAS) blockers, aspirin, and lipid-lowering agents on top of behavior modification had been proved to effectively reduce CV morbidity and mortality over a long-term follow-up in patients with persistent microalbuminuria [6]. Nowadays, a combination of optimal glycemic and blood pressure control by the use of RAAS system inhibitors is still the cornerstone for prevention and treatment of DKD [7].
Hypertension is a common condition in patients with diabetes and its prevalence in subjects with DKD is significantly higher than in general population and increases gradually according to glomerular filtration rate (GFR) decline, reaching the 90% in ESKD subjects [8]. Therefore, the management of hypertension plays a central role since an adequate blood pressure control is able to reduce the burden of albuminuria, the progression of DKD as well as CV risk burden. However, in this setting of intervention, blood pressure targets and pharmacological choices are still a matter of debate in clinical practice.
Antihypertensive treatment and renal protection in type 2 diabetes: the need for a patient centered approach
Albuminuria is a known marker of renal damage and has been shown to have a strong cardiovascular and renal predictive power. Furthermore, modifications of urinary albumin excretion under pharmacological treatment might impact CV and renal prognosis independent of blood pressure reduction [9,10,11]. In the classical, five-stage course of diabetic nephropathy, mostly derived from studies conducted in patients with type 1 diabetes, microalbuminuria has traditionally been taken to represent the first sign of renal involvement [12] (Fig. 1). According to this clinical paradigm, overtime, albuminuria eventually progresses to overt proteinuria, which in turn, precedes GFR decline. While the natural history of DKD in type 2 diabetes seems to be more heterogenous, the assessment of albuminuria has been traditionally considered the reference tool for diagnosis of diabetic nephropathy and the reduction of progression or regression of albuminuria has been the primary end-point in many clinical trials aiming at the evaluation of nephroprotection conferred by the pharmacological inhibition of RAAS [13].
More recent studies, however, have shown that DKD in both type 1 and type 2 diabetes has an heterogeneous histologic phenotype, with various involvement of glomeruli and the interstitium [14]. On the other hand, it has recently been reported that a considerable proportion of diabetic patients with reduced GFR have a normal or only slightly increased urinary albumin excretion indicating that GFR decline does not necessarily parallel changes in albuminuria [15, 16]. Both features of DKD, reduction of GFR and albuminuria, have an independent prognostic role in terms of CV and renal events and, therefore, the awareness and a better knowledge of these different conditions might have an impact in the management of DKD. Several studies as well as real-life observations have pointed out that a strict blood pressure control is associated to a reduction of albuminuria, but is not always associated to a benefit in terms of GFR preservation [17, 18]. Accordingly, it has been suggested that lower BP values might be beneficial in terms of renal protection in diabetic patients with an albuminuric phenotype, while in absence of albuminuria this benefit is uncertain [19]. Therefore, it seems that an individually tailored treatment strategy in terms of both optimal blood targets and choice of antihypertensive drugs combinations could be desirable on the basis of the presenting renal phenotype [20]. This issue needs to be verified by studies specifically conducted in patients with non-albuminuric renal impairment.
DCRecent International Guidelines [21] suggest that in patients with diabetes and hypertension, blood pressure targets should be individualized through a shared decision-making process that addresses cardiovascular risk among other variables. For individuals at high or very high cardiovascular risk (with atherosclerotic cardiovascular disease or 10-year Atherosclerotic Cardiovascular Disease risk ≥ 15%) a blood pressure target of < 130/80 mmHg seems to be appropriate, if it can be safely attained, while for those at lower risk for cardiovascular disease (10-year Atherosclerotic Cardiovascular Disease risk < 15%) a target of < 140/90 mmHg is recommended.
