Abstract
Purpose of Review
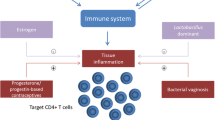
Recent technological developments have vastly improved our ability to study the host microbiome and its role in many disease states. Numerous other reviews have contributed to our understanding of single viruses and gut microbiota or immunological outcomes. Here, we report, in aggregate, the newest data on genital microbiota interactions with the three most common viral STIs.
Recent Findings
Four themes emerge: (1) the repeatability of specific community state types corresponding with infection risk, (2) a role for the microbiota as both therapeutic target and major player in treatment efficacy, (3) a need for models in which to study the mechanisms at play in microbiota/virus interactions, and (4) the impact of microbiota populating external genitalia on viral transmission.
Summary
The studies reviewed herein suggest a convoluted interplay between host microbiota and viral STIs. More mechanistic studies are needed in order to leverage these interactions to improve prevention and treatment strategies.
Similar content being viewed by others
Abbreviations
- ART:
-
Antiretroviral therapy
- CST:
-
Community state type
- HIV:
-
Human immunodeficiency virus
- HPV:
-
Human papillomavirus
- HSV:
-
Herpes simplex virus
- PrEP:
-
Preexposure prophylaxis
- STI:
-
Sexually transmitted infection
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–7. https://doi.org/10.1073/pnas.1002611107.
Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–911. https://doi.org/10.1056/NEJMoa043802.
Ribeiro ABDTM, Heimesaat MM, Bereswill S. Changes of the intestinal microbiome-host homeostasis in HIV-infected individuals - a focus on the bacterial gut microbiome. Eur J Microbiol Immunol (Bp). 2017;7:158–67. https://doi.org/10.1556/1886.2017.00016.
Gootenberg DB, Paer JM, Luevano J-M, Kwon DS. HIV-associated changes in the enteric microbial community: potential role in loss of homeostasis and development of systemic inflammation. Curr Opin Infect Dis. 2017;30:31–43. https://doi.org/10.1097/QCO.0000000000000341.
Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther. 2016;13:19. https://doi.org/10.1186/s12981-016-0103-1.
Bandera A, De Benedetto I, Bozzi G, Gori A. Altered gut microbiome composition in HIV infection: causes, effects and potential intervention. Curr Opin HIV AIDS. 2018;13:73–80. https://doi.org/10.1097/COH.0000000000000429.
Torcia MG. Interplay among vaginal microbiome, immune response and sexually transmitted viral infections. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20020266.
Brotman RM, Shardell MD, Gajer P, et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis. 2014;210:1723–33. https://doi.org/10.1093/infdis/jiu330.
Di Paola M, Sani C, Clemente AM, et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk human papillomavirus infection. Sci Rep. 2017;7:10200. https://doi.org/10.1038/s41598-017-09842-6.
Kero K, Rautava J, Syrjänen K, et al. Association of asymptomatic bacterial vaginosis with persistence of female genital human papillomavirus infection. Eur J Clin Microbiol Infect Dis. 2017;36:2215–9. https://doi.org/10.1007/s10096-017-3048-y.
Tuominen H, Rautava S, Syrjänen S, et al. HPV infection and bacterial microbiota in the placenta, uterine cervix and oral mucosa. Sci Rep. 2018;8:9787. https://doi.org/10.1038/s41598-018-27980-3.
Ritu W, Enqi W, Zheng S, et al. Evaluation of the associations between cervical microbiota and HPV infection, clearance, and persistence in cytologically normal women. Cancer Prev Res (Phila). 2019;12:43–56. https://doi.org/10.1158/1940-6207.CAPR-18-0233.
Kwasniewski W, Wolun-Cholewa M, Kotarski J, et al. Microbiota dysbiosis is associated with HPV-induced cervical carcinogenesis. Oncol Lett. 2018;16:7035–47. https://doi.org/10.3892/ol.2018.9509.
Arokiyaraj S, Seo SS, Kwon M, et al. Association of cervical microbial community with persistence, clearance and negativity of human papillomavirus in Korean women: a longitudinal study. Sci Rep. 2018;8:15479. https://doi.org/10.1038/s41598-018-33750-y.
Reimers LL, Mehta SD, Massad LS, et al. The cervicovaginal microbiota and its associations with human papillomavirus detection in HIV-infected and HIV-uninfected women. J Infect Dis. 2016;214:1361–9. https://doi.org/10.1093/infdis/jiw374.
