Abstract
The topic of intravenous (IV) fluids may be regarded as “reverse nephrology”, because nephrologists usually treat to remove fluids rather than to infuse them. However, because nephrology is deeply rooted in fluid, electrolyte, and acid-base balance, IV fluids belong in the realm of our specialty. The field of IV fluid therapy is in motion due to the increasing use of balanced crystalloids, partly fueled by the advent of new solutions. This review aims to capture these recent developments by critically evaluating the current evidence base. It will review both indications and complications of IV fluid therapy, including the characteristics of the currently available solutions. It will also cover the use of IV fluids in specific settings such as kidney transplantation and pediatrics. Finally, this review will address the pathogenesis of saline-induced hyperchloremic acidosis, its potential effect on outcomes, and the question if this should lead to a definitive switch to balanced solutions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In a sense, the topic of intravenous fluids is “reverse nephrology”. Nephrologists usually spend their days removing fluids, either by diuretics or by ultrafiltration, rather than infusing them. Still, nephrologists like to think of themselves as experts in fluid, electrolyte, and acid-base balance. This is directly linked to IV fluids as IV fluid therapy implies infusing fluid, electrolytes, and buffers directly into the extracellular fluid volume. IV fluids can be used to correct electrolyte or acid-base disorders, while—equally so—their inadequate use can cause these disorders (Tables 1, 2). Nephrologists are often asked for advice on IV fluids in consultative services, for example in the intensive care, surgical or medical wards. Regardless of the context, the proper prescription of IV fluids requires understanding of physiology. Or, stated more eloquently, “their appropriate use requires reverence for the fine balance that constitutes human homeostasis [1].” A useful approach is to consider IV fluids as drugs, including specific pharmacokinetic and pharmacodynamics properties (as recently reviewed by us [2]). In addition, to understand the current selection of the types of IV fluids, it is useful to briefly review the history of IV fluids in medicine. One of the pioneers in applying IV fluids was Dr. Thomas Latta who, during the cholera epidemic in 1832, instead of infusing fluid into the colon, decided to “throw it immediately in the circulation” [3]. He used a fluid consisting of “soda and two scruples of subcarbonate of soda in six pints of water”, which was remarkably effective, but also quickly forgotten [4]. Between 1882 and 1885 the British physiologist Dr. Sydney Ringer conducted his so-called “extravital investigations” and developed a fluid which enabled a frog’s heart to continue beating outside the body by using a solution comparable to blood plasma [5, 6]. In 1932 the American pediatrician Dr. Alexis Hartmann modified Ringer’s solution by adding the buffer lactate [7, 8]. The IV fluid lactated Ringer’s or Hartmann’s solution still reminds us today of these achievements and may be considered the first balanced solutions (Table 3). Around 1900 the Dutch physiologist Dr. Hartog Jacob Hamburger developed “physiological” or “normal” saline, i.e. 0.9% sodium chloride [9, 10]. In retrospect, the adjectives “physiological” and “normal” may be considered misnomers, because especially the chloride concentration in 0.9% NaCl is supraphysiological (154 vs. 100 mmol/l, Table 3) [11]. The fluids developed by Latta, Ringer, Hartmann, and Hamburger all qualify as crystalloids, water with electrolytes forming a solution. Crystalloids should be distinguished from colloids, in which—chemically speaking—insoluble particles are suspended, but not in solution. A naturally occurring colloid is albumin, which was first used to treat trauma casualties, including burn patients after the Pearl Harbor attack [12]. Today, albumin is occasionally used in severe hypovolemic shock, and in patients with liver failure [13]. Other colloids include dextrans, hydroxyethyl starches, and gelatins. Colloids will not be reviewed in detail here, because they are being used less frequently after several signals that they may cause harm (increased mortality [14] and acute kidney injury (AKI) [15]), especially after retraction of the articles by Dr. Joachim Boldt [16]. Instead, this review will focus on the evolving field of crystalloid IV fluids.
