Abstract
The pandemic of coronavirus disease 2019 (CoVID-19) has been an unprecedented period. The disease afflicts multiple organ systems, with acute kidney injury (AKI) a major complication in seriously ill patients. The incidence of AKI in patients with CoVID-19 is variable across numerous international studies, but the high incidence of AKI and its associated worse outcomes in the critical care setting are a consistent finding. A multitude of patterns and mechanisms of AKI have been elucidated, and novel strategies to address shortage of renal replacement therapy equipment have been implemented. The disease also has had consequences on longitudinal management of patients with chronic kidney disease and end stage kidney disease. Kidney transplant recipients may be especially susceptible to CoVID-19 as a result of immunosuppression, with preliminary studies demonstrating high mortality rates. Increased surveillance of disease with low threshold for testing and adjustment of immunosuppression regimen during acute periods of illness have been recommended.
Similar content being viewed by others
Background
The coronavirus disease 2019 (CoVID-19) pandemic has strained medical systems globally. The disease results from infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and it results in multi-organ injury including the kidneys. The disease has unique implications for patients developing acute kidney injury (AKI), as well as patients with chronic kidney disease (CKD) or end stage kidney disease (ESKD) and kidney transplant recipients (KTR). In this review, we provide a comprehensive overview of the effect of CoVID-19 on these aspects of kidney disease.
We elucidate the epidemiology and associated clinical characteristics of AKI by analyzing various studies that have reported outcomes related to AKI and renal replacement therapy (RRT). We also discuss the mechanisms and patterns of AKI. In the CKD and ESKD sections we discuss the controversy of renin angiotensin system blockade, along with recommendations for managing patients on chronic dialysis in the era of CoVID-19. Kidney transplantation requires particular emphasis during the pandemic due to concern for increased susceptibility to infection. The segment on kidney transplantation incorporates current outcomes of kidney transplant patients with CoVID-19 and recommendations for management of immunosuppression. Finally, we highlight a need for consideration of how the CoVID-19 pandemic is impacting socially disadvantaged populations known to experience worse outcomes in kidney disease.
Main text
Acute Kidney injury
AKI was first recognized as a complication of the severe acute respiratory syndrome novel coronavirus (SARS-CoV) in 2003 [1,2,3]. Based on studies at the time, AKI developed in 6.7% of patients at a median duration of 20 days (range 5–48 days) after onset of viral infection, with 30% requiring renal replacement therapy [4]. Mortality rates of up to 70% in patients with AKI have been reported. In both SARS-CoV and SARS-CoV-2, age, acute respiratory distress syndrome, and AKI were independent predictors of mortality [3, 5].
With respect to SARS-CoV-2, at the time of writing this review, numerous studies have reported a wide incidence of AKI. Three large retrospective studies, involving more than 1000 patients each from China, United Kingdom, and United States, have reported the development of AKI in 0.5–46% of patients [6,7,8].
Epidemiology & Clinical Characteristics
Currently available studies evaluating patient characteristics and disease course are from China, Europe and United States.
AKI appears to be more common in the elderly, males, and those with higher body mass index (median BMI ~ 30 for patients with AKI) [7, 9,10,11]. Patients with co-morbidities such as CKD, hypertension, coronary artery disease, heart failure, diabetes mellitus, and peripheral arterial disease were more likely to develop AKI [7, 10]. Non-survivors were more likely to have CKD and elevated creatinine during admission, with CKD being an independent predictor of AKI stage 3 (KDIGO criteria) [7, 12].
China
Most studies evaluating rates of AKI from China are from single hospitals, with a range of 52–1392 patients for a period of 2–9 weeks [8, 12,13,14,15,16,17,18,19,20,21,22,23] (Table 1). Reported rates of AKI vary between 0.5–50% [8, 12, 23, 32]. The heterogeneity in rates may be attributable to different definitions for AKI, variable cohort size, and inclusion of patients from different care settings (e.g., all hospitalized vs intensive care unit). The elderly, patients with comorbidities such as hypertension and cardiovascular disease, and ICU patients were more likely to develop AKI. A minority of patients were reported to have pre-existing CKD.
United Kingdom and France
Hospital groups from the UK and France have largely focused on patients in the critical care setting. In the study from the UK (n = 6143), 24% required renal replacement therapy, and of these, 95.3% required advanced respiratory support (invasive ventilation, extracorporeal respiratory support) and 71% died [6]. A smaller study from Bordeaux (n = 71) demonstrated higher mortality in patients with AKI, and 64% of surviving patients recovered kidney function by day 21 of follow up [24]. Similar to data from China, less than 6% of patients in both studies had CKD. A larger dataset from the UK (n = 20,133), however, found that 16.2% of patients with COVID-19 had CKD [26]. A prospective cohort study from Spain (n = 1603) demonstrated that an increased serum creatinine on arrival was a risk factor for poor outcome, whether it is present acutely or as a consequence of CKD [25].
United States
New York City and New Orleans, Louisiana, were 2 of the more intensely affected regions of the United States. In New York, 2 large cohorts revealed AKI rates in excess of 20%, with higher proportion of CKD patients affected (10–28%) in comparison to data from China and Europe. The New Orleans (n = 575), Mount Sinai (n = 3235), Montefiore (n = 3345), and Northwell (n = 5449) hospital groups have specifically assessed AKI and associated outcomes over 5–7 weeks, with the following findings:
-
1.
Male sex, African-American race, and age > 50 years are associated with higher risk of AKI.
-
2.
