Abstract
Background
Over recent years, a growing interest has developed in microbiota and in the concept of maintaining a special balance between Lactobacillus and other bacteria species in order to promote women’s well-being. The aim of our study was to confirm that vaginal Lactobacilli long-lasting implementation in women with HPV-infections and concomitant bacterial vaginosis or vaginitis might be able to help in solving the viral infection, by re-establishing the original eubiosis.
Methods
A total of 117 women affected by bacterial vaginosis or vaginitis with concomitant HPV-infections were enrolled at Department of Gynecological Obstetrics and Urological Sciences, La Sapienza University, Rome, Italy between February 2015 and March 2016. Women were randomized in two groups, standard treatment (metronidazole 500 mg twice a day for 7 days or fluconazole 150 mg orally once a day for 2 consecutive days) plus short-term (3 months) vaginal Lactobacillus implementation (group 1, short probiotics treatment protocol group, n = 60) versus the same standard treatment plus long-lasting (6 months) vaginal Lactobacillus rhamnosus BMX 54 administration (group 2, treatment group, n = 57).
Results
After a median follow up of 14 months (range 9–30 months) the chance to solve HPV-related cytological anomalies was twice higher in probiotic long-term users (group 2) versus short probiotics implementation group (group 1) (79.4% vs 37.5%, p = 0.041). Moreover, a total HPV-clearance was shown in 11.6% of short schedule probiotics implementation patients compared to a percentage of 31.2% in vaginal Lactobacilli long term users (p = 0.044), assessed as negative HPV-DNA test documented at the end of the study period.
Conclusions
The consistent percentage of clearance of PAP-smear abnormalities and HPV-clearance obtained in long-term treatment group has been interestingly high and encouraging. Obviously, larger and randomized studies are warranted to confirm these encouraging results, but we believe that eubiosis re-establishment is the key to tackle effectively even HPV-infection.
Trial registration
Retrospectively registered on PRS NCT03372395 (12/12/2017).
Similar content being viewed by others
Background
An increasing interest has been developed in microbiota, with the belief that probiotics could be able to promote women’s well-being and illnesses in several ways.
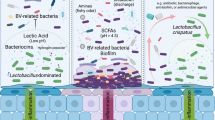
The vaginal microbiota of healthy women consists of a diversity of anaerobic and aerobic microorganisms (eubiosis). Lactobacilli are the most widespread and prevailing subpopulation. In some conditions, this balance can be compromised (dysbiosis) and other microorganisms may grow reducing anti-bacterial defence mechanisms. The loss of this delicate steadiness can move in numerous directions (pathobiosis), depending on different of factors (hormone levels, douching, sexual practices, bacterial interactions, host defences, and so on), promoting disorders such as bacterial vaginosis and yeast vaginitis, and then endorsing the occurrence of sexually transmitted diseases.
So, a steady vaginal ecosystem would have the ability to tackle infections, by maintaining a sort of local equilibrium between the different microbial subpopulations inhabiting vaginal micro-environment. Likewise, the microbial species that populate the vagina would play an important role in having Lactobacilli as the most remarkable protagonists of this process. The mechanisms by which Lactobacilli are able to stabilize the vaginal microbiota consist of the production of antimicrobial compounds (hydrogen peroxide, lactic acid, bacteriocin-like substances) and in the capability to adhere and compete for adhesion sites in the vagina with other pathogens [1,2,3].
Thus, we hypothesized that the development of any kind of disease/infection might be the result of a transitional process, beginning by compromising the physiological vaginal eubiosis, increasing Lactobacilli-mediated cytolysis and then reaching the stage of pathobiosis, when the vaginal ecosystem starts to be defenceless and becomes vulnerable to a variety of infections.
Although just putative relationships between dysbiosis/pathobiosis and cancer development/progression have been observed so far, the long duration of dysbiosis that precedes this condition and the hypothesis of possible combined effects with other risk factors, suggests the presence of greater clinical implications regarding the importance of microbiota conservation and balance [4,5,6]. Additionally, the association of microbial dysbiosis with several cancer types has been noted mainly in areas surrounded by mucosal membranes where bacteria live tightly [7, 8].
Infection with human papillomavirus (HPV) has been recognized as the major cause of cervical cancer development [9, 19]. Recently, a potential role of the vaginal dysbiosis/pathobiosis in promoting cervical HPV-related alterations and consequently precancerous lesions development through the elevation of pH has been reported [6, 8,9,10].