Indications for intravenous fluids
IV fluids are so ubiquitous in clinical medicine that one would almost forget considering its indications (Table 1). An important classification is the distinction between replacement and maintenance IV fluids. Patients requiring replacement IV fluids have a degree of volume depletion that may be due to hemorrhagic or non-hemorrhagic causes. For both categories, the rapid infusion of isotonic saline is indicated for resuscitation (e.g., 500 ml in 10 min, repeated as needed). Isotonic IV fluids expand the intravascular compartment more effectively than hypotonic IV fluids. No studies are available to address whether balanced crystalloids offer advantages in this setting [17]. Colloids are not recommended because of their adverse effects (discussed above) and also because they may be less effective [18]. In less severe volume depletion, the goal of replacement therapy is to correct existing abnormalities in fluid, electrolyte, or acid-base balance. Furthermore, IV fluids may be used in patients with pre-renal azotemia in the context of AKI or acute on chronic renal insufficiency. The fractional excretion of urea rather than sodium may be useful to select patients eligible for a trial of fluid repletion [19]. Maintenance IV fluids are usually given when the patient cannot drink for a prolonged period of time. Owing to immobility, hospitalized patients often require less than a liter of water per day. However, this rough estimate can change dramatically in circumstances with increased loss due to fever, sweating, burns, tachypnea, gastro-intestinal losses, drains, or polyuria. Conversely, non-osmotic stimuli may be present for the release of vasopressin (the antidiuretic hormone), resulting in renal water retention [20]. Changes in body weight and the serum sodium concentration (as measure of water balance) are useful parameters to assess water balance and, accordingly, plan initial IV fluid therapy. Other parameters should also guide the selection of IV fluid therapy, including blood pressure, acid-base status, kidney function, and the presence of diabetes. In general, isotonic IV fluids are recommended for maintenance [20], but specific settings may require tailored therapy. Recommended average rates of infusion are 100–120 ml/h, but should be decreased (25 ml/h in oligoanuric states, 40–60 ml/h in edematous states) or increased (>120 ml/h with urinary concentrating defect) depending on the clinical context [20]. The tendency towards isotonic maintenance IV fluids may be related to previous cases of acute hyponatremia, for example due to the combination of post-operative vasopressin release and the use of hypotonic IV fluids [21]. In addition, because IV fluid therapy can cause fluid overload, and a positive fluid balance in the ICU is associated with higher mortality [22], the need for giving maintenance fluids should always be critically reviewed [23]. Although IV fluids often contain glucose, it offers a poor source of long-term nutrition, which should usually come from enteral tube feeding. Alternatively, in patients with compromised gut function, total parental nutrition may be indicated. These considerations raise the question if glucose should be part of maintenance IV fluids. This practice differs per country and no large studies are available comparing isotonic maintenance fluids with or without dextrose. One randomized trial in the peri-operative setting did show that 72% of patients receiving IV fluids containing dextrose developed transient hyperglycemia, whereas those without dextrose remained normoglycemic [24]. Because hyperglycemia is associated with worse outcomes after acute neurological injury, dextrose may need to be avoided especially in this setting [25, 26]. When hypoglycemia is a potential risk (e.g., during surgery in children) dextrose levels can be safely reduced from 5 to 1% (Table 4) [27].
Etiology of saline-induced acidosis
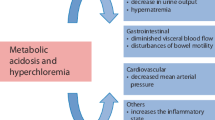
Normal saline has long been the dominant type of IV fluid both for replacement and maintenance. A unique side-effect of normal saline is hyperchloremic metabolic acidosis. A straightforward question without a clear answer is why “normal” saline causes hyperchloremic acidosis. Normal saline differs from other IV fluids in two regards: it does not contain a buffer and it has a higher chloride concentration. Indeed, both the change in serum chloride and the volume of infused 0.9% NaCl correlated with the degree of acidosis in one study [28]. One possibility, therefore, is that expansion of the extracellular fluid with a buffer-free fluid dilutes serum bicarbonate and therefore causes acidosis. This phenomenon of “dilution acidosis”, however, is not linear because experimental data showed that a 28% expansion of the extracellular fluid volume reduced serum bicarbonate by only 10% [29]. The degree of acidosis is likely attenuated by mobilization of intracellular bicarbonate (e.g., from bone) and binding of hydrogen ions by albumin and hemoglobin. Subsequent clinical cases, however, did find the degree of acidosis to be predictable by the amount of normal saline [30]. Both Gattioni et al. and Doberer et al. performed in vitro experiments to investigate the basis of dilution acidosis [31, 32]. They concluded that, for dilution acidosis to occur, the infused volume should largely exceed urine output, and an open CO2/HCO3 − buffer system should exist, where the buffer base (HCO3 −) but not the buffer acid (CO2) is diluted [31]. In a commentary accompanying both studies, Davenport offered yet another explanation focusing on the observation that hyperchloremic acidosis usually occurs in a phase of clinical improvement [33]. He suggested that improved organ perfusion results in a “wash out” of lactate, organic acids, and other intermediary metabolites. This, however, should increase the serum anion gap, which is usually normal in hyperchloremic acidosis. Dilution acidosis is also unlikely to be a renal phenomenon (i.e., due to reduced bicarbonate reabsorption or reduced proton excretion), because it has also been reported in patients with end-stage renal disease receiving normal saline [34], and the kidney responds with an appropriate increase in NH4+ excretion [35].