Presence of CKD and higher potassium levels were independent predictors of stage 3 AKI (KDIGO criteria) [7].
-
3.
Higher rates of AKI and RRT in patients with COVID-19 in comparison to historical controls (56.9% vs 25.1% for rates of AKI) [11].
-
4.
Patients with AKI were more likely to be admitted to the ICU, undergo mechanical ventilation, and require vasopressor support [7, 10, 11].
-
5.
AKI developed in 90% of patients on mechanical ventilation as compared to 22% of non-ventilated patients [10].
-
6.
Ninety seven percent of patients requiring RRT were on mechanical ventilation [10].
-
7.
Patients with AKI had higher levels of ferritin, d-dimer, C-reactive protein (CRP), procalcitonin and lactate dehydrogenase (LDH) [9, 11].
-
8.
Mortality in patients with AKI was 45% vs 7% in those without (ICU mortality: 52% in those with AKI vs 9% in those without) [7]. AKI in hospitalized patients is associated with significant risk for in-hospital death (incidence rate of in-hospital death: 37.5/1000 patient-days in those with AKI vs 10.8/1000 patient-days in those without) [33].
-
9.
43% (211/486) of patients with AKI had evidence of persistent abnormal kidney function at discharge [7].
Summary of outcomes
There is evidence of disparate incidence and outcomes of AKI across the 3 continents, with AKI reported in 0.5–46%. Reports from Europe and the United States describe a greater burden of co-morbid disease in association with the higher rates of AKI. Additionally, the prevalence of CKD, which is a risk factor for AKI, has not been reported in most studies from China, with a low proportion of disease (~ 2%) reported in recent studies. This could be explained by absence of baseline creatinine values and/or utilization of differing criteria for diagnosis.
AKI often develops early during hospitalization, with 37–57% of patients developing AKI within 0–4 days of admission [9, 10]. Severity of pneumonia was commonly associated with lower chance of renal recovery and lower remission of proteinuria or hematuria [22]. The highest incidence of AKI (19–90%) occurs in the critical care setting, with the majority of patients requiring RRT also supported with mechanical ventilation [10].
AKI is associated with increased length of stay and mortality [7, 13, 16, 22], and this is supported by 2 meta-analyses demonstrating AKI to be an unfavorable clinical predictor associated with high mortality [34, 35]. Development of AKI appears to be a consequence of severity of illness, and its occurrence portends a worse prognosis. Current studies demonstrate up to half of patients with AKI did not recover to baseline creatinine levels and may have persistent CKD [7, 22]. Given the lack of long-term follow-up of hospitalized patients to date, the AKI recovery rate remains unknown.
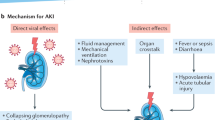
Mechanisms and patterns of injury
AKI in patients with CoVID-19 is postulated to occur via multiple, often co-existing mechanisms (Table 2).
Acute tubular injury is the most common etiology of AKI based on autopsy and biopsy reports [18, 22, 23, 40, 51, 52, 59], and lymphocytic infiltration is commonly present. Proximal tubular dysfunction has been demonstrated in a subset of patients with COVID-19, with resultant hypouricemia and inappropriate uricosuria correlating with disease severity and respiratory decompensation [60]. Microangiopathic injury has been infrequently observed in autopsy and biopsy series to date [52]. Hypercoagulability is a well-recognized feature of CoVID-19, although most attention has been focused on pulmonary microangiopathy, venous thromboembolism, and stroke. Terminal complement activation (C5b-9) has been demonstrated in kidney, lung, and skin, although the mechanisms underlying complement activation remain to be clarified. It has been suggested complement and neutrophil extracellular traps generate a coagulopathic milieu leading to formation of microthrombi, thereby leading to severe manifestations of COVID-19 such as lung and cardiac injury [61]. It is intriguing that despite the susceptibility of the kidney to thrombotic microangiopathy in general, most of the microangiopathic injury in CoVID-19 has been documented in extra-renal organs [18, 62, 63].
Hematuria (27–53%) and proteinuria (36–66%) are common, with higher rates reported in patients with AKI [13, 20, 22, 64]. Proteinuria is often transient, similar to Middle East Respiratory Syndrome (MERS)-CoV, although the mechanism is not known with certainty. It may be a consequence of fever, systemic inflammation, or possibly direct viral infection of renal epithelium [3, 22]. Nevertheless, the severity of proteinuria and hematuria is associated with an increased risk of mortality in patients with CoVID-19 [13]. Hematuria is often multifactorial in origin, and detailed studies on the mechanism of hematuria are lacking.
Lastly, collapsing glomerulopathy has been reported in the context of COVID-19 infection [9, 51,52,53,54]. Not surprisingly, this lesion has developed in patients homozygous for APOL1 G1 risk alleles. This pattern of injury is most strongly associated with viral infection, particularly HIV and parvovirus. This finding suggests the presence of a high risk APOL1 genotype and may increase the risk for interferon mediated podocyte injury due to CoVID-19. Homozygosity for high-risk APOL1 alleles is present in 14% of African Americans, who collectively represent 12.9% of the US population but have suffered an estimated 25.1% of U.S. COVID-19 deaths [53, 54]. It remains to be determined if infection will lead to increased rates of CKD/ESKD in this population as compared to other groups.
