Abstract
Purpose
While SARS-CoV-2 infection appears not to be clinically evident in the testes, indirect inflammatory effects and fever may impair testicular function. To date, few long-term data of semen parameters impairment after recovery and comprehensive andrological evaluation of recovered patients has been published. The purpose of this study was to investigate whether SARS-CoV-2 infection affect male reproductive health.
Methods
Eighty patients were recruited three months after COVID-19 recovery. They performed physical examination, testicular ultrasound, semen analysis, sperm DNA integrity evaluation (TUNEL), anti-sperm antibodies (ASA) testing, sex hormone profile evaluation (Total testosterone, LH, FSH). In addition, all patients were administered International Index of Erectile Function questionnaire (IIEF-15). Sperm parameters were compared with two age-matched healthy pre-COVID-19 control groups of normozoospermic (CTR1) and primary infertile (CTR2) subjects.
Results
Median values of semen parameters from recovered SARS-CoV-2 subjects were within WHO 2010 fifth percentile. Mean percentage of sperm DNA fragmentation (%SDF) was 14.1 ± 7.0%. Gelatin Agglutination Test (GAT) was positive in 3.9% of blood serum samples, but no positive semen plasma sample was found. Only five subjects (6.2%) had total testosterone levels below the laboratory reference range. Mean bilateral testicular volume was 31.5 ± 9.6 ml. Erectile dysfunction was detected in 30% of subjects.
Conclusion
Our data remark that COVID-19 does not seem to cause direct damage to the testicular function, while indirect damage appears to be transient. It is possible to counsel infertile couples to postpone the research of parenthood or ART procedures around three months after recovery from the infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
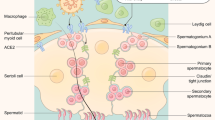
Epidemiological data have identified male gender as a risk factor for severe COVID-19 and increased mortality [1]. This inequality is likely due to mixture of behavioral/lifestyle patterns, gender-specific incidence of comorbidities, aging and intrinsic biological differences between the sexes [1] either on hormonal (i.e., the different effects of testosterone, oestrogens or progesterone) or genetical basis. For this reason, the SARS-CoV-2 outbreak has induced researchers to focus on two substantial male reproductive health issues: first, the possible presence of SARS-CoV-2 in seminal fluid and, second, its impact on testicular function. ACE2, the molecular target for SARS-CoV-2, is expressed in the human testis but its association with testicular infection and, consequently, with impaired spermatogenesis is controversial. Several authors have investigated the presence of SARS-CoV-2 in semen but to date the main consensus is that the chance of detection in this biological sample is negligible: collectively, published data indicate that SARS-CoV-2 has been reported in around 3% (12/386) of investigated semen samples [2]. We could then conclude that the infection appears not to be clinically evident in the testes, although SARS-CoV-2 could theoretically reach semen from the blood, through the blood–testis barrier. This can be explained by the fact that ACE2 and TMPRSS2 are not co-expressed in testicular tissue [3]. Furthermore, to date, few studies have found the presence of SARS-CoV-2 in blood [2]. It is possible that the protection of the blood–testis barrier combined to the absent/low level of viremia in COVID-19 patients might contribute to the SARS-CoV-2 absence in the testis [4]. Besides, testicular damage may be indirectly caused by COVID-19 disease and spermatogenesis may be subsequently impaired through different mechanisms.
Infection and inflammation of the reproductive tract are important factors of infertility [5]. Severe COVID-19 induce high serum levels of pro-inflammatory cytokines and in critical cases it induces the so-called “cytokine storm” with high likelihood of tissue damage [6]. Several studies have shown high levels of seminal pro- and anti-inflammatory cytokines [7, 8] markers of apoptosis and impaired antioxidant activity along with compromised spermatogenesis in male patients recovering from COVID-19 [7], suggesting the presence of an inflammatory condition in the male genital tract. Dysregulated cytokines and chemokines may trigger an autoimmune reaction with consequent alteration of the testicular tissue [9]; TNF-α and IL-1β, in particular, may induce oxidative stress in the Sertoli cells and compromise the blood–testis barrier integrity [10]. This may trigger an anti-sperm autoimmune response, capable of affecting both semen quality (sperm concentration, motility) and the fertilizing ability of spermatozoa and the fusion of the gametes [11, 12]. Pro-inflammatory IL-6 could be involved in the alteration of Leydig cell differentiation as increased LH levels and decreased T/LH and FSH/LH ratios were found in COVID-19 patients compared to healthy [13]. Additional data suggestive of a SARS-CoV-2 interference with gonadal function are consistent with low Testosterone levels in COVID-19 patients [14, 15]. It could, therefore, be hypothesized that an alteration in semen quality could be caused by dysregulated inflammatory mediators, seminal antioxidant defense system and gonadal hormone levels [7]. Fever may also induce changes in testicular temperature that can also negatively impact on germ cells development [16] and transiently affect semen quality and sperm DNA integrity [17, 18]. Therefore, fever induced by COVID-19 can alter sperm parameters even in the absence of the virus in the semen [19]. Several studies evaluated the effect of SARS CoV 2 on semen quality showing impaired semen parameters. It is noteworthy that most of these studies performed semen analyses after a median of 30–40 days from the recovery [7, 20,21,22]. Since a spermatogenetic cycle takes approximately 78 days to be completed, it seems appropriate to evaluate semen quality at least 3 months from the recovery. However, few studies have considered semen analyses at least three months after recovery; moreover, these studies are characterized by small caseloads, contrasting results and even fewer considered testicular functional features other than semen analysis [13, 23,24,25,26,27].