Comparing solutions
The development of hyperchloremic metabolic acidosis during IV fluid therapy has led to the search for alternative options. Continuous hemorrhage experiments in dogs showed that resuscitation with lactated Ringer’s kept blood pH stable at 7.40, while normal saline reduced it slightly to 7.36 [36]. In humans, a 2-h peri-operative infusion of 30 ml/kg/h normal saline but not lactated Ringer’s increased serum chloride (104 to 115 mmol/l) and reduced pH (7.40 to 7.28) [37]. The authors considered this phenomenon benign unless confused with hypoperfusion or when superimposed with respiratory acidosis by analgetics in the post-operative phase. In a double-blind randomized trial, patients who received normal saline while undergoing aortic reconstructive surgery required more bicarbonate supplementation and more blood products [38]. However, no differences in the duration of mechanical ventilation, intensive care or hospital stay, and incidence of complications were noted [38]. Subsequent studies confirmed the differential effects on coagulation of IV fluids, ascribing this both to a dilutional coagulopathy by normal saline [39] and the development of hypercoagulability with lactated Ringer’s [40]. Together, these head-to-head comparisons clearly show that normal saline causes hyperchloremic metabolic acidosis and impairs coagulation. Because these fluids were given for relatively short periods of time (peri-operatively or during resuscitation after trauma), long-term outcomes were more difficult to evaluate. Still, these studies likely spurred the development of newer balanced crystalloid solutions, including PlasmaLyte and Sterofundin (Table 3). For PlasmaLyte and Sterofundin other buffers than lactate are used (acetate and maleate) and these solutions are isotonic compared to the mildly hypotonic lactated Ringer’s [41]. Since their introduction, several studies have been performed with these newer balanced crystalloids. In healthy volunteers, 2 l of normal saline but not PlasmaLyte increased serum chloride and reduced renal artery flow velocity and renal cortical tissue perfusion (as measured by magnetic resonance imaging) [42]. This effect may be a direct consequence of hyperchloremia, which has been shown to produce renal vasoconstriction and a fall in glomerular filtration rate [43]. This effect on renal blood flow may have contributed to a greater expansion of the extracellular fluid volume with normal saline. Indeed, the excretion of both water and sodium are slower after 2 l of normal saline than after a balanced solution [11]. Because the hypertensive effect of sodium also depends on chloride, normal saline may increase blood pressure, especially in hypertensive patients [44]. Although observational, a study on postoperative IV fluids also favored PlasmaLyte over normal saline [45]. The use of normal saline after open abdominal surgery was associated with higher mortality, and more infections, blood transfusions, renal replacement therapy (RRT), electrolyte disorders, and acidosis [45]. One of the first large prospective studies was an open-label, sequential period pilot study comparing a so-called “chloride-liberal” IV fluid regimen (normal saline, gelatin, albumin) to a “chloride-restrictive” regimen (Hartmann’s solution, PlasmaLyte, chloride-poor albumin) [46]. The chloride-restrictive regimen nearly halved the risk of AKI and RRT. In a 6-month extension of this study these differences in renal outcomes remained [47]. In contrast, the more recent and controlled SPLIT trial (double-blind and double-crossover) compared normal saline with PlasmaLyte in 2278 ICU-patients and found no differences in the incidences of AKI or RRT [48]. Of note, this study did not report serum chloride concentrations and a relatively small volume (median of 2 l) was infused in patients with moderate severity of disease [49]. Finally, and quite surprisingly, no sample size calculations were performed [48].
Intravenous fluids in specific settings
Kidney transplantation
If high-chloride IV fluids indeed impair renal blood flow or even kidney function, the type of IV fluid during kidney transplantation may be particularly relevant. O’Malley et al. addressed this issue in a double-blind randomized trial comparing lactated Ringer’s to normal saline (primary outcome was serum creatinine at day 3) [50]. The study was terminated prematurely because significantly more patients in the normal saline group developed hyperchloremic acidosis and hyperkalemia (serum potassium >6 mmol/l) [50]. Of interest, urine output and graft function were also significantly worse in the normal saline group even a few days after transplantation. Recently, a Cochrane analysis was performed on this topic including the six studies that have been performed to date (477 patients, 70% live donor) [51]. In this analysis, the use of normal saline was associated with a higher risk of hyperchloremic acidosis, but not hyperkalemia or delayed graft function. No intervention studies in other solid organ transplantations are available. However, an observational study in 158 liver transplantations identified the use of >3.2 l of chloride-liberal fluids and a higher pre-operative model for end-stage liver disease (MELD) score as independent predictors for postoperative AKI (occurring in a third of the patients) [52].