Renal replacement therapy
Reported rates of RRT also vary widely, with overall rates of 2–73% in the critical care setting [6, 7, 14, 64]. The rapid surge of patients requiring RRT has led to shortages of staffing, dialysis machines, and dialysis fluid, particularly for continuous renal replacement therapy (CRRT). At the time of writing, several states in the US are expected to experience CRRT equipment shortages during the pandemic based on mathematical models [65]. This has accelerated interest in hybrid therapies such as prolonged intermittent RRT (PIRRT) with careful titration of therapy fluid rates to minimize waste [63, 66, 67]. Protocols for on-site preparation of CRRT therapy fluid have been described by multiple groups including Vanderbilt University Medical Center, Cleveland Clinic, and Johns Hopkins Hospital [68,69,70]. The pandemic has helped rejuvenate the utilization of acute peritoneal dialysis (PD), and data suggest the increased peritoneal pressure in setting of ARDS does not worsen hypoxemia or respiratory mechanics [71,72,73]. Acute PD, however, may not be suitable for prone patients. Nevertheless, even vascular access is challenging in prone patients, although skilled operators can often still insert internal jugular catheters and novel access sites such as the popliteal vein have been reported [74]. Low copies of viral RNA are present in the effluent of both PD and hemodialysis (HD) [75, 76], but there has not been isolation of infectious virus from these fluids. Current guidelines do not recommend special decontamination of effluent, and effluent should be discarded in accordance with standard practice.
Chronic kidney disease
While there has been extensive early reporting on the impact of CoVID-19 on the kidneys in the acute setting, and the association of worse short-term outcomes including in-hospital mortality in patients with any kidney involvement, much less has been published on the impact of CoVID-19 in patients with underlying CKD. However, prior research has demonstrated that patients with CKD, and particularly those with ESKD, have been found to have immune dysregulation and increased susceptibility for infections [77]. In addition, large national organizations such as the National Kidney Foundation have published general guidelines for the care of patients with CKD in the setting of CoVID-19 [78]. Patients with any degree of CKD, for instance, have been recommended to adhere strictly to guidelines on the importance of self-isolation, and when it becomes necessary, to use face masks in public and avoid or limit exposure to large gatherings of people.
For many patients with CKD, renin-angiotensin-aldosterone system (RAAS) blockade is a mainstay of therapy. However, there has been some controversy regarding the use of RAAS blockade in the setting of COVID-19. SARS-CoV-2, similar to the SARS-CoV-1 virus that originated in 2003, uses the angiotensin-converting enzyme 2 (ACE2) receptor for viral entry [79]. ACE2 is widely expressed in a number of tissues, including the type 2 alveolar cells of lung epithelium and renal tubular epithelial cells [79]. While the more commonly recognized ACE converts angiotensin I to angiotensin II, ACE2 converts angiotensin II to angiotensin- [1,2,3,4,5,6,7], which acts on the Mas receptor present in many tissues throughout the body. This pathway ultimately leads to vasodilation and the systemic reduction in inflammation, as a counter-regulatory system to the vasoconstriction induced by angiotensin-II binding to angiotensin 1 receptor (AT1R).
The use of ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), therefore, has come under more intense scrutiny in the face of this current pandemic [80]. Controversy surrounds the potential detrimental effect of ongoing ACEIs or ARBs use, with potential up regulation of the ACE2 receptor which could then increase the ability of the virus to enter cells. It is also possible that ACEIs help by blocking the ACE2 receptor and blocking viral entry. Over the past several months, a number of large studies have explored the potential association between ACEIs or ARBs use with adverse outcomes, including COVID-19 positivity and mortality, in detail [81, 82]. Mancia and colleagues performed a population-based case-control study in Italy and demonstrated that while ACEI and ARB use was more common among patients with COVID-19, they did not find any significant association between ACEI or ARB use with risk of COVID-19 [81]. Reynolds and colleagues conducted a retrospective review of all patients admitted to the New York University Langone Health system tested for COVID-19 from March 1 to April 15, 2020, and demonstrated no substantial increase in the likelihood of COVID-19 test positivity, or severe COVID-19, based on ACEI or ARB use [82].
Various professional societies and councils have also contributed to the debate, with the American Heart Association, American College of Cardiology, and Council on Hypertension of the European Society of Cardiology, all recommending the continued use of ACEIs and ARBs. Within the field of nephrology, investigators across several institutions jointly published a perspective emphasizing the lack of clear evidence for either benefit or risk with ACEIs/ARBs use and COVID-19 [80, 83]. Without additional and high-quality clinical trial data, clinical equipoise dictates the continued use of RAAS blockade in patients who are already on these medications for other indications.
End stage kidney disease
Among Medicare beneficiaries, patients with ESKD have the highest hospitalization rate for CoVID-19 at 1341 cases per 100,000 [84]. Patients with ESKD who are on home dialysis therapy, either PD or home HD have been able to continue dialysis while safely self-isolating. However, there are a number of unique challenges that patients undergoing in-center HD may face. While the U.S. Centers for Disease Control and Prevention (CDC) has published recommendations to reduce the spread of this highly contagious respiratory pathogen, patients undergoing in-center HD necessarily remain in dialysis units for 3 h or longer, 3 times per week, sometimes surrounded by 20 to 30 other patients, along with dialysis staff including nurses, technicians, and nephrologists. In addition to potential lack of appropriate spacing, patients with ESKD on in-center HD sometimes present to their dialysis units with symptoms of cough and shortness of breath. Such patients may then be denied entry to the HD center and require transfer to a dedicated dialysis unit and shift specific for patients under investigation (PUIs) or those with confirmed CoVID-19.