The aim of our study was to investigate if Lactobacillus rhamnosus BMX 54 (NORMOGIN ®) long-lasting vaginal application in women with dysbiosis and concomitant HPV-infections, might be able to have an advantageous effect on viral infection control, by restoring a stable microbiota to eubiosis.
To our knowledge this is the first trial in the literature to assess the efficacy of microbiota balance maintenance against HPV infections and related alterations.
Methods
This is a pilot study, performed between February 2015 and March 2016 at Department of Gynecological Obstetrics and Urologic Sciences, “Sapienza” University of Rome. Inclusion criteria were: age > 18 years, documented BV or yeast vaginitis associated with HPV-infection documented as PAP-smear abnormalities (ASCUS, L-SIL or H-SIL histologically demonstrated as CIN1) and/or positive for HPV-DNA. Exclusion criteria were: pregnancy or breastfeeding, previous abnormal PAP-smear, CIN2–3, concomitant malignancies, immunological diseases, severe comorbidities, prolonged corticosteroid treatment.
Bacterioscopic exam, PAP-smear, HPV DNA test and colposcopy were performed for every enrolled patient at intake and every 3 months of follow up, when indicated. Bacterioscopic exam was performed every time by the same investigator as follows: each vaginal sample was rehydrated with normal saline solution and plotted under a phase contrast microscope with 400× magnification and an area of 0.016 mm2. The presence of bacteria, clue cells, number of vaginal leucocytes and other signs of possible microbial balance alteration/infections were recorded [11]. Evaluation of pH was made with Litmus paper test.
PAP-smears were evaluated by dedicated expert pathologists using the three-tiered CIN classification and Bethesda terminology [9]. An adequate smear was defined as a sample of adequate squamous cells with evidence of transformation zone noted on histologic examination.
HPV infection was detected using polymerase chain reaction (PCR) amplification of the viral DNA, followed by dot blot hybridization to identify its relevant subtypes including HPV 16, 18 and collectively other high-risk HPV subtypes (31–33–35-39-45-51-52-56-58-59-68).
Colposcopy assessment was performed on the basis of the terminology introduced by the Nomenclature Committee of International Federation for Cervical Pathology and Colposcopy in 2011 [12], identifying as Grade 1 (minor changes) the occurrence of a fine mosaic, fine punctation, a thin acetowhite epithelium, or an irregular, geographic border and as Grade 2 (major changes) the presence of a sharp border, an inner border sign, a ridge sign, a dense acetowhite epithelium, a coarse mosaic/punctation, or cuffed crypt openings. Atypical vessels or other suspicious signs of invasion (such as fragile vessels, irregular surface, exophytic lesion, necrosis, ulceration, tumor or gross neoplasm) were included under Grade 2. In the absence of abnormal findings, the colposcopic impression was designated as normal. The squamo-columnar junction visibility was classified as completely visible or non-completely visible. The transformation zone type was classified as type 1 and 2 (completely visible) or type 3 (not fully visible). Endocervical curettage was performed when the squamo-columnar junction was not completely visible. Lesion location was classified on the basis of histological results as follows: ectocervical (when the lesion was only present on ectocervical biopsy), endocervical (when the lesion was present on endocervical curettage), or ectocervical and endocervical (when the lesion was present on both).
Patients were consecutively randomized in two groups, standard treatment plus short-term (3 months) vaginal Lactobacilli implementation (group 1, n = 60) vs standard treatment plus long-lasting (6 months) Lactobacilli administration (group 2, n = 57). Standard initial treatment for bacterial or yeast infections was metronidazole 500 mg (orally twice a day for 7 days) or fluconazole 150 mg (orally once a day for two consecutive days), respectively (Fig. 1).
We decided to administer probiotics as support to standard treatment in the short treatment protocol group as well, considering our encouraging results in vaginal infections control obtained in a previous study [13].
Lactobacilli were administered by vaginal tablets containing 104 CFU/tablet freeze-dried Lactobacillus rhamnosus BMX 54, NORMOGIN ® as follows: once a day for 10 days, once every 3 days for 20 days and then once every 5 days for other 2 months in all patients (probiotics implementation for 3 months comprehensively). On the other hand, patients belonging to long-term treatment arm (group 2) continued using the same vaginal tablets once a week for a 5-month period, instead of just 2 months as others. All patients followed a strict follow-up (every 3 months for 9 months). HPV-DNA test was repeated at the end of the study period (Fig. 1).