Pediatrics
Until recently IV fluids in pediatrics were usually hypotonic. Maintenance requirements for water were traditionally based on caloric expenditure [53]. This usually resulted in glucose-containing solutions with less than 0.9% NaCl (e.g., 0.67, 0.45 or 0.2% NaCl). Because glucose is metabolized to carbon dioxide and water, the net result is a hypotonic solution. This may become problematic if there is a reason for the release of vasopressin. A myriad of non-osmotic stimuli for vasopressin is recognized, including hypovolemia, nausea, pain, the postoperatieve state, several drugs or underlying disease (ranging from infections to malignancy) [20]. Indeed, several cases of iatrogenic acute hyponatremia due to hypotonic IV fluids have been reported in children [54]. In a previous observational study, we showed that almost half of the children with hyponatremia developed this in-hospital while receiving more electrolyte-free water in IV fluids [55]. In children with gastroenteritis plasma levels of vasopressin were frequently high due to vomiting, hypovolemia, hypoglycemia, or “stress” [56]. Consequently, most children had a urinary tonicity that exceeded that of 0.45% saline, predisposing them to the development of hyponatremia. In a randomized trial, the same group subsequently showed that isotonic IV fluids in children with gastroenteritis effectively treat hyponatremia present on admission while preventing hospital-acquired hyponatremia [57]. More rapid infusion of IV fluids, however, did not correct dehydration faster [57]. When considering the pediatric patient population as a whole, a systematic review (including six studies) confirmed that hypotonic solutions greatly increased the risk of developing hyponatremia [58]. One of the first randomized controlled trials that compared 0.45% NaCl with 0.9% NaCl in 5% dextrose as maintenance IV-fluids, however, did not detect an effect on serum sodium, but likely lacked power [59]. A larger trial comparing the same solutions postoperatively did demonstrate a greater risk for hyponatremia with hypotonic fluids [60]. Two meta-analyses, both including ten randomized controlled trials, again confirmed the risk of hyponatremia with hypotonic fluids [61, 62]. Despite this increasing body of evidence, two additional trials were conducted recently [63, 64]. Friedman and colleagues randomized 110 children to receive isotonic (0.9% NaCl in 5% dextrose) or hypotonic IV fluids (0.45% NaCl in 5% dextrose) at maintenance rates for 48 h [63]. The difference in serum sodium concentration after 48 h was minimal (1.4 mmol/l), although the two children developing hyponatremia were both in the hypotonic IV fluid group. The study by McNab et al. had a similar set-up, but is of interest for two reasons [64]. First, the sample size was large: 690 children who required IV fluids for over 6 h. Second, PlasmaLyte was used as isotonic IV fluid and was compared with a hypotonic IV fluid containing 77 mmol/l sodium. Patients in the isotonic group developed hyponatremia significantly less often (11% vs. 4%). Although cerebral edema was not more common in the hypotonic group, a trend towards more seizures was observed (7 vs. 1 children, p = 0.07). In all of these studies isotonic IV fluids did not increase the risk of hypernatremia or fluid overload [65]. Overall, the message is crystal clear: similar to adults, children also require isotonic maintenance IV fluids, and the focus should now be on the implementation of this compelling evidence [66].