The inhibition of RAAS along the renal continuum
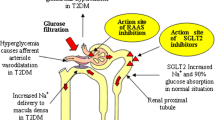
DKD is a complex disease, wherein various pathological changes including glomerular hypertrophy with progressive mesangial expansion and tubulointerstitial fibrosis are associated with multiple pathophysiological abnormalities such as inappropriate activation of the RAAS at the systemic and tissue level [22, 23]. Hypertension and hyperglycaemia play a central role in the development and progression of renal damage contributing to structural and functional alterations. In particular, at the renal level increase intraglomerular pressure, cellular growth, and fibroblast differentiation have been shown to favor the development of interstitial fibrosis and glomerular sclerosis. Reducing intraglomerular pressure with an angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) can minimize, or even prevent, glomerular disease and albumin excretion. Furthermore, several experimental, animal, and in vivo studies have shown that the pharmacologic inhibition of RAAS leads to attenuation of interstitial fibrosis and glomerular sclerosis by down-regulation of advanced glycation end-products, TGF-β, NADPH oxidase, reactive oxygen species, reduced expression of receptors for advanced glycation end-products, reduced type IV collagen excretion and reduced mesangial extracellular matrix accumulation [24]. For all these reasons RAAS inhibitors have traditionally represented the mainstay treatment of hypertension in diabetic patients [25]. In fact, the effect of pharmacological RAAS blockade at various levels, by using ACEIs, ARBs, direct renin inhibitors, and mineralocorticoid antagonists, has been evaluated along the renal continuum.
Potentials of RAAS Inhibition
Main clinical trials that have evaluated renal protective effect of RAAS blockade across the renal continuum in DKD are reported in Table 1.
Patients with normal albuminuria In diabetic patients without microalbuminuria RAAS blockade has been studied to evaluate the effect on the transition from normoalbuminuria to microalbuminuria. Trials conducted on type 1 diabetic patients, such as Renin-Angiotensin System Study (RASS) [26] and Diabetic Retinopathy Candesartan Trial (DIRECT)-Prevent 1, and DIRECT-Protect 1 [27] failed to show any benefit in the prevention of the development of microalbuminuria. In patients with type 2 diabetes results are inconclusive. In the HOPE (Heart Outcomes Prevention Evaluation) trial ramipril was not effective [28], while in the the Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) [29] the 2 arms containing trandolapril showed similar benefit in preventing the development of albuminuria, and their effect seemed to be independent of blood pressure reduction. Furthermore, in the Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) [30] the use of olmesartan prevented or delayed the onset of microalbuminuria, but difference in blood pressure between the olmesartan and placebo arms were statistically different.
Patients with moderately increased albuminuria As for the transition from microalbuminuria to overt proteinuria in the IRMA-2 (Effect of Irbesartan in the Development of Diabetic Nephropathy in Patients With T2DM) trial [31] irbesartan reduced the risk for the development of overt proteinuria (defined as albumin excretion > 200 mg/day) and the effect was dose-dependent.
Patients with severely increased albuminuria More solid data support the use of RAAS inhibitors in DKD with overt albuminuria. The Collaborative study group evaluated the impact of captopril versus placebo on CKD progression in patients with type 1 diabetes and proteinuria [32]. Despite similar blood pressure control the captopril group had a significantly lower rates of chronic kidney disease progression or ESKD. Similarly, the data from the RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan) [33] and IDNT (Irbesartan Diabetic Nephropathy Trial) [34] trials confirmed the nephroprotective effect of ARBs, in patients with DKD with proteinuria.
Therefore, the use of RAAS blockers has been considered for many years the gold standard therapy to reduce cardiovascular risk and to slow the progression of diabetic nephropathy and previous guidelines recommended the use of RAAS blockers in all patients with diabetes without distinction [35]. However, while lower BP values and pharmacologic inhibition of the RAAS have consistently been shown to improve renal outcome in diabetic patients presenting with the traditional “albuminuric” renal phenotype, it is uncertain whether a similar therapeutic strategy should be applied in the absence of albuminuria. In fact, RCTs as well as real-life reports have demonstrated that the use of RAAS inhibiting drugs in patients with diabetic nephropathy has a clinical significant benefit in slowing renal progression only in people with increased albuminuria.