For these reasons, we aimed to perform a comprehensive evaluation of the male reproductive health of COVID-19 patients after 3 months from the recovery, investigating:
-
testicular function evaluating both semen parameters and hormone profile
-
molecular aspects of spermatozoa by evaluation of DNA integrity
-
the integrity of the blood–testis barrier analyzing the presence of anti-sperm antibodies.
-
testicular morphological features through ultrasound evaluation
-
sexual functioning through the self-administered questionnaire IIEF-15
Materials and methods
Patients
The study was approved by the Ethics Committee “Sapienza” (Prot. 0282/2021). Written informed consent was obtained from all study participants. Patients with previous SARS-CoV-2 infection occurred between July 2020 and January 2021 (before the opening of the Italian vaccination campaign to the whole population) were recruited in two Departments of Infectious Disease: AOU Policlinico Umberto I Hospital—“Sapienza” University of Rome and Santa Maria Goretti Hospital Latina (ASL Latina). Each patient was asked to perform an andrological screening to evaluate possible COVID-19 consequences.
Patients were recruited according to the following criteria:
-
previous nasopharyngeal swab positive for SARS-CoV-2 between July 2020 and January 2021;
-
3 months after disease recovery (first negative nasopharyngeal swab);
-
age from 18 to 65 years.
Men with andrological and systemic diseases, Klinefelter’s syndrome and other chromosomal conditions, genetic syndromes, diabetes, hypogonadism (total testosterone below 8 nmol/l), neoplasms, or previous chemotherapy and/or radiotherapy treatments, recent urinary tract infections, clinically relevant varicocele (clinical grade III) or any other andrological condition known to affect semen parameters and sperm DNA integrity were excluded from the study. COVID-19 severity was classified according to the WHO classification (Mild, Moderate, Severe, Critical) [28]. Three months after recovery, patients performed physical examination, testicular ultrasound and were asked to provide a semen and a blood sample. Medical history and other relevant clinical and biochemical data were retrieved from medical records of the subjects.
Control groups
Semen parameters of COVID19 recovered subjects were compared with healthy controls recruited between 2018 and 2019 (before the SARS-CoV-2 appearance). In particular, we retrospectively selected:
Control Group 1 (CTR1)—Healthy normozoospermic subjects with no andrological diseases who attended the Laboratory of Seminology, Sperm Bank “Loredana Gandini” Department of Experimental Medicine—Sapienza University of Rome before the COVID-19 outbreak (2018–2019) for a pre-conceptional screening and, therefore, none of the Control group 1 subjects had children prior of semen analysis.
Control group 2 (CTR2)—Patients with idiopathic infertility but otherwise healthy who attended the Laboratory of Seminology, Sperm Bank “Loredana Gandini” between 2018 and 2019 for semen analysis as a part of an andrological work-up for couple infertility, in the absence of a detectable male or female factor (their partner simultaneously attended the gynecological dept. of our hospital).
Centralized assessment
The principal bias in a multicentre study is the interlaboratory difference in analysis evaluation. For this reason, we centralized at the Laboratory of Seminology- Sperm Bank—“Sapienza” University of Rome the assessment of several parameters: sperm morphology (May-Grünwald–Giemsa staining), hormone analyses (FSH, LH, Testosterone), antisperm antibodies (indirect tests), sperm DNA fragmentation (TUNEL assay). The laboratory is currently a recognized andrological and seminological training center, accredited by major national and international Scientific Societies.
Semen analysis
Semen samples were collected by masturbation after 2–7 days’ abstinence. All samples were allowed to liquefy at 37 °C for 60 min and were then assessed according to WHO (2010) [29]. The following variables were taken into consideration: volume (ml), total sperm number (n × 106 per ejaculate), progressive motility (%), and morphology (% abnormal forms). A sperm viability test was carried out to differentiate cell death from immotility by staining with eosin Y 0.5% in saline solution. As semen analysis was performed in two different Andrology Centres (Rome and Latina), standardization of analyses was achieved by the participation of each Centre in external quality control (EQC) programs and the execution of a routinary internal quality control (IQC).
Antisperm antibodies (ASA) detection
Direct ASA test—Autoimmune reaction was evaluated on the sperm surface by the SpermMar test (FertiPro, Belgium) (WHO 2010). Direct tests could not be performed in hypokinetic or oligozoospermic samples. Light microscopy at 400 × was used to evaluate the percentage of motile sperm that presented latex particles (coated with human IgG or IgA) bound and the site of the bond (head, midpiece, tail).
Indirect ASA test—Autoimmune reaction was evaluated in blood serum and seminal plasma by the Gelatin Agglutination Test (GAT) [30]. All indirect tests were performed twice with different antigens.
Positivity was defined as a direct SpermMar test showing binding > 20%, but clinical relevance was considered with a binding percentage of > 50%. For indirect tests (GAT), an antibody titer of 1:32 or more in blood serum was considered clinically significant (1:16 in seminal plasma) [11].