Intravenous fluids to correct electrolyte or acid-base disorders
In addition to replacing fluid loss or maintaining fluid balance, IV fluids may also be used specifically to correct an existing electrolyte or acid-base disorder. IV fluids are instrumental in the treatment of certain subtypes of hyponatremia, hypernatremia, metabolic acidosis, and metabolic alkalosis, which will be discussed here. IV fluids can also be used as vehicle to correct other electrolyte disorders (hypokalemia, hypocalcemia, hypomagnesemia, and hypophosphatemia) but this is beyond the scope of this review. Because hyponatremia always indicates a positive water balance, water restriction or approaches to increase renal water excretion are usually the mainstay of treatment. However, patients with hyponatremia can be truly hypovolemic (e.g., due to vomiting or diarrhea), in which case water balance is less negative (relatively positive) in comparison to sodium balance. In these patients hyponatremia can be corrected by isotonic IV fluids. Because vasopressin release is driven by hypovolemia, a sudden water diuresis may occur when the extracellular volume normalizes during IV fluid therapy with the risk of (too) rapid correction of hyponatremia [67]. In addition, acute or symptomatic hyponatremia is usually treated by specific IV fluids, namely hypertonic saline (usually 3% NaCl). In this context, the tonicity rather than the volume of the IV fluid is relevant, as this hypertonic solution will attract water from the intracellular compartment. As such, hypertonic saline can be used to treat cerebral edema in hyponatremic encephalopathy. The amount of hypertonic saline that should be administered can be calculated by the Adrogué–Madias formula [68]. More recently, the approach of giving a bolus of hypertonic saline has been advocated because it can be given rapidly in emergency situations while avoiding calculations [69, 70]. However, a strong evidence base for these recommendations is lacking. Ayus et al. did recently report their experience with a protocol of uniformly infusing 500 ml 3% hypertonic saline in 6 h in 64 patients with hyponatremic encephalopathy [71]. Although this approach appeared safe and effective, the patients had severe hyponatremia (average serum sodium 114 ± 0.8 mmol/l), in whom the distinction between acute or chronic (or acute on chronic) hyponatremia is often difficult to make. In addition, it is important to factor in body weight [72]. In contrast to hyponatremia, hypernatremia should be treated with hypotonic fluids [73]. If possible, increasing oral water intake is preferable, but otherwise half-isotonic or 5% dextrose can be used (while avoiding hyperglycemia). Of note, patients presenting with hypernatremia can be severely hypovolemic and may require initial resuscitation with isotonic saline (that is still relatively hypotonic to serum sodium). In patients in whom volume depletion is accompanied by metabolic acidosis, sodium bicarbonate rather than sodium chloride may be the preferred solution. This strategy may be particularly effective in patients who lost bicarbonate due to gastrointestinal losses. Intravenous sodium bicarbonate is usually reserved for acute metabolic acidosis, but it remains controversial when to initiate treatment (usually when pH <7.1). In addition, the focus should be on the underlying cause of the acidosis (e.g., ketoacids, lactate, or intoxications). If intravenous sodium bicarbonate is given, calculating the bicarbonate space helps to assess the amount of sodium bicarbonate. Commercially available solutions with preset concentrations of sodium bicarbonate may be used (e.g., 1.4, 4.2 or 8.4%, Table 3). Depending on the concentration of sodium bicarbonate used, hypernatremia is a potential side-effect [74]. An alternative solution in this regard is tris-hydroxymethylaminomethase (THAM), an amino alcohol which buffers acids and carbon dioxide [75]. In patients with lactic acidosis due to sepsis or a low-flow state, sodium bicarbonate does not improve outcomes [76, 77]. Experimentally, the negative effects of sodium bicarbonate (lowering of serum calcium and generation of carbon dioxide) can be effectively targeted by calcium supplementation and hyperventilation, but clinical trials are lacking [76, 78]. The much-criticized high chloride concentration of normal saline may actually benefit patients with so-called chloride depletion metabolic alkalosis [79, 80]. This usually concerns patients who lost large amounts of hydrogen chloride from their upper gastrointestinal tract. In these patients hypochloremia is central to the pathogenesis of metabolic alkalosis, as it will directly impair renal bicarbonate excretion. Thus, whatever the place of the newer balanced crystalloid solution may become, normal saline will still remain the treatment of choice for chloride depletion metabolic alkalosis.
Conclusions
Electrolyte solutions are part of our daily clinical practice. The use of isotonic rather than hypotonic maintenance IV fluids is now well established, especially in pediatrics (Table 4) [64]. The comparison between normal saline and balanced crystalloid solutions, however, is still undecided. Although balanced crystalloid IV fluids have physiological appeal given their resemblance to human plasma (Table 3), strong evidence in favor of their general use is still lacking (Table 4). Normal saline can cause hyperchloremic acidosis, which, in turn, may negatively affect renal blood flow [42, 43]. Although preliminary studies indeed showed a higher degree of AKI and RRT in patients treated with normal saline compared to balanced crystalloids [46, 47], a recent large randomized controlled trial failed to confirm these differences [48]. Such differences in outcome may be especially evident in patients receiving large volumes of IV fluids, providing a potential focus for future trials. Similarly, in the setting of kidney transplantation, prevention of hyperchloremic acidosis may be most relevant in patients receiving a cadaveric donor, in whom delayed graft function is a common problem. Regardless of these interesting developments in the field of IV fluids, the first critical assessment should be whether the patient actually requires IV fluids, and if so, to tailor this to the specific characteristics of that individual patient.