Accordingly, more recently, guidelines do not recommend routine use of RAAS blockers in diabetic subjects unless in the presence of nephropathy with an urinary albumin-to-creatinine ratio greater than 30 mg/g creatinine in order to reduce the risk of progressive kidney disease [21, 36, 37]. Either an ACEI or an ARB are recommended, as these drug classes have shown similar nephroprotective effect. ARB have been studied more extensively than ACEi in patients with type 2 diabetes; on the other hand, no study has been completed with ARB in type 1 diabetes. As for dosage, in the absence of side effects the maximum tolerated dose approved for blood pressure treatment is usually recommended. While in the absence of albuminuria a less strict blood pressure control could be advisable and RAAS inhibitors may not necessarily represents the preferred antihypertensive drugs and treatment for hypertension should include drug classes demonstrated to reduce cardiovascular events in patients with diabetes (ACE inhibitors, angiotensin receptor blockers, thiazide-like diuretics, or dihydropyridine calcium channel blockers) without cogent preference [21, 25].
Limitations of RAAS inhibition
Several studies have explored the potential benefit of maximal inhibition of the RAAS by means of high doses monotherapy or combination drug. Dual RAAS blockade is based on the pathophysiological rationale of a more profound inhibition of the RAAS cascade which may counteract the well known “aldosterone escape” phenomenon observed in patients treated with a single agent. However, disappointing results from several mega trials with hard endpoints have subsided the initial enthusiasm on the promising effects of dual RAAS blockade on surrogate outcomes. In fact, the combination of two RAAS blocking drugs did not provide considerable benefit in terms of long term renal protection and was accompanied by higher incidence of acute kidney injury and hyperkaliemia as compared to single-drug therapy [38,39,40]. It is likely that dual/multilevel RAAS blockade completely abolishes the protective role of this system in maintaining renal haemodynamics under stressful conditions and, therefore, may favor AKI in hypovolemic patients. Accordingly, international guidelines currently discourage the use of dual RAAS blockade for the prevention and treatment of DKD [21, 25].
Finally, mineralocorticoid receptor inhibitors (MRI) such as spironolactone or eplerenone might offer an additional opportunity for RAAS intervention by counteracting the “aldosterone breakthrough” that occurs in a subset of patients on RAAS-inhibiting therapy. Aldosterone is known to affect volume status by the regulating renal sodium reabsorption and exert profibrotic effects through increased production of TGF-beta, reactive oxygen, species, PAI-1 and increased collagen gene expression and synthesis [41, 42] and, the use of MRI has been demonstrated to reduce inflammation and fibrosis at kidney level [43,44,45]. Several studies have shown that the addition of aldosterone receptor blockade to ACEI or ARB blockade can lead to further reduction in albuminuria including in DKD [46,47,48], however long-term data on the efficacy of mineralocorticoid receptor antagonists on hard endpoints, for example the development of ESKD or patient survival, are still lacking.
Conclusions
Multiple trials have demonstrated that the use of RAAS blocking drugs can slow progression to ESKD in patients with diabetic nephropathy and proteinuria > 300 mg/day, however single agent RAAS blockade is not sufficient in slowering progression in many patients. In fact, significant residual risk remains in patients with DN and many patients progress to ESKD despite standard recommended care, including optimal control of blood pressure, glycemia and lipids and the use of RAAS blockade [49, 50]. Furthermore, a paradoxical J-curve relationship between BP reduction and renal morbidity may limit the benefit of aggressive treatment strategies, especially in elderly patients with a renal dysfunction without albuminuria [51]. Thus, different individually tailored therapeutic targets, that take into account specific cardiovascular and renal phenotype should guide therapeutic strategies.