The study was approved by the Ethical Committee and written informed consent was obtained from all patients.
Statistical analysis was performed using SPSS software package (SPSS Inc., Chicago, IL, USA) given as mean (SD) or number (%) of cases, with 95% confidence intervals (CIs), when suitable. Comparison of quantitative variables in the two groups considered was performed using the 2-sample Chi-square and Fisher’s test. The measures were indicated as mean ± SD and 95% confidence interval. All tests used were 2-tailed. A p value of <0.05 was considered as statistically significant.
Results
A total of 117 women were included in the study (Fig. 1). Bacterial vaginosis was diagnosed following Amsel criteria [14]. Women were diagnosed with yeast vaginitis by the presence of vaginal discharge in association with at least one of the distinctive symptoms and signs of the disease (itching, burning, dyspareunia and dysuria) and by the presence of yeast infection by at least one of the following processes: wet mount preparation, Gram-staining or culture. Only patients with recognized yeast or bacterial infection and concomitant PAP smear alteration or HPV found by PCR analysis were enrolled in the study. Most of the patients had simultaneous bacterial vaginosis (55 and 54.4% in Group 1 and 2 respectively). Pap smear abnormalities were associated with HPV DNA positivity in 58.3 and 57.8% of cases in Group 1 and 2 respectively. Patients were consecutively randomized in two group as previously explained (Group 1, n = 60 and Group 2, n = 57).
Mean age in Group 1 was 32.4 years (10.5 SD, 95% CI 29.7–35.1), while in Group 2 it was 29.1 years (8.9 SD, 95% CI 26.7–31.5). Patients’ baseline characteristics are shown in Table 1. No significant differences were seen for any of them between Groups 1 and 2. A diagram of follow up schedule was shown in Fig. 1.
Of all participants 84% (n = 98) reported being in a monogamous heterosexual relationship and 11 women reported same-sex relationships and eight declared not having had intercourses at the moment of interview. Compliance was excellent. All participants came back for all follow up visits.
Median follow up time was 14 months (range 9–30 months). After three months, there were no statistically significant differences between the two groups: remission rates of symptoms and bacterial or yeast infections related clinical findings were similar in the two cohorts (Group 1 93.3%, n = 56 and Group 2 96.4%, n = 55 respectively).
At the end of the study period, the chance to solve HPV-related cytological anomalies was twice higher in Lactobacillus rhamnosus BMX 54 long-term vaginal users (79.4%, n = 31 vs 37.5%, n = 15; p = 0.041) as shown in Fig. 2. Moreover, a total HPV-clearance was shown in 11.6% (n = 7) of short treatment protocol patients compared to a percentage of 31.2% (n = 18) in long-term probiotic users (as negative HPV-DNA test) after 9 months since treatment starting (p = 0.044; Fig. 3).
HPV clearance at the end of follow up period (t9) in the two groups (group 1 – short probiotics implementation group, n = 60; group 2 – long probiotics implementation group, n = 57). HPV clearance was significantly higher in long term probiotics users than in the other group (p = 0.044). t0: before treatment; t9: after 9 months follow up
Furthermore, long term probiotics administration determined also a reduction in vaginal infections recurrences as shown in Table 2.
Discussion
The vaginal microbiota plays a significant role in the female reproductive tract wellness maintenance. Although the human papilloma-virus is highly widespread, only a small number of women have recurrent HPV infection and subsequently develop precancerous lesions. An increased variety of vaginal microbiota, combined with a reduction of Lactobacillus spp., is linked with HPV infection acquisition and persistence and development of cervical pre-cancerous lesions and after years cancer [15,16,17]. Cervical cancer is the most common cancer among women in developing countries and the second most frequent female tumour worldwide [16]. Commonly, it progresses through a sequence of premalignant lesions known as cervical intraepithelial neoplasia (CIN) 1, 2 and 3 [9]. A normal cervical epithelial cell needs around 10–20 years to become malignant and just a few women with CIN lesions develop invasive cancer. HPV is a known risk factor for cervical cancer, but despite the high prevalence of HPV infection, CIN occurrence and evolution rates in untreated CIN lesions are quite low [17, 18]. As acknowledged from the literature, over 90% of HPV infections and infection-induced lesions are transient or recurrent and resolve spontaneously [19, 20]. Although HPV infection plays a key role in cervical cancer pathogenesis and develpoment, other environmental and host factors are involved in endorsing the process, as local microflora imbalance, immune response deficiency and consequently concomitant vaginal infections [21–24].