Sperm DNA fragmentation (SDF)
SDF was evaluated using TUNEL assay (Roche, In Situ Cell Death Detection Kit, Fluorescein, Roche, Basel, Switzerland). After assessment of semen parameters, the samples were centrifuged and evaluated as previously described by [31]. The samples were then analysed under fluorescent microscope (Leica DMR; Leica, Wetzlar, Germany), counting at least 500 cells.
Hormone evaluation
Recruited subjects provided a peripheral blood sample at around 8 a.m. after overnight fasting. Serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), Prolactin (PRL) and total testosterone were quantified by Chemiluminescent Microparticle ImmunoAssay (CMIA, Architect System; Abbott Laboratories, Abbott Park, IL, USA). Detection limits, intra- and inter-assay coefficients of variation and normal ranges were previously described [32]
Sexual function (IIEF- 15 questionnaires)
Sexual function was evaluated through self-administered questionnaires as described elsewhere [34;35].
Testicular ultrasonography (US)
Testicular US examinations were performed in both Rome and Latina centers using standardized views for testicular and epidydimal evaluation with 7–15 MHz wideband linear transducers as described in Pozza et al. 2020 [35]. Testicular volume was estimated using the formula for a prolate ellipsoid: length (L) x width (W) x height (H) × 0.52 [36].
Statistical analysis
Continuous variables are presented as mean and standard deviations or median and interquartile range, based on data distribution as evaluated by Kolmogorov–Smirnov test. Differences between groups are evaluated by Mann Whitney U or Kruskal–Wallis test, as appropriate. Where multiple comparisons are performed, post-hoc results are adjusted according to the Bonferroni method. Categorical variables are presented as counts and percentages and are compared by χ2 test. Statistically significant correlations among the variables examined were evaluated using Spearman’s rank correlation test. For analyses, we grouped patients in two severity grades: “Severe/Critical” and “Mild/Moderate”. The probability values are 2-sided and a p-value < 0.05 was considered statistically significant. All computations were carried out with Statistical Package for the Social Sciences (SPSS) 25.0 (SPSS Inc., Chicago, USA).
Results
Based on inclusion/exclusion criteria, we have been able to recruit 80 patients recovered from SARS-CoV-2 (45 subjects from Latina and 35 from Rome). Additionally, two control groups who were never positive to SARS-CoV-2 have been retrospectively selected: 98 normozoospermic subjects with no previous andrological pathologies (CTR1) and 98 infertile subjects (CTR2) who previously attended the Reproductive Medicine center for couple infertility of Policlinico Umberto I-Rome. The two populations of the cases group were comparable by age (Rome: 40.8 ± 13.1 years vs Latina 45.6 ± 10.0 years; p = 0.156) and BMI (Rome: 25.6 ± 3.9 vs Latina: 27.4 ± 4.3; p = 0.063). Table 1 shows relevant demographics and comorbidities of the investigated population. In particular, 38/80 subjects (47.5%) already had achieved fatherhood. Subject’s occupations are described in Supplementary Table 1. Furthermore, treatments listed in the medical records of each subject were quite heterogeneous and included the following drugs: hydroxychloroquine, lopinavir/ritonavir, darunavir/cobicistat, tocilizumab, azithromycin, paracetamol, ibuprofen, corticosteroids. According to COVID 19 severity patients were classified as: 32 patients Mild, 22pts Moderate, 15pts Severe and 11 pts Critical. Around eighty-eight percent of patients reported the presence of fever during the disease: in particular, the ten afebrile subjects all had a mild disease.
Semen parameters
Median of semen parameters from recovered SARS-CoV-2 subjects were within WHO 2010 fifth percentile. Sperm eosin vitality test showed that mean sperm viability was 63.8 ± 15.0%. No significant difference was found when comparing parameters from the normozoospermic control group (CTR1) (Table 2). In fact, total sperm number and percentage of abnormal forms between these two groups was comparable (p = 0.287 and p = 0.070, respectively), while these same parameters in post COVID-19 subjects were significantly better than infertile controls (CTR2) (p = 0.004 and p < 0.001, respectively) (Figs. 1, 2).
Moreover, while overall oligozoospermic subjects were 13/80 (16.2%), we could observe that when stratified for COVID-19 severity the prevalence of oligoozoospermia nearly doubles in severe cases (12.7% in mild subjects vs. 24.0% in severe subjects) although this does not reach statistical significance (Fisher Exact test p = 0.098).
Regarding progressive motility, we could not detect differences in semen samples from SARS-CoV-2 recovered subjects vs both Normozoospermic (CTR1) and Infertile controls (CTR2) (Table 2 and Fig. 3). Additionally, no significant association was detected between sperm parameters and both the previous presence of fever or COVID-19 severity at three months from recovery (data not shown).
Antisperm antibodies (ASA) evaluation
The presence of ASA has been evaluated with both direct and indirect tests. Direct assays (SpermMar IgG and IgA) were performed in 62 subjects (18 samples were excluded due to oligoozoospermia or asthenozoospermia): only 1/62 subjects (1.6%) were found positive to IgG class. Indirect testing, Gelatin Agglutination Test (GAT), was performed in all subjects in both semen plasma and blood serum: we detected 3/77 (3.9%) positive blood serum samples, but no positive semen plasma sample (Supplementary Table 2). Of note, all subjects with positive blood serum samples had severely altered semen parameters and, because of this, direct tests could not have been performed in these subjects.