Given the steady increase in the prevalence of diabetes worldwide and the soaring costs related to its management, prevention and treatment of DKD remains an unmet clinical need. Within this context, recently developed therapeutic agents such as sodium-glucose co-transporter-2 inhibitors and glucagone like peptide-1 receptor agonist have shown promising therapeutic potential not only to reduce CV risk but also to slow the progression of kidney disease with beneficial effect on albuminuria as well as on GFR [52,53,54,55]. As a matter of fact, the nephroprotective effect of these drug classes, especially SGLT2-is, was shown to be additive to RAAS-is and consistent in various studies completed so far, irrespective of baseline GFR and the presence of albuminuria. Several mechanisms, both glycemic and extra glycemic, have been proposed to account for the observed benefit on renal and cardiovascular events [56,57,58,59].
Because of their remarkable antiproteinuric and renoprotective activity, confirmed in many large trials, SGLT2i may soon become, in association with RAASi, the standard of care to prevent DKD and retard its progression.
Take home messages
-
Diabetic kidney disease (DKD) is the leading cause of end-stage kidney disease and represent a significant cause of morbidity and mortality worldwide due to the related cardiovascular risk burden.
-
The standard of care for management of DKD over the last three decades has been the control of cardiovascular risk factors and the pharmacologic inhibition of renin–angiotensin–aldosterone system (RAAS).
-
Nowadays, non-albuminuric DKD is the prevailing phenotype. The nephroprotective role of intensive blood pressure control and the use of RAAS inhibitor in these patients is at present unclear.
-
Recent evidence supports a renal protective effect of sodium-glucose cotransporter 2 (SGLT2) inhibitors independent of their hypoglycemic properties.
-
We suggest that co-treatment with RAAS and SGLT2 inhibitors may represent the new era of therapeutic options for DKD.
References
Thomas B (2019) The global burden of diabetic kidney disease: time trends and gender gaps. Curr Diabetes Rep 19:18. https://doi.org/10.1007/s11892-019-1133-6
Umanath K, Lewis JB (2018) Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis 71:884–895. https://doi.org/10.1053/j.ajkd.2017.10.026
De Cosmo S, Rossi MC, Pellegrini F, Lucisano G, Bacci S, Gentile S, Ceriello A, Russo G, Nicolucci A, Giorda C, Viazzi F, Pontremoli R, AMD-Annals Study Group (2014) Kidney dysfunction and related cardiovascular risk factors among patients with type 2 diabetes. Nephrol Dial Transpl 29:657–662. https://doi.org/10.1093/ndt/gft506
Viazzi F, Russo GT, Ceriello A, Fioretto P, Giorda C, De Cosmo S, Pontremoli R (2019) Natural history and risk factors for diabetic kidney disease in patients with T2D: lessons from the AMD-annals. J Nephrol 32:517–525. https://doi.org/10.1007/s40620-018-00561-3
Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, UKPDS Group (2003) Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63:225–232. https://doi.org/10.1046/j.1523-1755.2003.00712.x
Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O (2003) Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 348:383–893. https://doi.org/10.1056/NEJMoa021778
Chan GC, Tang SC (2016) Diabetic nephropathy: landmark clinical trials and tribulations. Nephrol Dial Transpl 31:359–368. https://doi.org/10.1093/ndt/gfu411(Epub 2015 Jan 29)
Bethesda, Md, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (2010). U.S. Renal Data System 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in United States. http://www.usrds.org/atlas10.aspx. Accessed 17 Feb 2020
Viazzi F, Muiesan ML, Schillaci G, Salvetti M, Pucci G, Bonino B, Signori A, Pontremoli R (2016) Changes in albuminuria and cardiovascular risk under antihypertensive treatment: a systematic review and meta-regression analysis. J Hypertens 34:1689–1697. https://doi.org/10.1097/HJH.0000000000000991