Additionally, since the start of the pandemic, the use of telemedicine has become increasingly common for the care of patients with ESKD on home therapies [85, 86]. Indeed, the International Society of Peritoneal Dialysis has provided recommendations on management of patients receiving PD in the setting of COVID-19 [87]. Overall, the use of telemedicine has allowed for continued follow-up of patients on a monthly basis with specific outlines for how to manage suspected or confirmed COVID-19, in order to minimize exposure risk.
Current recommendations for patients receiving in-center HD include screening upon entry to their dialysis unit to evaluate symptoms (e.g., shortness of breath, cough) and objective measures such as temperature checks [88]. For patients who are cleared, they should be kept at a safe distance from one another for the duration of their HD treatment. While the use of gowns, gloves, and masks have been routinely used by HD center staff at the start and end of each HD treatment, even greater emphasis should be placed on the use of personal protective equipment and hand hygiene, including masking all patients and staff during treatment. Additionally, patients with confirmed CoVID-19 or under investigation for CoVID-19 have been isolated at the level of the HD center. Among HD centers designated to take care of such patients, PUIs and patients with confirmed cases have been dialyzed on separate shifts (typically the last shift of the day), possibly on separate days depending on patient volume at the dialysis center. Another major consideration for these patients is transportation. Public transportation and dedicated mobility transportation, upon which many patients rely to and from HD centers, may not be available to those with confirmed or suspected CoVID-19 infection.
Kidney transplantation
CoVID-19 has posed challenges to the practice of organ transplantation. Kidney transplant recipients (KTRs) are at high risk for illness, due to chronic immunosuppression and co-morbidities [89, 90]. A significant temporal association has been observed between increase in CoVID-19 infections and reduction in overall solid organ transplantation procedures [91]. This reduction in transplantation rates has been mostly seen in kidney transplantation, even in regions where CoVID-19 cases are low.
Current experience
At the time of writing this review, there are currently five case series along with cohort studies from US and Europe, that have reported clinical course of KTRs with COVID-19 infection (n = 10–1216) [85, 89, 92,93,94,95,96,97,98]. Fever and cough remain the most common symptoms of presentation, although atypical initial presentation with gastrointestinal symptoms has also been reported [99]. These series show a preponderance of male recipients, with median age 51–62 years (Table 3). Duration elapsed since transplantation to presentation with viral disease is highly variable with a range of 2–13 years. Of note, in two of the series, 2 patients had evidence of COVID-19 within 3 months of transplantation [92, 94]. Most of the KTRs on presentation were on maintenance immunosuppression comprising of tacrolimus (FK), mycophenolate mofetil (MMF) and prednisone. A high proportion of patients experienced AKI (30–57%) with variable rates of RRT (5–43%). Even accounting for a sizeable number of KTRs still being inpatient at the time of publication of these case series, mortality is as high as 32% [95].
Management of immunosuppression
In-vitro studies have demonstrated that calcineurin inhibitors (CNIs) such as cyclosporine and tacrolimus strongly inhibit the growth of coronavirus via inhibition of cyclophilin and immunophilin pathways [100, 101]. It is not known whether this effect of CNIs translates into clinical efficacy against the virus, but the current recommendation is to continue the CNI in KTRs since cessation may lead to increased risk of rejection and greater use of corticosteroids [102,103,104].
Lymphopenia is a common sign of viral infections including coronavirus, suggesting that anti-metabolites such as MMF and azathioprine be decreased or discontinued. A systematic review from 2011 showed that, in general, there is weak evidence that reduction or cessation of MMF may increase rejection rate and graft loss [103]. As such, the risk of rejection with discontinuing the anti-metabolite may be outweighed by the potential benefit in countering infection.
Based on experience from (SARS)-CoV and MERS-CoV, steroid use has been associated with delayed clearance from blood and respiratory tract [105, 106]. If steroids are a part of maintenance immunosuppression, cessation would not be recommended, although increment in dose would not be deemed beneficial unless compelling indication exists.
Future directions
It is difficult to ascertain the incidence and impact of COVID-19 in KTRs based on the aforementioned data. Initial reports from our center have shown an incidence of rate of 0.67% (n = 20), in a cohort of about 3000 patients followed with a low threshold for viral disease testing. This probably reflects that the incidence rates might be similar to normal population, but still warrants analysis of larger cohorts. It is also encouraging to note that many outpatient KTRs with known or suspected COVID-19 infection had symptomatic resolution without requiring hospitalization [107]. There is a need, however, for development of anti-viral therapy since it appears that a subset of KTRs may especially be susceptible to the disease. While hydroxychloroquine, azithromycin and tocilizumab were employed as therapy in studies discussed, it cannot be deduced that these agents are beneficial in KTRs and trials are ongoing to ascertain efficacy. Delay in transplantation is ultimately deleterious to patients in the long term, and a concerted effort on part of transplant societies and government agencies is currently paramount in overcoming this crisis.
Socially disadvantaged populations
It is important to note that in many settings throughout the world, rates of kidney disease and the provision of its care are defined by socioeconomic, cultural, and political factors, leading to profound disparities in kidney disease burden across social strata [108]. Similarly, CoVID-19 is disproportionately impacting socially disadvantaged individuals. Such persons are often overrepresented in low-wage, public-service professions that raise risk of exposure to CoVID-19. Moreover, they may dwell in crowded, poor-quality housing that limits ability to physically distance from others to reduce infection risk [109]. In many communities, socially disadvantaged persons may also face barriers to CoVID-19 testing, fragmented access to health care, and disruptions in social services as well as greater risk of the economic consequences of CoVID-19. We will likely find that these challenges lead to delayed presentation to nephrology care (e.g. presenting with more advanced disease) and worsening of disparities in CKD and ESKD [110].