Sperm DNA fragmentation
Chromatin integrity analysis showed that the mean percentage of sperm DNA fragmentation (%SDF) in SARS-CoV-2 recovered subjects was 14.1 ± 7.0% (median 12.4%). We compared this to a previously published normozoospermic control population, and we observed that both values were comparable (Carlini et al. 2017—12.8 ± 5.3%, median 12.2%) [37]. Additionally, %SDF did not differ significantly between COVID-19 severity groups (p = 0.538) (Fig. 4) or between subjects with/without fever (p = 0.939) but correlated significantly with patients’ age (ρ = 0.282; p = 0.031).
Hormone profile
Table 3 shows the hormone profile of recruited COVID-19 recovered subjects. Remarkably, mean levels of investigated hormones (LH, FSH, total testosterone and prolactin) were well within normal ranges. We could detect that only five subjects (6.2%) had total testosterone levels below the laboratory reference range (< 10.4 nmol/l). The prevalence of biochemical hypogonadism was comparable between the two participating centers. Moreover, it should be stressed that testosterone levels did not differ significantly between COVID-19 severity groups (p = 0.423), and the pattern of testosterone levels among groups is shown in Supplementary Fig. 1.
Testicular ultrasonography
Testicular ultrasound evaluation of COVID-19 recovered subjects showed that all patients had normal testicular volume, ultrasound echotexture and echogenicity and, in general, all ultrasound findings were consistent with patients’ age. No subject showed ultrasound signs of testicular damage or suggestive of previous orchitis. Mean bilateral testicular volume was 31.5 ± 9.6 ml (median 30.9). In seven subjects, we could detect a unilateral left varicocele (grade I-II) in absence of significant testicular asymmetry.
Sexual function—IIEF-15
Sexual function investigated through IIEF-15 and Table 4 shows the scores of the various domains of the questionnaire. Erectile dysfunction (Erectile function domain score < 26) was detected in 30% of subjects. Even though we did not find significant differences in IIEF-15 domains among COVID-19 severity scores (erectile function domain scores p = 0.473 Mild vs Severe), we could observe a trend of reduction of EF domain score in highest severity grades as well as a trend of increase in prevalence of erectile dysfunction (Supplementary Fig. 2).
Discussion
From December 2019, the COVID-19 became pandemic; and in the last two years, infected billions of people worldwide marking a still active planetary emergency. Up to February 2022 more than 12 millions of Italian cases have been reported with nearly 150 thousands COVID-19 deaths (website ISS EpiCentro, last accessed 22-02-2022). While clinical characteristics of the disease have slightly changed during the years due to the circulation of new variants and, above all, the intensive vaccination campaign, there is still great concern for infected patients. Attention has moved towards possible long-term consequences of the infection in terms of cardiovascular, pneumological, neurological and endocrine health. In particular, concerns for male reproductive health have been raised in terms both of direct and indirect testicular damage. Since the outbreak, epidemiological data of SARS-CoV-2 showed a higher incidence and severity in men. Despite a likely multi-factorial etiology, ACE2 and TMPRSS2 expression in the male genital tract might in part explain these earlier observations. Nonetheless, there is paucity if in vivo evidence of COVID-19 related orchitis. Furthermore, we recently reported that SARS-CoV-2 has a negligible chance of detection in semen [2], thus weakening the hypothesis of direct testicular damage.
This, however, does not exclude possible indirect effects on testicular function. COVID-19 pathophysiology includes a dysregulated immune response [38]. While cytokines are essential immune mediators to contrast the infection, their dysregulation might induce harmful consequences, and might also play a relevant role in the induction of testicular side effects mainly through stimulation of local inflammation and oxidative stress.
Recent illnesses, especially when the patient has reported fever or the utilization of certain drugs, are known to possibly affect the ejaculate (WHO 2021) and, thus, semen quality [39]. Antiviral drugs and immunomodulators are currently the mainstay of COVID-19 treatment, especially in moderate to severe cases, but in early days of the pandemic due to limited knowledge the use of heterogeneous drug protocols have been reported as it can also be noted in our caseload, where we registered a wide use of corticosteroids, antibiotics and hydroxychloroquine. Corticosteroids might alter testicular hormone axis and increase SHBG levels. Although evidence is generally of low quality, all these drugs are known to transiently affect semen parameters [40] and their negative effects on spermatogenesis might be synergistic with the indirect effects of fever and COVID-19 itself. Since these effects might endure for a full spermatogenic cycle, evidence of post COVID-19 alteration in semen parameters should be interpreted in regards of distance from recovery as it is likely that post infection semen quality might be transiently affected. Few papers have investigated post COVID-19 semen parameters, and can be roughly divided into those which have analysed semen parameters within a median of 30–40 days [7, 20,21,22, 41] (Table 5a) and those who reported data on recovered subject from more than 60 days [13, 23, 24, 26, 27] (Table 5b). Short term data show a wide range of semen outcomes, from a higher incidence of azoospermia to normozoospermia (Table 5a). Holtmann et al. [20] and Guo et al. [41] reported that recovered patients had semen parameters within WHO 2010 5th percentile, but it must be remarked that most subject recruited had only a mild disease. On the other hand, Gacci et al. [22] reported a prevalence of 18.6% of azoospermia and 7.0% of severe oligoasthenoteratozoospermia, and a correlation