Change history
28 January 2020
In Table 3 on Page 486 the term osmolality should��have read��osmolarity.
28 January 2020
In Table 3 on Page 486 the term osmolality should��have read��osmolarity.
References
Goldfarb DS (2010) The normal saline ceremony. Am J Kidney Dis 56(2):A28–A29
Severs D, Rookmaaker MB, Hoorn EJ (2015) Intravenous solutions in the care of patients with volume depletion and electrolyte abnormalities. Am J Kidney Dis 66(1):147–153
Latta TA (1832) Malignant cholera. Documents communicated by the Central Board of Health, London, relative to the treatment of cholera by the copious injection of aqueous and saline fluids into the veins. The Lancet 18:274–280
Cosnett JE (1989) The origins of intravenous fluid therapy. The Lancet 1(8641):768–771
Miller DJ (2004) Sydney Ringer; physiological saline, calcium and the contraction of the heart. J Physiol 555(Pt 3):585–587
Ringer S. Regarding the action of the hydrate of soda, hydrate of ammonia, and the hydrate of potash on the ventricle of the frog’s heart. J Physiol (London) 1880/82;3:195–202.
Lee JA (1981) Sydney Ringer (1834–1910) and Alexis Hartmann (1898–1964). Anaesthesia 36(12):1115–1121
Hartmann AF, Senn MJ (1932) Studies in the metabolism of sodium R-lactate. I. Response of normal human subjects to the intravenous injection of sodium R-lactate. J Clin Invest 11(2):327–335
Hamburger HJ (1902) Osmotischer Druck und Ionenlehre in den medicinischen Wissenschaften: Zugleich Lehrbuch physikalisch-chemischer Methoden. J. F. Bergmann, Wiesbaden
Awad S, Allison SP, Lobo DN (2008) The history of 0.9% saline. Clin Nutr 27(2):179–188
Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP (2003) (Ab)normal saline and physiological Hartmann’s solution: a randomized double-blind crossover study. Clin Sci (Lond) 104(1):17–24
Caironi P, Gattinoni L (2009) The clinical use of albumin: the point of view of a specialist in intensive care. Blood Transfus 7(4):259–267
Finfer S, Bellomo R, Boyce N et al (2004) A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 350(22):2247–2256
Zarychanski R, Abou-Setta AM, Turgeon AF et al (2013) Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA 309(7):678–688
Myburgh JA, Finfer S, Bellomo R et al (2012) Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 367(20):1901–1911
Wise J (2013) Boldt: the great pretender. BMJ 346:f1738
Rizoli S (2011) PlasmaLyte. J Trauma 70(5 Suppl):17–18
Smorenberg A, Groeneveld AB (2015) Diuretic response to colloid and crystalloid fluid loading in critically ill patients. J Nephrol 28(1):89–95
Carvounis CP, Nisar S, Guro-Razuman S (2002) Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int 62(6):2223–2229
Moritz ML, Ayus JC (2015) Maintenance intravenous fluids in acutely Ill patients. N Engl J Med 373(14):1350–1360
Arieff AI (1986) Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med 314(24):1529–1535
Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA (2011) Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 39(2):259–265
Lief L (2016) Maintenance intravenous fluids in acutely Ill patients. N Engl J Med 374(3):289–290
Chin KJ, Macachor J, Ong KC, Ong BC (2006) A comparison of 5% dextrose in 0.9% normal saline versus non-dextrose-containing crystalloids as the initial intravenous replacement fluid in elective surgery. Anaesth Intensive Care 34(5):613–617
Petzold A, Citerio G, Mayer SA (2016) Maintenance intravenous fluids in acutely Ill patients. N Engl J Med 374(3):290
Seners P, Turc G, Oppenheim C, Baron JC (2015) Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry 86(1):87–94
Sumpelmann R, Mader T, Eich C, Witt L, Osthaus WA (2010) A novel isotonic-balanced electrolyte solution with 1% glucose for intraoperative fluid therapy in children: results of a prospective multicentre observational post-authorization safety study (PASS). Paediatr Anaesth 20(11):977–981
Waters JH, Miller LR, Clack S, Kim JV (1999) Cause of metabolic acidosis in prolonged surgery. Crit Care Med 27(10):2142–2146
Garella S, Chang BS, Kahn SI (1975) Dilution acidosis and contraction alkalosis: review of a concept. Kidney Int 8(5):279–283
Prough DS, Bidani A (1999) Hyperchloremic metabolic acidosis is a predictable consequence of intraoperative infusion of 0.9% saline. Anesthesiology 90(5):1247–1249
Gattinoni L, Carlesso E, Maiocchi G, Polli F, Cadringher P (2009) Dilutional acidosis: where do the protons come from? Intensive Care Med 35(12):2033–2043
Doberer D, Funk GC, Kirchner K, Schneeweiss B (2009) A critique of Stewart’s approach: the chemical mechanism of dilutional acidosis. Intensive Care Med 35(12):2173–2180
Davenport A (2009) Dilutional acidosis or uncovered cellular metabolism? Intensive Care Med 35(12):2009–2011
Mirza BI, Sahani M, Leehey DJ, Patel SB, Yang VL, Ing TS (1999) Saline-induced dilutional acidosis in a maintenance hemodialysis patient. Int J Artif Organs 22(10):676–678
Wilcox CS, Granges F, Kirk G, Gordon D, Giebisch G (1984) Effects of saline infusion on titratable acid generation and ammonia secretion. Am J Physiol 247(3 Pt 2):F506–F519.