Physiologically, a healthy vaginal ecosystem is dominated by Lactobacilli and most of them had also a local immune system and inflammation response modulation [25, 26] and by controlling cell proliferation/apoptosis [27,28,29].
Hydrogen peroxide, lactate and bacteriocins made by Lactobacilli play an essential role in vaginal flora balance and eubiosis by inhibiting further pathogens’ colonization [13, 30]. Lactobacilli inhibit pathogen overgrowth in the whole urogenital tract, being a vital support in achieving a good reproductive and general health in women.
Importance of chronic inflammation in the development of pre-cancerous lesions has conquered increasing attention in the recent years and vaginal infections have been extensively debated in the literature as a risk factor for cervical dysplasia [31, 32].
Owing to a defined antitumor effect of probiotics, their fundamental role in vaginal ecosystem, their inhibitory effects on pathogens overgrowth and the relationship between vaginal infections and CIN, we assumed a possible relationship between vaginal Lactobacilli deficiency and HPV infections and consequently with HPV related cervical dysplasia.
Lactobacillus rhamnosus vaginal administration was chosen because it represents the exclusive Lactobacillus strain that has been recognized as able to colonize human vaginal microbiota once exogenously applied [26]. Thus, Lactobacilli BMX 54 – a specific selected Lactobacillus strain deposited to Pasteur Institute under Budapest Treaty –has shown clinical evidence of effectiveness when applied vaginally by restoring vaginal balanced ecosystem [33,34,35,36].
This study has some limitations the small sample size and the short follow up even if the statistically significant results obtained by using long-term probiotic vaginal administration are interesting and promising. In this study, cytological and viral endpoints were used to evaluate a potential effect of vaginal Lactobacilli long-term implementation in solving cervical abnormalities, through the establishment of the physiological vaginal balance (eubiosis). The consistent percentage of clearance of PAP-smear abnormalities obtained in vaginal Lactobacilli long term users group is interesting and encouraging and it supports our vaginal eubiosis versus pathobiosis theory.
Conclusions
To our knowledge, this is the first study showing a control of HPV infection using probiotics. From our data, probiotics implementation for at least 6 months may be useful in re-establishing vaginal microflora and due to the recreation of vaginal balance, seems to benefit in controlling HPV infection.
Abbreviations
- ASCUS:
-
Atypical Squamous Cells of Undetermined Significance
- CFU:
-
Colony-Forming Unit
- CIN:
-
Cervical Intraepithelial Neoplasia
- HPV:
-
Human Papilloma Virus
- H-SIL:
-
High Grade Intraepithelial Lesion
- L-SIL:
-
Low Grade Intraepithelial Lesion
- PCR:
-
Polymerase Chain Reaction
References
Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, Gurguis A, Faro S. Defense factors of vaginal lactobacilli. Am J Obstet Gynecol. 2001;185:375–9.
Conti C, Malacrino C, Mastromarino P. Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J Physiol Pharmacol. 2009;60:19–26.
Matu MN, Orinda GO, Njagi ENM, Cohen CR, Bukusi EA. Vitro inhibitory activity of human vaginal lactobacilli against pathogenic bacteria associated with bacterial vaginosis in Kenyan women. Anaerobe. 2010;16:210–5.
Bultman SJ. Emerging roles of the microbiome in cancer. Carcinogenesis. 2014;35:249–55.
Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907.
Clarke MA, Rodriguez AC, Gage JC, et al. A large, population-based study of age-related associations between vaginal pH and human papillomavirus infection. BMC Infect Dis. 2012;12:33.
Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Imm. 2009;9:799–809.
Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–706.
Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda system: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9.
Lee JE, Lee S, Lee H, et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One. 2013;8:e63514.
Holst E. Bacterial vaginosis microbiological and clinical findings. Eur J Clin Microbiol. 1987;6:536–41.
Bornstein J, Bentley J, Bösze P, et al. 2011 colposcopic terminology of the International Federation for Cervical Pathology and Colposcopy. Obstet Gynecol. 2012;120:166–72.