Conclusion
CoVID-19 has been a unique challenge to the field of nephrology. Not only has it shown to be associated with high rates and vast array of presentations of AKI, it has also overwhelmed capabilities of medical systems to provide acute and chronic renal replacement therapies. Mortality in CoVID-19 patients afflicted with AKI and those with kidney transplants is high, with a high requirement for mechanical ventilation. Importantly, the disease has also further exposed glaring disparities in care of the socially disadvantaged. As our understanding of CoVID-19 will continue to evolve, there is an impetus for innovation to overcome these obstacles and develop therapeutics to support this vulnerable population.
Availability of data and materials
Not applicable.
Abbreviations
- CoVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus-2
- AKI:
-
Acute kidney injury
- CKD:
-
Chronic kidney disease
- ESKD:
-
End stage kidney disease
- BMI:
-
Body mass index
- ICU:
-
Intensive care unit
- CRP:
-
C-reactive protein
- LDH:
-
Lactate dehydrogenase
- MERS-CoV:
-
middle East Respiratory Syndrome
- CRRT:
-
Continuous renal replacement therapy
- PIRRT:
-
Prolonged intermittent RRT
- PD:
-
Peritoneal dialysis
- HD:
-
Hemodialysis
- ACE2:
-
Angiotensin-converting enzyme 2
- AT1R:
-
Angiotensin-II binding to angiotensin 1 receptor
- ACEIs:
-
Angiotensin-converting enzyme inhibitors
- ARBs:
-
Angiotensin receptor blockers
- PUIs:
-
Patients under investigation
- KTRs:
-
Kidney transplant recipients
- FK:
-
Tacrolimus
- MMF:
-
Mycophenolate mofetil
- CNIs:
-
Calcineurin inhibitors
References
Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, Ghabashi A, et al. Clinical course and Outcomes of Critically Ill Patients With Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014 Mar 18;160(6):389–97.
ALKINDI F, Boobes Y, Nair SC, Hashmey R. SAT-028 ACUTE KIDNEY INJURY ASSOCIATED WITH MIDDLE EAST RESPIRATORY SYNDROME CORONAVIRUS (MERS-CoV) INFECTION. Kidney Int Rep. 2020;5:S13 p. Available from. https://doi.org/10.1016/j.ekir.2020.02.033.
Cha R-H, Joh J-S, Jeong I, Lee JY, Shin H-S, Kim G, et al. Renal complications and their prognosis in Korean Patients with Middle East respiratory syndrome-coronavirus from the central MERS-CoV designated hospital. J Korean Med Sci. 2015;30(12):1807–14.
Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67(2):698–705.
Alsolamy S. Middle East respiratory syndrome: knowledge to date. Crit Care Med. 2015 Jun;43(6):1283–90.
ICNARC report on COVID-19 in critical care. Available from: https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports. Accessed 20 Aug 2020.
Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Baweja M, et al. Acute Kidney Injury in Hospitalized Patients with COVID-19. JASN 2020 DOI: doi: https://doi.org/10.1681/ASN.2020050615. 2020.
Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-20. https://doi.org/10.1056/NEJMoa2002032.
Mohamed MM, Lukitsch I, Torres-Ortiz AE, Walker JB, Varghese V, Hernandez-Arroyo CF, et al. Acute Kidney Injury Associated with Coronavirus Disease 2019 in Urban New Orleans. Kidney360. 2020. https://doi.org/10.34067/KID.0002652020.
Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD. Northwell COVID-19 research consortium; northwell nephrology COVID-19 research consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209-218. https://doi.org/10.1016/j.kint.2020.05.006. Epub 2020 May 16.
Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TS, et al. AKI in Hospitalized Patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020 Sep;31(9):2145–57.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–62.
Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020 May;97(5):829–38.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061.
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81.
Xiao G, Hu H, Wu F, Sha T, Huang Q, Li H, et al. Acute kidney injury in patients hospitalized with COVID-19 in Wuhan, China: A single-center retrospective observational study. medRxiv. 2020;2020:20055194.
Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu Province: A Multicenter Descriptive Study. Clin Infect Dis. 2020;71(15):706-712. https://doi.org/10.1093/cid/ciaa199.
Diao B, Wang C, Wang R, Feng Z, Tan Y, Wang H, et al. Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. medRxiv. 2020;2020:20031120.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 Feb 15;395(10223):507–13.
Cao M, Zhang D, Wang Y, Lu Y, Zhu X, Li Y, et al. Clinical Features of Patients Infected with the 2019 Novel Coronavirus (COVID-19) in Shanghai, China. medRxiv. 2020;2020:20030395.
Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, et al. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. medRxiv. 2020;2020:20030452.
Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia. J Am Soc Nephrol. 2020;ASN:2020030276.
Xia P, Wen Y, Duan Y, Su H, Cao W, Xiao M, et al. Clinicopathological features and Outcomes of acute kidney injury in Critically Ill COVID-19 with prolonged disease course: a retrospective cohort. J Am Soc Nephrol. 2020 Sep;31(9):2205–21.
Rubin S, Orieux A, Prevel R, Garric A, Bats M-L, Dabernat S, et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin Kidney J. 2020;13(3):354–61.