Cervera AL, Moss G (1975) Dilutional re-expansion with crystalloid after massive hemorrahage: saline versus balanced electrolyte solution for maintenance of normal blood volume and arterial pH. J Trauma 15(6):498–503
Scheingraber S, Rehm M, Sehmisch C, Finsterer U (1999) Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology 90(5):1265–1270
Waters JH, Gottlieb A, Schoenwald P, Popovich MJ, Sprung J, Nelson DR (2001) Normal saline versus lactated Ringer’s solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg 93(4):817–822
Todd SR, Malinoski D, Muller PJ, Schreiber MA (2007) Lactated Ringer’s is superior to normal saline in the resuscitation of uncontrolled hemorrhagic shock. J Trauma 62(3):636–639
Kiraly LN, Differding JA, Enomoto TM et al (2006) Resuscitation with normal saline (NS) vs. lactated ringers (LR) modulates hypercoagulability and leads to increased blood loss in an uncontrolled hemorrhagic shock swine model. J Trauma 61(1):57–64 (discussion 5)
Severs D, Hoorn EJ, Rookmaaker MB (2015) A critical appraisal of intravenous fluids: from the physiological basis to clinical evidence. Nephrol Dial Transplant 30(2):178–187
Chowdhury AH, Cox EF, Francis ST, Lobo DN (2012) A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 256(1):18–24
Wilcox CS (1983) Regulation of renal blood flow by plasma chloride. J Clin Invest 71(3):726–735
Hsieh BS, Wang TC, Chen YM, Wu KD (1994) Blood pressure, circulating atrial natriuretic peptide and sodium excretion responses during acute saline infusion in patients with essential hypertension. J Formos Med Assoc 93(7):576–581
Shaw AD, Bagshaw SM, Goldstein SL et al (2012) Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg 255(5):821–829
Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M (2012) Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 308(15):1566–1572
Yunos NM, Bellomo R, Glassford N, Sutcliffe H, Lam Q, Bailey M (2015) Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med 41(2):257–264
Young P, Bailey M, Beasley R et al (2015) Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA 314(16):1701–1710
Kellum JA, Shaw AD (2015) Assessing toxicity of intravenous crystalloids in critically Ill patients. JAMA 314(16):1695–1697
O’Malley CM, Frumento RJ, Hardy MA et al (2005) A randomized, double-blind comparison of lactated Ringer’s solution and 0.9% NaCl during renal transplantation. Anesth Analg 100(5):1518–1524 (table of contents)
Wan S, Roberts MA, Mount P. Normal saline versus lower-chloride solutions for kidney transplantation. Cochrane Database Syst Rev 2016(8):CD010741
Nadeem A, Salahuddin N, El Hazmi A et al (2014) Chloride-liberal fluids are associated with acute kidney injury after liver transplantation. Crit Care 18(6):625
Holliday MA, Segar WE (1957) The maintenance need for water in parenteral fluid therapy. Pediatrics 19(5):823–832
Halberthal M, Halperin ML, Bohn D (2001) Lesson of the week: acute hyponatraemia in children admitted to hospital: retrospective analysis of factors contributing to its development and resolution. BMJ 322(7289):780–782
Hoorn EJ, Geary D, Robb M, Halperin ML, Bohn D (2004) Acute hyponatremia related to intravenous fluid administration in hospitalized children: an observational study. Pediatrics 113(5):1279–1284
Neville KA, Verge CF, O’Meara MW, Walker JL (2005) High antidiuretic hormone levels and hyponatremia in children with gastroenteritis. Pediatrics 116(6):1401–1407
Neville KA, Sandeman DJ, Rubinstein A, Henry GM, McGlynn M, Walker JL (2010) Prevention of hyponatremia during maintenance intravenous fluid administration: a prospective randomized study of fluid type versus fluid rate. J Pediatr 313-9(2):e1–e2