Savarese G, Dei Cas A, Rosano G, D’Amore C, Musella F, Mosca S, Reiner MF, Marchioli R, Trimarco B, Perrone-Filardi P (2014) Reduction of albumin urinary excretion is associated with reduced cardiovascular events in hypertensive and/or diabetic patients. A meta-regression analysis of 32 randomized trials. Int J Cardiol 172:403–410. https://doi.org/10.1016/j.ijcard.2014.01.065
Viazzi F, Ceriello A, Fioretto P, Giorda C, Guida P, Russo G, Greco E, De Cosmo S, Pontremoli R, AMD-Annals Study Group (2018) Changes in albuminuria and renal outcome in patients with type 2 diabetes and hypertension: a real-life observational study. J Hypertens 36:1719–1728. https://doi.org/10.1097/HJH.0000000000001749
Mogensen CE, Christensen CK, Vittinghus E (1983) The stages in diabetic renal disease With emphasis on the stage of incipient diabetic nephropathy. Diabetes 32(Suppl 2):64–78. https://doi.org/10.2337/diab.32.2.s64
de Zeeuw D (2007) Albuminuria: a target for treatment of type 2 diabetic nephropathy. Semin Nephrol 27(2):172–181. https://doi.org/10.1016/j.semnephrol.2007.01.002
Fioretto P, Mauer M (2010) Diabetic nephropathy: diabetic nephropathy-challenges in pathologic classification. Nat Rev Nephrol 6:508–510. https://doi.org/10.1038/nrneph.2010.96
Viazzi F, Piscitelli P, Giorda C, Ceriello A, Genovese S, Russo GT, Fioretto P, Guida P, De Cosmo S, Pontremoli R, AMD-Annals Study Group (2017) Association of kidney disease measures with risk of renal function worsening in patients with hypertension and type 2 diabetes. J Diabetes Complications 31:419–426. https://doi.org/10.1016/j.jdiacomp.2016.10.030
Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, Trevisan R, Vedovato M, Gruden G, Cavalot F, Cignarelli M, Laviola L, Morano S, Nicolucci A, Pugliese G, Renal Insufficiency And Cardiovascular Events (RIACE) Study Group (2011) Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens 29:1802–1809. https://doi.org/10.1097/HJH.0b013e3283495cd6
De Cosmo S, Viazzi F, Piscitelli P, Giorda C, Ceriello A, Genovese S, Russo G, Guida P, Fioretto P, Pontremoli R, AMD-Annals Study Group (2016) Blood pressure status and the incidence of diabetic kidney disease in patients with hypertension and type 2 diabetes. J Hypertens 34:2090–2098. https://doi.org/10.1097/HJH.0000000000001045BB
Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K (2016) Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 387:957–967. https://doi.org/10.1016/S0140-6736(15)01225-8
Beddhu S, Greene T, Boucher R, Cushman WC, Wei G, Stoddard G, Ix JH, Chonchol M, Kramer H, Cheung AK, Kimmel PL, Paul K, Whelton PK, Chertow GM (2018) Intensive systolic blood pressure control and incident chronic kidney disease in people with and without diabetes mellitus: secondary analyses of two randomised controlled trials. Lancet Diabetes Endocrinol 6:555–563. https://doi.org/10.1016/S2213-8587(18)30099-8
Viazzi F, Leoncini G, Grassi G, Pontremoli R (2018) Antihypertensive treatment and renal protection: is there a J-curve relationship? J Clin Hypertens (Greenwich) 20:1560–1574. https://doi.org/10.1111/jch.13396
American Diabetes Association (2020) Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care 43(Suppl 1):S111–S134. https://doi.org/10.2337/dc20-S010
Cao Z, Cooper ME (2011) Pathogenesis of diabetic nephropathy. J Diabetes Investig 2:243–247. https://doi.org/10.1111/j.2040-1124.2011.00131
Navarro-González JF, Mora-Fernández C, de Fuentes MM, García-Pérez J (2011) Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 7(6):327–340. https://doi.org/10.1038/nrneph.2011.51
Koszegi S, Molnar A, Lenart L, Hodrea J, Balogh DB, Lakat T, Szkibinszkij E, Hosszu A, Sparding N, Genovese F, Wagner L, Vannay A, Szabo AJ, Fekete A (2019) RAAS inhibitors directly reduce diabetes-induced renal fibrosis via growth factor inhibition. J Physiol 597:193–209. https://doi.org/10.1113/JP277002