Portolés J, Marques M, López-Sánchez P, de Valdenebro M, Muñez E, Serrano ML, et al. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dial Transplant. 2020 Aug 1;35(8):1353–61.
Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985.
Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington state. JAMA. 2020 Mar 19;323(16):1612–4.
Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020 Jun;395(10239):1763–70.
Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;29:m1996.
Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA Intern Med. 2020; [cited 2020 Sep 24]; Available from: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2768602.
Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area [published correction appears in JAMA. 2020;323(20):2098]. JAMA. 2020;323(20):2052–9. https://doi.org/10.1001/jama.2020.6775.
Cheng Y, Luo R, Wang X, Wang K, Zhang N, Zhang M, et al. The Incidence, Risk Factors, and Prognosis of Acute Kidney Injury in Adult Patients with Coronavirus Disease 2019. Clin J Am Soc Nephrol. 2020;CJN:04650420.
Ng JH, Hirsch JS, Hazzan A, Wanchoo R, Shah HH, Malieckal DA, et al. Outcomes Among Patients Hospitalized With COVID-19 and acute kidney injury. Am J Kidney Dis. 2020;S0272638620309987.
Ali H, Daoud A, Mohamed MM, Salim SA, Yessayan L, Baharani J, et al. Survival rate in acute kidney injury superimposed COVID-19 patients: a systematic review and meta-analysis. Ren Fail. 2020;42(1):393–7.
Robbins-Juarez SY, Qian L, King KL, Stevens JS, Husain SA, Radhakrishnan J, et al. Outcomes for Patients With COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep. 2020 Aug;5(8):1149–60.
Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol JASN. 2006;17(11):3067–75.
Serfozo P, Wysocki J, Gulua G, Schulze A, Ye M, Liu P, et al. Ang II (angiotensin II) conversion to angiotensin-(1-7) in the circulation is POP (Prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension. 2020 Jan;75(1):173–82.
Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46(6):1114–16. https://doi.org/10.1007/s00134-020-06026-1.
Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, et al. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–2. https://doi.org/10.1056/NEJMc2011400. Epub 2020 May 13.
Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–27. https://doi.org/10.1016/j.kint.2020.04.003. Epub 2020 Apr 9.
Martinez-Rojas MA, Vega-Vega O, Bobadilla NA. Is the kidney a target of SARS-CoV-2? Am J Physiol-Ren Physiol. 2020;318(6):F1454–62.
Braun F, Lütgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, et al. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet. 2020 Aug;396(10251):597–8.
Goldsmith CS, Miller SE. Caution in Identifying Coronaviruses by Electron Microscopy. J Am Soc Nephrol. 2020;ASN:2020050755.
Roufosse C, Curtis E, Moran L, Hollinshead M, Cook T, Hanley B, et al. Electron microscopic investigations in COVID-19: not all crowns are coronas. Kidney Int. 2020 Aug;98(2):505–6.
Vijayan A. Humphreys BD. SARS-CoV-2 in the kidney: bystander or culprit? Nat Rev Nephrol. 2020; [cited 2020 Sep 25]; Available from: http://www.nature.com/articles/s41581-020-00354-7.
Creel-Bulos C, Hockstein M, Amin N, Melhem S, Truong A, Sharifpour M. Acute Cor Pulmonale in Critically Ill Patients with Covid-19. N Engl J Med. 2020:e70. Available from:. https://doi.org/10.1056/NEJMc2010459.
Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463-71. https://doi.org/10.1016/j.hrthm.2020.05.001. Epub 2020 May 5.
Upadhyaya VD, Shariff MZ, Mathew RO, Hossain MA, Asif A, Vachharajani TJ. Management of Acute Kidney Injury in the setting of acute respiratory distress syndrome: review focusing on ventilation and fluid management strategies. J Clin Med Res. 2020 Jan;12(1):1–5.
Panitchote A, Mehkri O, Hastings A, Hanane T, Demirjian S, Torbic H, et al. Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann Intensive Care. 2019 Jul 1;9(1):74.
Lee SA, Cozzi M, Bush EL, Rabb H. Distant organ dysfunction in acute kidney injury: a review. Am J Kidney Dis. 2018 Dec;72(6):846–56.
Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing Glomerulopathy in a Patient With Coronavirus Disease 2019 (COVID-19). Kidney Int Rep. 2020; Available from:. https://doi.org/10.1016/j.ekir.2020.04.002.
Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, et al. COVID-19–Associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020 Sep;31(9):1948–58.
Kissling S, Rotman S, Gerber C, et al. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98(1):228–31. https://doi.org/10.1016/j.kint.2020.04.006.
Peleg Y, Kudose S, D’Agati V, Siddall E, Ahmad S, Kisselev S, et al. Acute kidney injury due to collapsing Glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;28.
Nasr SH, Kopp JB. COVID-19-Associated Collapsing Glomerulopathy: An Emerging Entity. Kidney Int Rep. 2020; Available from:. https://doi.org/10.1016/j.ekir.2020.04.030.
Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, et al. Kidney biopsy findings in Patients with COVID-19. J Am Soc Nephrol JASN. 2020;31(9):1959–68.
Suwanwongse K, Shabarek N. Rhabdomyolysis as a Presentation of 2019 Novel coronavirus disease. Cureus. 2020;12(4):e7561. https://doi.org/10.7759/cureus.7561. Published 2020 Apr 6.
Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26(7):1618–20. https://doi.org/10.3201/eid2607.200445. Epub 2020 Jun 21.