• Palma E, Recine N, Domenici L, et al. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: a promising solution against HPV-infection. BMC Infect Dis. 2018;18:13. https://doi.org/10.1186/s12879-017-2938-z Demonstrates utility of probiotics as a potential therapeutic in HPV infection.
Masese L, Baeten JM, Richardson BA, et al. Changes in the contribution of genital tract infections to HIV acquisition among Kenyan high-risk women from 1993 to 2012. AIDS. 2015;29:1077–85. https://doi.org/10.1097/QAD.0000000000000646.
Wessels JM, Lajoie J, Vitali D, et al. Association of high-risk sexual behaviour with diversity of the vaginal microbiota and abundance of Lactobacillus. PLoS One. 2017;12:e0187612. https://doi.org/10.1371/journal.pone.0187612.
Lennard K, Dabee S, Barnabas SL, et al. Microbial composition predicts genital tract inflammation and persistent bacterial vaginosis in South African adolescent females. Infect Immun. 2018. https://doi.org/10.1128/IAI.00410-17.
McClelland RS, Lingappa JR, Srinivasan S, et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. Lancet Infect Dis. 2018;18:554–64. https://doi.org/10.1016/S1473-3099(18)30058-6.
Gosmann C, Anahtar MN, Handley SA, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity. 2017;46:29–37. https://doi.org/10.1016/j.immuni.2016.12.013.
Curty G, Costa RL, Siqueira JD, et al. Analysis of the cervical microbiome and potential biomarkers from postpartum HIV-positive women displaying cervical intraepithelial lesions. Sci Rep. 2017;7:17364. https://doi.org/10.1038/s41598-017-17351-9.
Benning L, Golub ET, Anastos K, et al. Comparison of lower genital tract microbiota in HIV-infected and uninfected women from Rwanda and the US. PLoS One. 2014;9:e96844. https://doi.org/10.1371/journal.pone.0096844.
Borgdorff H, Tsivtsivadze E, Verhelst R, et al. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J. 2014;8:1781–93. https://doi.org/10.1038/ismej.2014.26.
Moss JA, Srinivasan P, Smith TJ, et al. Pharmacokinetics and preliminary safety study of pod-intravaginal rings delivering antiretroviral combinations for HIV prophylaxis in a macaque model. Antimicrob Agents Chemother. 2014;58:5125–35. https://doi.org/10.1128/AAC.02871-14.
• Vincent KL, Moss JA, Marzinke MA, et al. Safety and pharmacokinetics of single, dual, and triple antiretroviral drug formulations delivered by pod-intravaginal rings designed for HIV-1 prevention: a phase I trial. PLoS Med. 2018;15:e1002655. https://doi.org/10.1371/journal.pmed.1002655 Demonstrates potential efficacy of intravaginal rings for HIV PrEP.
• Hardy L, Jespers V, De Baetselier I, et al. Association of vaginal dysbiosis and biofilm with contraceptive vaginal ring biomass in African women. PLoS One. 2017;12:e0178324. https://doi.org/10.1371/journal.pone.0178324 Biofilm production on intravaginal rings may affect pharmokinetics.
Heffron R, McClelland RS, Balkus JE, et al. Efficacy of oral pre-exposure prophylaxis (PrEP) for HIV among women with abnormal vaginal microbiota: a post-hoc analysis of the randomised, placebo-controlled Partners PrEP Study. Lancet HIV. 2017;4:e449–56. https://doi.org/10.1016/S2352-3018(17)30110-8.
Klatt NR, Cheu R, Birse K, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356:938–45. https://doi.org/10.1126/science.aai9383.
• Donahue Carlson R, Sheth AN, Read TD, et al. The female genital tract microbiome is associated with vaginal antiretroviral drug concentrations in human immunodeficiency virus-infected women on antiretroviral therapy. J Infect Dis. 2017;216:990–9. https://doi.org/10.1093/infdis/jix420 Genital microbiota influence tissue concentration of antiretroviral drugs.
Shilaih M, Angst DC, Marzel A, et al. Antibacterial effects of antiretrovirals, potential implications for microbiome studies in HIV. Antivir Ther (Lond). 2018;23:91–4. https://doi.org/10.3851/IMP3173.
Zalenskaya IA, Joseph T, Bavarva J, et al. Gene expression profiling of human vaginal cells in vitro discriminates compounds with pro-inflammatory and mucosa-altering properties: novel biomarkers for preclinical testing of HIV microbicide candidates. PLoS One. 2015;10:e0128557. https://doi.org/10.1371/journal.pone.0128557.