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, Authors/Task Force Members (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 36:1953–2041. https://doi.org/10.1097/HJH.0000000000001940
Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R (2009) Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 361:40–51. https://doi.org/10.1056/NEJMoa0808400
Bilous R, Chaturvedi N, Sjølie AK, Fuller J, Klein R, Orchard T, Porta M, Parving HH (2009) Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Ann Intern Med 151(11–20):W3–W4. https://doi.org/10.7326/0003-4819-151-1-200907070-00120
Heart Outcomes Prevention Evaluation (HOPE) Study Investigators (2000) Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 355:253–259. https://doi.org/10.1016/S0140-6736(99)12323-7
Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M, Ene-Iordache B, Gaspari F, Perna A, Bossi A, Trevisan R, Dodesini AR, Remuzzi G, Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) Investigators (2004) Preventing microalbuminuria in type 2 diabetes. N Engl J Med 351:1941–1951. https://doi.org/10.1056/NEJMoa042167
Haller H, Ito S, Izzo JL Jr, Januszewicz A, Katayama S, Menne J, Mimran A, Rabelink TJ, Ritz E, Ruilope LM, Rump LC, Viberti G, ROADMAP Trial Investigators (2011) Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 364:907–917. https://doi.org/10.1056/NEJMoa1007994
Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P, Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group (2001) The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345:870–878. https://doi.org/10.1056/NEJMoa011489
Lewis EJ, Hunsicker LG, Bain RP, Rohde RD (1993) The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329:1456–1462. https://doi.org/10.1056/NEJM199311113292004(Erratum in: N Engl J Med 1993 Jan 13;330:152)
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators (2001) Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345:861–869. https://doi.org/10.1056/NEJMoa011161
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Group (2001) Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345(12):851–860. https://doi.org/10.1056/NEJMoa011303
American Diabetes Association (2012) Standards of medical care in diabetes—2012. Diabetes Care 35(Suppl 1):S11–S63. https://doi.org/10.2337/dc12-s011
Taler SJ, Agarwal R, Bakris GL, Flynn JT, Nilsson PM, Rahman M, Sanders PW, Textor SC, Weir MR, Townsend RR (2013) KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis 62:201–213. https://doi.org/10.1053/j.ajkd.2013.03.018
American Diabetes Association (2020) Microvascular complications and foot care: standards of medical care in diabetes—2020. Diabetes Care 43(Suppl 1):S135–S151. https://doi.org/10.2337/dc20-S011
Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O’Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P, VA NEPHRON-D Investigators; VA NEPHRON-D Investigators (2013) Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369:1892–1903. https://doi.org/10.1056/NEJMoa1303154
Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S, ONTARGET investigators (2008) Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372:547–553. https://doi.org/10.1016/S0140-6736(08)61236-2
Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA, ALTITUDE Investigators (2012) Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367:2204–2213. https://doi.org/10.1056/NEJMoa1208799
Hostetter TH, Rosenberg ME, Ibrahim HN, Juknevicius I (2001) Aldosterone in progressive renal disease. Semin Nephrol 21:573–579. https://doi.org/10.1053/snep.2001.26797
Greene EL, Kren S, Hostetter TH (1996) Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 98(4):1063–1068. https://doi.org/10.1172/JCI118867