Golmai P, Larsen CP, DeVita MV, Wahl SJ, Weins A, Rennke HG, et al. Histopathologic and Ultrastructural findings in postmortem kidney biopsy material in 12 Patients with AKI and COVID-19. J Am Soc Nephrol. 2020;31(9):1944–7.
Werion A, Belkhir L, Perrot M, et al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020;S0085–2538(20)3091–1. https://doi.org/10.1016/j.kint.2020.07.019.
Java A, Apicelli AJ, Liszewski MK, Coler-Reilly A, Atkinson JP, Kim AHJ, et al. The complement system in COVID-19: friend and foe? JCI Insight. 2020;5(15):e140711.
Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020; Available from:. https://doi.org/10.1016/j.trsl.2020.04.007.
Recommendations on the care of hospitalized patients With COVID-19 and Kidney Failure Requiring Renal Replacement Therapy. Available from: https://www.asn-online.org/g/blast/files/Handouts_Webinar_4.21.20.pdf. Accessed 7 July 2020.
RUBIN S, Orieux A, Prevel R, Garric A, Bats M-L, Dabernat S, et al. Characterisation of Acute Kidney Injury in Critically Ill Patients with Severe Coronavirus Disease-2019 (COVID-19). medRxiv. 2020;2020:20069872.
Reddy YNV, Walensky RP, Mendu ML, Green N, Reddy KP. Estimating shortages in capacity to deliver continuous kidney replacement therapy during the COVID-19 pandemic in the United States. Am J Kidney Dis. 2020;76(5):696–709.e1. https://doi.org/10.1053/j.ajkd.2020.07.005.
Fisher M, Prudhvi K, Brogan M, Golestaneh L. Providing care to patients with acute kidney injury and COVID-19 infection: Experience of front line nephrologists in New York. Kidney360. 2020. https://doi.org/10.34067/KID.0002002020.
The Division of Nephrology, Columbia University Vagelos College of Physicians Working Group, New York, New York. Disaster Response to the COVID-19 Pandemic for Patients with Kidney Disease in New York City. J Am Soc Nephrol. 2020;ASN:2020040520.
Burgner A, Ikizler TA, Dwyer JP. COVID-19 and the Inpatient Dialysis Unit: Managing Resources During Contingency Planning Pre-Crisis. Clin J Am Soc Nephrol. 2020;CJN:03750320.
Cleveland Clinic Dialysate Preparation. https://www.youtube.com/watch?v=1ektoZGu83M.70. Accessed 7 July 2020.
Johns Hopkins Dialysate Preparation https://docs.google.com/document/d/17oXLTEqvOtymh_aiR8cU39uylud2w_QRQCjW2-NXmrA/edit. Accessed 7 July 2020.
Almeida CP, Ponce D, de Marchi AC, Balbi AL. Effect of peritoneal Dialysis on respiratory mechanics in acute kidney injury Patients. Perit Dial Int J Int Soc Perit Dial. 2014 Jul;34(5):544–9.
Cullis B, Abdelraheem M, Abrahams G, Balbi A, Cruz DN, Frishberg Y, et al. Peritoneal Dialysis for acute kidney injury. Perit Dial Int J Int Soc Perit Dial. 2014 Jul;34(5):494–517.
Sourial MY, Sourial MH, Dalsan R, et al. Urgent peritoneal Dialysis in Patients With COVID-19 and acute kidney injury: a single-center experience in a time of crisis in the United States. Am J Kidney Dis. 2020;76(3):401–6. https://doi.org/10.1053/j.ajkd.2020.06.001.
Adams E, Mousa AY. Achieving a popliteal venous access for renal replacement therapy in critically ill COVID-19 patient in prone position. J Vasc Surg Cases Innov Tech. 2020;6(2):266–8. https://doi.org/10.1016/j.jvscit.2020.04.003. Published 2020 Apr 22.
Candellier A, Goffin É. Letter regarding "SARS-CoV-2 in the peritoneal waste in a patient treated with peritoneal dialysis". Kidney Int. 2020;98(2):512. https://doi.org/10.1016/j.kint.2020.05.034.
Katagiri D, Ishikane M, Ogawa T, Kinoshita N, Katano H, Suzuki T, et al. Continuous renal replacement therapy for a Patient with severe COVID-19. Blood Purif. 2020;11:1–3.
Syed-Ahmed M, Narayanan M. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis. 2019 Jan;26(1):8–15.
Kidney disease & COVID-19 [Internet]. Available from: [https://www.kidney.org/coronavirus/kidney-disease-covid-19]. Accessed 7 July 2020.
South AM, Tomlinson L, Edmonston D, Hiremath S, Sparks MA. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020 Jun;16(6):305–7.
Patel AB, Verma A. COVID-19 and Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers: What Is the Evidence? JAMA. 2020; [cited 2020 Jun 23]; Available from: https://jamanetwork.com/journals/jama/fullarticle/2763803.
Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020 Jun 18;382(25):2431–40.
Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020 Jun 18;382(25):2441–8.
Sparks MA, South A, Welling P, Luther JM, Cohen J, Byrd JB, et al. Sound science before quick Judgement regarding RAS blockade in COVID-19. Clin J Am Soc Nephrol. 2020 May 7;15(5):714–6.
Medicare COVID-19 Data Release Blog [Internet]. 2020. Available from: https://www.cms.gov/blog/medicare-covid-19-data-release-blog. Accessed 7 July 2020.
Nair V, Jandovitz N, Hirsch JS, Nair G, Abate M, Bhaskaran M, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;n/a. Available from. https://doi.org/10.1111/ajt.15967.