Choong K, Kho ME, Menon K, Bohn D (2006) Hypotonic versus isotonic saline in hospitalised children: a systematic review. Arch Dis Child 91(10):828–835
Saba TG, Fairbairn J, Houghton F, Laforte D, Foster BJ (2011) A randomized controlled trial of isotonic versus hypotonic maintenance intravenous fluids in hospitalized children. BMC Pediatr 11:82
Choong K, Arora S, Cheng J et al (2011) Hypotonic versus isotonic maintenance fluids after surgery for children: a randomized controlled trial. Pediatrics 128(5):857–866
Foster BA, Tom D, Hill V (2014) Hypotonic versus isotonic fluids in hospitalized children: a systematic review and meta-analysis. J Pediatr 163-9(1):e2
Wang J, Xu E, Xiao Y (2014) Isotonic versus hypotonic maintenance IV fluids in hospitalized children: a meta-analysis. Pediatrics 133(1):105–113
Friedman JN, Beck CE, DeGroot J, Geary DF, Sklansky DJ, Freedman SB (2015) Comparison of isotonic and hypotonic intravenous maintenance fluids: a randomized clinical trial. JAMA Pediatr 169(5):445–451
McNab S, Duke T, South M et al (2015) 140 mmol/L of sodium versus 77 mmol/L of sodium in maintenance intravenous fluid therapy for children in hospital (PIMS): a randomised controlled double-blind trial. The Lancet 385(9974):1190–1197
Moritz ML, Ayus C (2009) Isotonic maintenance fluids do not produce hypernatraemia. Arch Dis Child 94(2):170
Duke T (2016) Maintenance intravenous fluids for children: enough evidence, now for translation and action. Paediatr Int Child Health (London) 36(3):165–167
Liamis G, Kalogirou M, Saugos V, Elisaf M (2006) Therapeutic approach in patients with dysnatraemias. Nephrol Dial Transplant 21(6):1564–1569
Adrogue HJ, Madias NE (2000) Hyponatremia. N Engl J Med 342(21):1581–1589
Spasovski G, Vanholder R, Allolio B et al (2014) Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol Dial Transplant 29(Suppl 2):i1–i39
Moritz ML, Ayus JC (2010) 100 cc 3% sodium chloride bolus: a novel treatment for hyponatremic encephalopathy. Metab Brain Dis 25(1):91–96
Ayus JC, Caputo D, Bazerque F, Heguilen R, Gonzalez CD, Moritz ML (2015) Treatment of hyponatremic encephalopathy with a 3% sodium chloride protocol: a case series. Am J Kidney Dis 65(3):435–442
Spital A (2015) Treatment of hyponatremic encephalopathy. Am J Kidney Dis 66(3):540
Sterns RH (1999) Hypernatremia in the intensive care unit: instant quality–just add water. Crit Care Med 27(6):1041–1042
Hoorn EJ, Betjes MG, Weigel J, Zietse R (2008) Hypernatraemia in critically ill patients: too little water and too much salt. Nephrol Dial Transplant 23(5):1562–1568
Hoste EA, Colpaert K, Vanholder RC et al (2005) Sodium bicarbonate versus THAM in ICU patients with mild metabolic acidosis. J Nephrol 18(3):303–307
Kraut JA, Madias NE (2016) Lactic acidosis: current treatments and future directions. Am J Kidney Dis 68(3):473–482
Jung B, Rimmele T, Le Goff C et al (2011) Severe metabolic or mixed acidemia on intensive care unit admission: incidence, prognosis and administration of buffer therapy. A prospective, multiple-center study. Crit Care 15(5):R238
Kimmoun A, Ducrocq N, Sennoun N et al (2014) Efficient extra- and intracellular alkalinization improves cardiovascular functions in severe lactic acidosis induced by hemorrhagic shock. Anesthesiology 120(4):926–934
Luke RG, Galla JH (2012) It is chloride depletion alkalosis, not contraction alkalosis. J Am Soc Nephrol 23(2):204–207
van Noord C, Zietse R, van den Dorpel MA, Hoorn EJ (2012) The case mid R: a 62-year-old man with severe alkalosis. Kidney Int 81(7):711–712
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hoorn, E.J. Intravenous fluids: balancing solutions. J Nephrol 30, 485–492 (2017). https://doi.org/10.1007/s40620-016-0363-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-016-0363-9