between azoospermia and COVID-19 severity. However, in this study patients with more severe disease were also enrolled, and this might have had a direct impact on reported results. Finally, when comparing COVID-19 recovered patient to healthy controls, recovered subjects were reported to have worse semen parameters [7, 21]. Overall, these studies reported cases with a median recovery from around one month and the lack of pre-COVID semen analyses and longer follow-up do not allow to exclude that patients with severe alterations of spermatogenesis either had preexisting damage to spermatogenesis or have only a transient damage. In this sense, Hajizadeh Maleki and Tartibian [7] also reported a higher %SDF in patients closer to recovery, which improves during follow-up (up to 60 days). The impact of fever associated with SARS-CoV-2 (present in up to 88% of our infected cases) was only reported in two studies, but with contrasting results (Table 5a). Transient effects of fever on semen quality and sperm DNA integrity have already been described [17, 18, 42]. It has been observed that semen parameters return comparable to baseline 79 days after the fever [18]. Moreover, Evenson et al. (2000) studied sperm chromatin structural integrity in a fertile man who contracted influenza, demonstrating an altered chromatin structure 18–66 days post-fever that returned to normal value near the completion of the spermatogenesis (74 days) [43]. Short delay after recovery in most studies probably does not allow to ascertain real effects of the infection on spermatogenesis and limiting the seminological assessment to semen analysis further limits the evaluation. Erbay et al. [23], in particular, showed that a caseload of 69 patients had worse semen parameters more than 90 days after disease recovery. The strength of this study is the presence of pre-COVID-19 semen analyses, but all patients were selected from an infertility clinic and could possibly represent a subgroup of population of infertile subjects whose spermatogenesis was more vulnerable to the direct/indirect effects of the virus. Other studies, however, did not confirm these findings [13, 24]. Donders et al. [27], provided a thorough seminological evaluation of 118 patients, including sperm DNA integrity and ASA evaluation. While incidence of ASA was only 3/119 subjects, semen parameters and %SDF was significantly lower close to recovery and progressively improved returning to normal after three months from recovery. Results from Ruan et al. [26] confirmed the absence of %SDF alterations over 3 months from recovery but semen parameters were nonetheless worse than healthy controls, in absence of significant hormone alterations. Furthermore, testicular US parameters appeared well within the normal values detected in fertile patients [44].
Our results showed that overall andrological health appears not to be compromised 3 months after COVID-19 recovery. In particular, it can be assumed that after a full spermatogenetic cycle from recovery semen parameters and SDF% present no significant long-term impairment and no sperm autoimmune response has taken place. Likewise, hormone profile did not show relevant alterations. To further support these findings, the ultrasound study did not show the presence of any testicular parenchymal damage. Remarkably, investigated parameters were not significantly associated with severity of COVID-19, further strengthening the hypothesis that, once clinical recovery has taken place, SARS-CoV-2 is unlikely to be causative of potential alterations of andrological parameters.
We did find, however, the presence of erectile dysfunction in roughly one-third of subjects. This aspect of post-COVID andrological health aims to be purely descriptive of a relatively under-investigated issue that will undoubtedly require further specific investigations. This first post COVID-19 screening of sexual functioning may be an important point to remark, as it may represent a long-term effect of drugs and/or COVID-19 related distress and may impact on both reproductive health and quality of life [45,46,47,48,49]. Recent reports highlighted the association of SARS-CoV-2 infection and increased prevalence of ED [50, 51]. Sivitrepe et al. investigated the presence of ED after three months of hospital discharge for COVID-19 detecting a further worsening of IIEF scores compared to the scores at hospital admission, linking this worsening to IL-6 levels [52]. Unfortunately, the recruited subject also had high glycemic levels, suggesting diabetes and cardiometabolic comorbidities as possible confounders. In our caseload, despite the lack of pre-COVID IIEF scores, the prevalence of ED appears age-dependent in absence of symptomatic metabolic diseases. Therefore, a viral-induced inflammatory state and endothelial dysfunction might suggest some degree of COVID-19 contribution to the ED in the recovery phase [53], but it is also likely that this might be associated to underlying metabolic disorders.
It is our opinion that the investigation of post COVID-19 sexual functioning might reveal those subjects who will likely require a more careful andrological follow-up in the possibility of post-COVID persistent effects, but this requires more in-depth analysis in future studies.
Strengths of the present study are the complete andrological evaluation of patients and their recruitment from a general population of SARS-CoV-2 infected subjects from Infectious Diseases Departments, at least partially overcoming selection biases from other studies recruiting an infertile population. Furthermore, the inclusion of sexological evaluation, testicular ultrasonography, hormone profile, ASA and sperm DNA integrity evaluation in the work up of post COVID-19 patients allowed to perform a comprehensive andrological evaluation of these subjects. A possible limitation to generalization is the absence of pre-infection data, but comparisons with both infertile and normozoospermic pre-COVID-19 subjects more than compensate for this limitation.