Fujisawa G, Okada K, Muto S, Fujita N, Itabashi N, Kusano E, Ishibashi S (2004) Spironolactone prevents early renal injury in streptozotocin-induced diabetic rats. Kidney Int 66:1493–1502. https://doi.org/10.1111/j.1523-1755.2004.00913.x
Han SY, Kim CH, Kim HS, Jee YH, Song HK, Lee MH, Han KH, Kim HK, Kang YS, Han JY, Kim YS, Cha DR (2006) Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol 17:1362–1372. https://doi.org/10.1681/ASN.2005111196
Pessôa BS, Peixoto EBMI, Papadimitriou A, Lopes de Faria JM, Lopes de Faria JB (2012) Spironolactone improves nephropathy by enhancing glucose-6-phosphate dehydrogenase activity and reducing oxidative stress in diabetic hypertensive rat. J Renin Angiotensin Aldosterone Syst 13:56–66. https://doi.org/10.1177/1470320311422581
Alexandrou ME, Papagianni A, Tsapas A, Loutradis C, Boutou A, Piperidou A, Papadopoulou D, Ruilope L, Bakris G, Sarafidis P (2019) Effects of mineralocorticoid receptor antagonists in proteinuric kidney disease: a systematic review and meta-analysis of randomized controlled trials. J Hypertens 37:2307–2324. https://doi.org/10.1097/HJH.0000000000002187
Bolignano D, Palmer SC, Navaneethan SD (2014) Strippoli GF (2014) Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev 4:CD007004. https://doi.org/10.1002/14651858.CD007004.pub3
Sun LJ, Sun YN, Shan JP, Jiang GR (2017) Effects of mineralocorticoid receptor antagonists on the progression of diabetic nephropathy. J Diabetes Investig 8:609–618. https://doi.org/10.1111/jdi.12629
Oellgaard J, Gæde P, Rossing P, Persson F, Parving HH, Pedersen O (2017) Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long-term renal benefits. Kidney Int 91:982–988. https://doi.org/10.1016/j.kint.2016.11.023
Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH (2016) Clinical manifestations of kidney disease among US adults with diabetes. 1988–2014. JAMA 316:602–610. https://doi.org/10.1001/jama.2016.10924
Viazzi F, Piscitelli P, Ceriello A, Fioretto P, Giorda C, Guida P, Russo G, De Cosmo S, Pontremoli R, AMD-Annals Study Group (2017) Resistant hypertension, time-updated blood pressure values and renal outcome in type 2 diabetes mellitus. J Am Heart Assoc. 6(9):e006745. https://doi.org/10.1161/JAHA.117.006745
Ninčević V, Omanović Kolarić T, Roguljić H, Kizivat T, Smolić M, Bilić Ćurčić I (2019) Renal benefits of SGLT 2 inhibitors and GLP-1 receptor agonists: evidence supporting a paradigm shift in the medical management of type 2 diabetes. Int J Mol Sci 20(23):E5831. https://doi.org/10.3390/ijms20235831
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS (2019) Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose co-transporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus: a systematic review and meta-analysis of cardiovascular outcomes trials. Circulation 139:2022–2031. https://doi.org/10.1161/CIRCULATIONAHA.118.038868
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW, CREDENCE Trial Investigators (2019) Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380:2295–2306. https://doi.org/10.1056/NEJMoa1811744
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. https://doi.org/10.1016/S0140-6736(18)32590-X
Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI (2018) Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int 94:26–39. https://doi.org/10.1016/j.kint.2017.12.027
Heerspink HJ, Perkins BA, Fitchett D, Husain M, Cherrney DZ (2016) Sodium glucose cotransporter 2 Inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134:752–772. https://doi.org/10.1161/CIRCULATIONHA.116.021887
Yaribeygi H, Katsiki N, Butler AE, Sahebkar A (2019) Effects of antidiabetic drugs on NLRP3 inflammasome activity, with a focus on diabetic kidneys. Drug Discov Today 24:256–262. https://doi.org/10.1016/j.drudis.2018.08.005