Pyles RB, Vincent KL, Baum MM, et al. Cultivated vaginal microbiomes alter HIV-1 infection and antiretroviral efficacy in colonized epithelial multilayer cultures. PLoS One. 2014;9:e93419. https://doi.org/10.1371/journal.pone.0093419.
Ñahui Palomino RA, Zicari S, Vanpouille C, et al. Vaginal Lactobacillus inhibits HIV-1 replication in human tissues ex vivo. Front Microbiol. 2017;8:906. https://doi.org/10.3389/fmicb.2017.00906.
Tyssen D, Wang Y-Y, Hayward JA, et al. Anti-HIV-1 activity of lactic acid in human cervicovaginal fluid. mSphere. 2018. https://doi.org/10.1128/mSphere.00055-18.
Malik S, Petrova MI, Imholz NCE, et al. High mannose-specific lectin Msl mediates key interactions of the vaginal Lactobacillus plantarum isolate CMPG5300. Sci Rep. 2016;6:37339. https://doi.org/10.1038/srep37339.
Hearps AC, Tyssen D, Srbinovski D, et al. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017;10:1480–90. https://doi.org/10.1038/mi.2017.27.
Nunn KL, Wang Y-Y, Harit D, et al. Enhanced trapping of HIV-1 by human cervicovaginal mucus is associated with Lactobacillus crispatus-dominant microbiota. MBio. 2015;6:e01084–15. https://doi.org/10.1128/mBio.01084-15.
Liu CM, Prodger JL, Tobian AAR, et al. Penile anaerobic dysbiosis as a risk factor for HIV infection. MBio. 2017. https://doi.org/10.1128/mBio.00996-17.
• Liu CM, Hungate BA, Tobian AAR, et al. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. MBio. 2015;6:e00589. https://doi.org/10.1128/mBio.00589-15 Demonstration that bacterial vaginosis may be sexually transmittable.
Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009;200:42.e1–7. https://doi.org/10.1016/j.ajog.2008.07.069.
Liu CM, Osborne BJW, Hungate BA, et al. The semen microbiome and its relationship with local immunology and viral load in HIV infection. PLoS Pathog. 2014;10:e1004262. https://doi.org/10.1371/journal.ppat.1004262.
Amerson-Brown MH, Miller AL, Maxwell CA, et al. Cultivated human vaginal microbiome communities impact Zika and herpes simplex virus replication in ex vivo vaginal mucosal cultures. Front Microbiol. 2019. https://doi.org/10.3389/fmicb.2018.03340.
Mousavi E, Makvandi M, Teimoori A, et al. Antiviral effects of Lactobacillus crispatus against HSV-2 in mammalian cell lines. J Chin Med Assoc. 2018;81:262–7. https://doi.org/10.1016/j.jcma.2017.07.010.
Li Y-T, Wang P-H. The Lactobacillus and herpes simplex virus type 2 infection. J Chin Med Assoc. 2018;81:757–8. https://doi.org/10.1016/j.jcma.2018.01.004.
Oh JE, Kim B-C, Chang D-H, et al. Dysbiosis-induced IL-33 contributes to impaired antiviral immunity in the genital mucosa. Proc Natl Acad Sci U S A. 2016;113:E762–71. https://doi.org/10.1073/pnas.1518589113.
Schroeder HA, Nunn KL, Schaefer A, et al. Herpes simplex virus-binding IgG traps HSV in human cervicovaginal mucus across the menstrual cycle and diverse vaginal microbial composition. Mucosal Immunol. 2018;11:1477–86. https://doi.org/10.1038/s41385-018-0054-z.
Ursell LK, Gunawardana M, Chang S, et al. Comparison of the vaginal microbial communities in women with recurrent genital HSV receiving acyclovir intravaginal rings. Antivir Res. 2014;102:87–94. https://doi.org/10.1016/j.antiviral.2013.12.004.
Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4:132ra52. https://doi.org/10.1126/scitranslmed.3003605.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Influences of Hormones and Other Microorganisms on Genital Tract Pathogens
Rights and permissions
About this article
Cite this article
Whitlow, A., Herndon, M.K., Bova, J. et al. Interactions Between Genital Microbiota and Viral Sexually Transmitted Infections: Transmission, Prevention, and Treatment. Curr Clin Micro Rpt 6, 59–66 (2019). https://doi.org/10.1007/s40588-019-00115-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-019-00115-6