Conclusions
Post COVID-19 subjects appear as a possibly vulnerable population to long-term systemic effects of the infection. Nonetheless, our data further remark that the virus does not seem to cause direct damage to the testicular function, while indirect damage due to inflammation, drugs and fever appear to be transient. This provides a strong and reassuring indication to couples that are attempting to conceive either naturally or artificially. Nonetheless, a careful monitoring of these subjects appears necessary [54]. In particular, due to evidence of transient alterations of sperm DNA integrity close to the recovery, it is possible to counsel infertile couples to postpone the research of parenthood or ART procedures around three months after recovery from the infection to maximize their reproductive chances.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Haitao T, Vermunt JV, Abeykoon J, Ghamrawi R, Gunaratne M, Jayachandran M, Narang K, Parashuram S, Suvakov S, Garovic VD (2020) COVID-19 and sex differences: mechanisms and biomarkers. Mayo Clin Proc 95:2189–2203. https://doi.org/10.1016/j.mayocp.2020.07.024
Paoli D, Pallotti F, Nigro G, Mazzuti L, Hirsch MN, Valli MB, Colangelo S, Mastroianni CM, Antonelli G, Lenzi A et al (2021) Molecular diagnosis of SARS-CoV-2 in seminal fluid. J Endocrinol Invest 44:2675–2684. https://doi.org/10.1007/s40618-021-01580-x
Stanley KE, Thomas E, Leaver M, Wells D (2020) Coronavirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril 114:33–43. https://doi.org/10.1016/j.fertnstert.2020.05.001
He Y, Wang J, Ren J, Zhao Y, Chen J, Chen X (2021) Effect of COVID-19 on male reproductive system - a systematic review. Front Endocrinol (Lausanne) 12:677701. https://doi.org/10.3389/fendo.2021.677701
Schuppe HC, Meinhardt A (2005) Immune privilege and inflammation of the testis. Chem Immunol Allergy 88:1–14. https://doi.org/10.1159/000087816
Cao X (2020) COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol 20:269–270. https://doi.org/10.1038/s41577-020-0308-3
Hajizadeh Maleki B, Tartibian B (2021) COVID-19 and male reproductive function: a prospective, longitudinal cohort study. Reproduction 161:319–331. https://doi.org/10.1530/REP-20-0382
Morselli S, Sebastianelli A, Liaci A, Zaccaro C, Pecoraro A, Nicoletti R, Manera A, Bisegna C, Campi R, Pollini S et al (2021) Male reproductive system inflammation after healing from coronavirus disease 2019. Andrology. https://doi.org/10.1111/andr.13138
Xu J, He L, Zhang Y, Hu Z, Su Y, Fang Y, Peng M, Fan Z, Liu C, Zhao K et al (2021) Severe acute respiratory syndrome coronavirus 2 and male reproduction: relationship, explanations, and clinical remedies. Front Physiol 12:651408. https://doi.org/10.3389/fphys.2021.651408
Olaniyan OT, Dare A, Okotie GE, Adetunji CO, Ibitoye BO, Bamidele OJ, Eweoya OO (2020) Testis and blood-testis barrier in Covid-19 infestation: role of angiotensin-converting enzyme 2 in male infertility. J Basic Clin Physiol Pharmacol. https://doi.org/10.1515/jbcpp-2020-0156
Paoli D, Gilio B, Piroli E, Gallo M, Lombardo F, Dondero F, Lenzi A, Gandini L (2009) Testicular tumors as a possible cause of antisperm autoimmune response. Fertil Steril 91:414–419. https://doi.org/10.1016/j.fertnstert.2007.11.084
Cui D, Han G, Shang Y, Liu C, Xia L, Li L, Yi S (2015) Antisperm antibodies in infertile men and their effect on semen parameters: a systematic review and meta-analysis. Clin Chim Acta 444:29–36. https://doi.org/10.1016/j.cca.2015.01.033
Ma L, Xie W, Li D, Shi L, Ye G, Mao Y, Xiong Y, Sun H, Zheng F, Chen Z et al (2021) Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol 93:456–462. https://doi.org/10.1002/jmv.26259
Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, Spreafico F, Greco GF, Cervi G, Pecoriello A et al (2021) Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology 9:88–98. https://doi.org/10.1111/andr.12821
Salonia A, Pontillo M, Capogrosso P, Gregori S, Tassara M, Boeri L, Carenzi C, Abbate C, Cignoli D, Ferrara AM et al (2021) Severely low testosterone in males with COVID-19: A case-control study. Andrology 9:1043–1052. https://doi.org/10.1111/andr.12993
Xu J, Qi L, Chi X, Yang J, Wei X, Gong E, Peh S, Gu J (2006) Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod 74:410–416. https://doi.org/10.1095/biolreprod.105.044776
Carlsen E, Andersson AM, Petersen JH, Skakkebaek NE (2003) History of febrile illness and variation in semen quality. Hum Reprod 18:2089–2092. https://doi.org/10.1093/humrep/deg412
Sergerie M, Mieusset R, Croute F, Daudin M, Bujan L (2007) High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil Steril 88:970.e1–7. https://doi.org/10.1016/j.fertnstert.2006.12.045
Bendayan M, Boitrelle F (2021) What could cause the long-term effects of COVID-19 on sperm parameters and male fertility? QJM 114:287. https://doi.org/10.1093/qjmed/hcab028
Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, Kruessel JS, Bielfeld AP (2020) Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril 114:233–238. https://doi.org/10.1016/j.fertnstert.2020.05.028
Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, Lu W, Pan F, Meng T, Zhao K et al (2020) Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 28:100604. https://doi.org/10.1016/j.eclinm.2020.100604
Gacci M, Coppi M, Baldi E, Sebastianelli A, Zaccaro C, Morselli S, Pecoraro A, Manera A, Nicoletti R, Liaci A et al (2021) Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum Reprod 36:1520–1529. https://doi.org/10.1093/humrep/deab026
Erbay G, Sanli A, Turel H, Yavuz U, Erdogan A, Karabakan M, Yaris M, Gultekin MH (2021) Short-term effects of COVID-19 on semen parameters: A multicenter study of 69 cases. Andrology 9:1060–1065. https://doi.org/10.1111/andr.13019
Gul A, Zengin S, Dundar G, Ozturk M (2021) Do SARS-CoV-2 infection (COVID-19) and the medications administered for its treatment impair testicular functions? Urol Int 105:944–948. https://doi.org/10.1159/000517925
Pazir Y, Eroglu T, Kose A, Bulut TB, Genc C, Kadihasanoglu M (2021) Impaired semen parameters in patients with confirmed SARS-CoV-2 infection: A prospective cohort study. Andrologia 53:e14157. https://doi.org/10.1111/and.14157
Ruan Y, Hu B, Liu Z, Liu K, Jiang H, Li H, Li R, Luan Y, Liu X, Yu G et al (2021) No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: A perspective and urogenital evaluation. Andrology 9:99–106. https://doi.org/10.1111/andr.12939
Donders GGG, Bosmans E, Reumers J, Donders F, Jonckheere J, Salembier G, Stern N, Jacquemyn Y, Ombelet W, Depuydt CE (2022) Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: a prospective, observational study and validation of the SpermCOVID test. Fertil Steril 117:287–296. https://doi.org/10.1016/j.fertnstert.2021.10.022
World Health Organisation (WHO) Living guidance for clinical management of COVID-19: Living guidance, 23 November 2021 Update. - https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 (last accessed 13–03–2022)
World Health Organisation (WHO) WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th edn. Geneva: Switzerland, 2010
Kibrick S, Belding DL, Merrill B (1952) Methods for the detection of antibodies against mammalian spermatozoa. II. A gelatin agglutination test. Fertil Steril 3:430–438. https://doi.org/10.1016/s0015-0282(16)31026-3
Paoli D, Pecora G, Pallotti F, Faja F, Pelloni M, Lenzi A, Lombardo F (2019) Cytological and molecular aspects of the ageing sperm. Hum Reprod 34:218–227. https://doi.org/10.1093/humrep/dey357
Pallotti F, Senofonte G, Pelloni M, Cargnelutti F, Carlini T, Radicioni AF, Rossi A, Lenzi A, Paoli D, Lombardo F (2020) Androgenetic alopecia: effects of oral finasteride on hormone profile, reproduction and sexual function. Endocrine 68:688–694. https://doi.org/10.1007/s12020-020-02219-2
Pallotti F, Petrozzi A, Cargnelutti F, Radicioni AF, Lenzi A, Paoli D, Lombardo F (2019) Long-term follow up of the erectile function of testicular cancer survivors. Front Endocrinol (Lausanne) 10:196. https://doi.org/10.3389/fendo.2019.00196
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A (1997) The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 49:822–830. https://doi.org/10.1016/s0090-4295(97)00238-0
Pozza C, Kanakis G, Carlomagno F, Lemma A, Pofi R, Tenuta M, Minnetti M, Tarsitano MG, Sesti F, Paoli D et al (2020) Testicular ultrasound score: A new proposal for a scoring system to predict testicular function. Andrology 8:1051–1063. https://doi.org/10.1111/andr.12822
Lenz S, Giwercman A, Elsborg A, Cohr KH, Jelnes JE, Carlsen E, Skakkebaek NE (1993) Ultrasonic testicular texture and size in 444 men from the general population: correlation to semen quality. Eur Urol 24(2):231–238. https://doi.org/10.1159/000474300
Carlini T, Paoli D, Pelloni M, Faja F, Dal Lago A, Lombardo F, Lenzi A, Gandini L (2017) Sperm DNA fragmentation in Italian couples with recurrent pregnancy loss. Reprod Biomed Online 34:58–65. https://doi.org/10.1016/j.rbmo.2016.09.014
van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, Derde L, Leavis H, van Crevel R, Engel JJ, Wiersinga WJ, Vlaar APJ, Shankar-Hari M et al (2022) A guide to immunotherapy for COVID-19. Nat Med 28:39–50. https://doi.org/10.1038/s41591-021-01643-9
World Health Organisation (WHO) WHO Laboratory Manual for the Examination and Processing of Human Semen. 6th edn. Geneva: Switzerland, 2021
Semet M, Paci M, Saïas-Magnan J, Metzler-Guillemain C, Boissier R, Lejeune H, Perrin J (2017) The impact of drugs on male fertility: a review. Andrology 5:640–663. https://doi.org/10.1111/andr.12366
Guo TH, Sang MY, Bai S, Ma H, Wan YY, Jiang XH, Zhang YW, Xu B, Chen H, Zheng XY et al (2021) Semen parameters in men recovered from COVID-19. Asian J Androl 23:479–483. https://doi.org/10.4103/aja.aja_31_21
Bendayan M, Robin G, Hamdi S, Mieusset R, Boitrelle F (2021) COVID-19 in men: With or without virus in semen, spermatogenesis may be impaired. Andrologia 53:e13878. https://doi.org/10.1111/and.13878