Woods TC, Satou R, Miyata K, Katsurada A, Dugas CM, Klingenberg NC, Fonseca VA, Navar LG (2019) Canagliflozin prevents intrarenal angiotensinogen augmentation and mitigates kidney injury and hypertension in mouse model of type 2 diabetes mellitus. Am J Nephrol 49:331–342. https://doi.org/10.1159/000499597
Ravid M, Brosh D, Levi Z, Bar-Dayan Y, Ravid D, Rachmani R (1998) Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med 128:982–988. https://doi.org/10.7326/0003-4819-128-12
Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B (2007) Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trialADVANCE Collaborative Group. Lancet 370:829–840. https://doi.org/10.1016/S0140-6736(07)61303-8
de Galan BE, Perkovic V, Ninomiya T, Pillai A, Patel A, Cass A, Neal B, Poulter N, Harrap S, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Glasziou P, Grobbee DE, MacMahon S, Chalmers J, ADVANCE Collaborative Group (2009) Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol 20:883–892. https://doi.org/10.1681/ASN.2008070667
The Microalbuminuria Captopril Study Group (1996) Captopril reduces the risk of nephropathy in IDDM patients with microalbuminuria. Diabetologia 39:587–593. https://doi.org/10.1007/BF00403306. https://doi.org/10.1016/S0140-6736(99)12323-7
Viberti G, Nigel M, Wheeldon NM, MicroAlbuminuria Reduction With VALsartan (MARVAL) Study Investigators (2002) Microalbuminuria Reduction With Valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation 106:672–678. https://doi.org/10.1161/01.cir.0000024416.33113.0a
Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, Mustonen J, Diabetics Exposed to Telmisartan and Enalapril Study Group (2004) Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 351:1952–1961. https://doi.org/10.1056/NEJMoa042274
Makino H, Haneda M, Babazono T, Moriya T, Ito S, Iwamoto Y, Kawamori R, Takeuchi M, Katayama S, INNOVATION Study Group (2007) Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care 30:1577–1578. https://doi.org/10.2337/dc06-1998
Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, DeFerrari G, Drury P, Locatelli F, Wiegmann TB, Lewis EJ (2005) Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 45:281–287. https://doi.org/10.1053/j.ajkd.2004.10.019
Imai E, Chan JC, Ito S, Yamasaki T, Kobayashi F, Haneda M, Makino H, ORIENT study investigators (2011) Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia 54:2978–2986. https://doi.org/10.1007/s00125-011-2325-z
Mogensen CE, Neldam S, Tikkanen I, Oren S, Viskoper R, Watts RW, Cooper ME (2000) Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the Candesartan and Lisinopril Microalbuminuria (CALM) study. Br Med J 321:1440–1444. https://doi.org/10.1056/NEJMoa042274
Jacobsen P, Andersen S, Rossing K, Jensen BR, Parving HH (2003) Dual blockade of the renin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int 63:1874–1880. https://doi.org/10.1046/j.1523-1755.2003.00940.x
Bakris GL, Ruilope L, Locatelli F, Ptaszynska A, Pieske B, de Champlain J, Weber MA, Raz I (2007) Treatment of microalbuminuria in hypertensive subjects with elevated cardiovascular risk: results of the IMPROVE trial. Kidney Int 72:879–885. https://doi.org/10.1038/sj.ki.5002455
Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C, ONTARGET Investigators (2008) Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358:1547–1559. https://doi.org/10.1056/NEJMoa0801317
Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK, AVOID Study Investigators (2008) Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 358:2433–2446. https://doi.org/10.1056/NEJMoa0708379
Acknowledgements
Open access funding provided by Università degli Studi di Genova within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leoncini, G., Viazzi, F., De Cosmo, S. et al. Blood pressure reduction and RAAS inhibition in diabetic kidney disease: therapeutic potentials and limitations. J Nephrol 33, 949–963 (2020). https://doi.org/10.1007/s40620-020-00803-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00803-3