Ronco C, Manani SM, Giuliani A, Tantillo I, Reis T, Brown EA. Remote patient management of peritoneal dialysis during COVID-19 pandemic. Perit Dial Int J Int Soc Perit Dial. 2020;40(4):363–7.
ISPD: Strategies regarding COVID-19 in PD patients. https://ispd.org/strategies-covid19/.
Watnick S, McNamara E. On the frontline of the COVID-19 outbreak: keeping Patients on long-term Dialysis safe. Clin J Am Soc Nephrol. 2020 May 7;15(5):710–3.
Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020; Available from:. https://doi.org/10.1016/j.kint.2020.04.002.
AST Resources For Transplant Professionals. Available from: https://www.myast.org/covid-19-informatio#. Accessed 7 July 2020.
Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. Available from. https://doi.org/10.1016/S0140-6736(20)31040-0.
Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020; Available from. https://doi.org/10.1016/j.kint.2020.03.018.
Early Description of Coronavirus 2019 Disease in Kidney Transplant Recipients in New York. J Am Soc Nephrol. 2020;ASN:2020030375.
Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, et al. Covid-19 and Kidney Transplantation. N Engl J Med. 2020; Available from. https://doi.org/10.1056/NEJMc2011117.
Cravedi P, Suraj SM, Azzi Y, Haverly M, Farouk S, Pérez-Sáez MJ, et al. COVID-19 and Kidney Transplantation: Results from the TANGO International Transplant Consortium. Am J Transplant. 2020;ajt:16185.
Lubetzky M, Aull MJ, Craig-Schapiro R, Lee JR, Marku-Podvorica J, Salinas T, et al. Kidney allograft recipients, immunosuppression, and coronavirus disease-2019: a report of consecutive cases from a New York City transplant center. Nephrol Dial Transplant. 2020 Jul 1;35(7):1250–61.
Caillard S, Anglicheau D, Matignon M, et al. An initial report from the French SOT COVID registry suggests high mortality due to Covid-19 in recipients of kidney transplants. Kidney Int. 2020;S0085-2538(20)30961-3. https://doi.org/10.1016/j.kint.2020.08.005. Published online ahead of print, 2020 Aug 24.
Elias M, Pievani D, Randoux C, Louis K, Denis B, Delion A, et al. COVID-19 Infection in Kidney Transplant Recipients: Disease Incidence and Clinical Outcomes. J Am Soc Nephrol. 2020;ASN:2020050639.
Guillen E, Pineiro GJ, Revuelta I, Rodriguez D, Bodro M, Moreno A, Campistol JM, Diekmann F, Ventura-Aguiar P. Case report of COVID-19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20(7):1875–8. https://doi.org/10.1111/ajt.15874. Epub 2020 Apr 9.
Carbajo-Lozoya J, Müller MA, Kallies S, Thiel V, Drosten C, von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012 Apr;165(1):112–7.
Ma-Lauer Y, Zheng Y, Malešević M, von Brunn B, Fischer G, von Brunn A. Influences of cyclosporin a and non-immunosuppressive derivatives on cellular cyclophilins and viral nucleocapsid protein during human coronavirus 229E replication. Antivir Res. 2020 Jan;173:104620.
Moore J, Middleton L, Cockwell P, Adu D, Ball S, Little MA, et al. Calcineurin inhibitor sparing With Mycophenolate in kidney transplantation: a systematic review and meta-analysis. Transplantation. 2009 Feb 27;87(4):591–605.
Su VC, Greanya ED, Ensom MH. Impact of Mycophenolate Mofetil dose reduction on allograft Outcomes in kidney transplant recipients on Tacrolimus-based regimens: a systematic review. Ann Pharmacother. 2011;45(2):248–57.
Willicombe M, Thomas D, McAdoo S. COVID-19 and Calcineurin Inhibitors: Should They Get Left Out in the Storm? J Am Soc Nephrol. 2020;ASN:2020030348.
Lee N, Chan KCA, Hui DS, Ng EKO, Wu A, Chiu RWK, et al. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304–9.
Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020 Feb 15;395(10223):473–5.
Husain SA, Dube G, Morris H, Fernandez H, Chang J-H, Paget K, et al. Early Outcomes of outpatient Management of Kidney Transplant Recipients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020 Aug 7;15(8):1174–8.
Crews DC, Bello AK, Saadi G, Li PKT, Garcia-Garcia G, Andreoli S, et al. Burden, access, and disparities in kidney disease. Kidney Int. 2019 Feb;95(2):242–8.
Social Distancing Is a Privilege. Available from: https://www.nytimes.com/2020/04/05/opinion/coronavirus-social-distancing.html. Accessed 7 Aug 2020.
Crews DC, Purnell TS. COVID-19, Racism, and Racial Disparities in Kidney Disease: Galvanizing the Kidney Community Response. J Am Soc Nephrol. 2020;ASN:2020060809.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
SK and SPM drafted the manuscript with input from all authors. CJS was a major contributor in editing the manuscript. MH, DMF, DCC, DCB, CJS and BGJ provided critical feedback. BGJ supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
BGJ is a section editor and DCC is an editorial advisor for BMC Nephrology.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kant, S., Menez, S.P., Hanouneh, M. et al. The COVID-19 nephrology compendium: AKI, CKD, ESKD and transplantation. BMC Nephrol 21, 449 (2020). https://doi.org/10.1186/s12882-020-02112-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-020-02112-0