Evenson DP, Jost LK, Corzett M, Balhorn R (2000) Characteristics of human sperm chromatin structure following an episode of influenza and high fever: a case study. J Androl 21:739–746
Lotti F, Frizza F, Balercia G, Barbonetti A, Behre HM, Calogero AE et al (2021) The European academy of andrology (EAA) ultrasound study on healthy, fertile men: Scrotal ultrasound reference ranges and associations with clinical, seminal, and biochemical characteristics. Andrology 9:559–576. https://doi.org/10.1111/andr.12951
Sansone A, Mollaioli D, Ciocca G, Limoncin E, Colonnello E, Vena W, Jannini EA (2021) Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J Endocrinol Invest 44(2):223–231. https://doi.org/10.1007/s40618-020-01350-1
Lisco G, Giagulli VA, De Pergola G, De Tullio A, Guastamacchia E, Triggiani V (2021) Covid-19 in man: a very dangerous affair. Endocr Metab Immune Disord Drug Targets 21:1544–1554. https://doi.org/10.2174/1871530321666210101123801
Mazzilli R, Zamponi V, Faggiano A (2022) Letter to the editor: how the COVID-19 pandemic has changed outpatient diagnosis in the andrological setting. J Endocrinol Invest 45:463–464. https://doi.org/10.1007/s40618-021-01673-7
Sansone A, Mollaioli D, Limoncin E, Ciocca G, Bắc NH, Cao TN, Hou G, Yuan J, Zitzmann M, Giraldi A, Jannini EA (2022) The sexual long COVID (SLC): erectile dysfunction as a biomarker of systemic complications for COVID-19 long haulers. Sex Med Rev 10:271–285. https://doi.org/10.1016/j.sxmr.2021.11.001
Masoudi M, Maasoumi R, Bragazzi NL (2022) Effects of the COVID-19 pandemic on sexual functioning and activity: a systematic review and meta-analysis. BMC Public Health 22:189. https://doi.org/10.1186/s12889-021-12390-4
Sansone A, Mollaioli D, Ciocca G, Colonnello E, Limoncin E, Balercia G, Jannini EA (2021) (2021) “Mask up to keep it up”: Preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology 9(4):1053–1059. https://doi.org/10.1111/andr.13003
Katz J, Yue S, Xue W, Gao H (2022) Increased odds ratio for erectile dysfunction in COVID-19 patients. J Endocrinol Invest 45:859–864. https://doi.org/10.1007/s40618-021-01717-y
Sivritepe R, Uçak Basat S, Baygul A, Küçük EV (2022) The effect of interleukin-6 level at the time of hospitalisation on erectile functions in hospitalised patients with COVID-19. Andrologia 54:e14285. https://doi.org/10.1111/and.14285
Kresch E, Achua J, Saltzman R, Khodamoradi K, Arora H, Ibrahim E, Kryvenko ON, Almeida VW, Firdaus F, Hare JM, Ramasamy R (2021) COVID-19 endothelial dysfunction can cause erectile dysfunction: histopathological, immunohistochemical, and ultrastructural study of the human penis. World J Mens Health 39:466–469. https://doi.org/10.5534/wjmh.210055
Nguyen TT, Hulme J, Tran HD, Vo TK, Vo GV (2022) The potential impact of COVID-19 on male reproductive health. J Endocrinol Invest 45:1483–1495. https://doi.org/10.1007/s40618-022-01764-z
Acknowledgements
None.
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. This work was supported by a grant from the Italian Ministry of Education and Research (MIUR-PRIN 2017S9KTNE_003) and the “Sapienza” University of Rome Faculty of Medicine.
Author information
Authors and Affiliations
Contributions
FP and DP conceived the study design and drafted the article; AA performed hormone analyses; DG, CP, IM performed testicular ultrasound; FF, FR, SB, AS, CF, LC performed antisperm antibodies and samples preparation; FF, DP performed molecular analyses; GN, PP provided patient care; DP and LC performed semen analyses; FP analysed the data; and FL, AL, CMM, MRC, ML revised the article; DP, FP, PS assisted in data interpretation. All authors revised the manuscript critically.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Ethical approval
Clinical data were collected in accordance with the ethical standards of the institution. The study was performed in line with the principles of the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

40618_2022_1887_MOESM1_ESM.jpg
Supplementary file1 (JPG 231 KB) Supplementary Fig. 1 Comparison of total testosterone (nmol/l) levels of SARS-CoV-2 recovered subjects stratified per COVID-19 Severity. (Mann Whitney U test)

40618_2022_1887_MOESM2_ESM.jpg
Supplementary file2 (JPG 172 KB) Supplementary Fig. 2 Comparison of Erectile Function domain scores of SARS-CoV-2 recovered subjects stratified per COVID-19 Severity. (Mann Whitney U test)
40618_2022_1887_MOESM4_ESM.docx
Supplementary file4 (DOCX 13 KB) Supplementary Table 2 Summary of ASA positive subjects. “*” indicates that direct testing could not be performed due to severe oligoasthenoteratozoospermia (OAT)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paoli, D., Pallotti, F., Anzuini, A. et al. Male reproductive health after 3 months from SARS-CoV-2 infection: a multicentric study. J Endocrinol Invest 46, 89–101 (2023). https://doi.org/10.1007/s40618-022-01887-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01887-3