Recine N, Palma E, Domenici L, et al. Restoring vaginal microbiota: biological control of bacterial vaginosis. A prospective case-control study using Lactobacillus rhamnosus BMX 54 as adjuvant treatment against bacterial vaginosis. Arch Gynecol Obstet. 2016;293:101–7.
Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22.
Mitra A, MacIntyre DA, Marchesi JR, Lee YS, Bennett PR, Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. 2016;4:58.
Song D, Kong WM, Zhang TQ, Jiao SM, Chen J, Han C, Liu TT. The negative conversion of high-risk human papillomavirus and its performance in surveillance of cervical cancer after treatment: a retrospective study. Arch Gynecol Obstet. 2017;295:197–203.
Gillet E, Meys JF, Verstraelen H, Verhelst R, De Sutter P, Temmerman M, Vanden Broeck D. Association between bacterial vaginosis and cervical intraepithelial neoplasia: systematic review and meta-analysis. PLoS One. 2012;7:e45201.
Nam KH, Kim YT, Kim SR, Kim SW, Kim JW, Lee MK, Nam EJ, Jung YW. Association between bacterial vaginosis and cervical intraepithelial neoplasia. J Gynecol Oncol. 2009;20:39–43.
Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65.
Motevaseli E, Shirzad M, Akrami SM, Mousavi AS, Mirsalehian A, Modarressi MH. Normal and tumour cervical cells respond differently to vaginal lactobacilli, independent of pH and lactate. J Med Microbiol. 2013;62:1065–72.
Pendharkar S, Magopane T, Larsson PG, de Bruyn G, Gray GE, Hammarström L, Marcotte H. Identification and characterisation of vaginal lactobacilli from south African women. BMC Infect Dis. 2013;13:43.
Jespers V, van de Wijgert J, Cools P, et al. Vaginal biomarkers study group. The significance of lactobacillus crispatus and L. Vaginalis for vaginal health and the negative effect of recent sex: a cross-sectional descriptive study across groups of African women. BMC Infect Dis. 2015;15:115.
De Backer E, Verhelst R, Verstraelen H, et al. Quantitative determination by real-time PCR of four vaginal lactobacillus species, Gardnerella vaginalis and Atopobium vaginae indicates an inverse relationship between L. Gasseri and L. Iners. BMC Microbiol. 2007;7:115.
Spurbeck RR, Arvidson CG. Lactobacilli at the front line of defense against vaginally acquired infections. Future Microbiol. 2011;6:567–82.
Iyer C, Kosters A, Sethi G, Kunnumakkara AB, Aggarwal BB, Versalovic J. Probiotic lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NF-kappaB and MAPK signalling. Cell Microbiol. 2008;10:1442–52.
Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6:39–51.
Orlando A, Messa C, Linsalata M, Cavallini A, Russo F. Effects of lactobacillus rhamnosus GG on proliferation and polyamine metabolism in HGC-27 human gastric and DLD-1 colonic cancer cell lines. Immunopharmacol Immunotoxicol. 2009;31:108–16.
Orlando A, Refolo MG, Messa C, Amati L, Lavermicocca P, Guerra V, Russo F. Antiproliferative and proapoptotic effects of viable or heat-killed lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr Cancer. 2012;64:1103–11.
Sharma S, Singh RL, Kakkar P. Modulation of Bax/Bcl-2 and caspases by probiotics during acetaminophen induced apoptosis in primary hepatocytes. Food Chem Toxicol. 2011;49:770–9.
Tamrakar R, Yamada T, Furuta I, Cho K, Morikawa M, Yamada H, Sakuragi N, Minakami H. Association between lactobacillus species and bacterial vaginosis-related bacteria, and bacterial vaginosis scores in pregnant Japanese women. BMC Infect Dis. 2007;7:128.
Boccardo E, Lepique AP, Villa LL. The role of inflammation in HPV carcinogenesis. Carcinogenesis. 2010;31:1905–12.
Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450–4.
YaW Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebocontrolled study. Am J Obstet Gynecol. 2010;203:120–5.
Reid G, Burton J. Use of lactobacillus to prevent infection by pathogenic bacteria. Microbes Infect. 2002;4:319–24.
Cadieux P, Burton J, Gardiner G, Braunstein I, Bruce AW, Kang CY, Reid G. Lactobacillus strains and vaginal ecology. JAMA. 2002;287:1940–1.
Reid GK, Mills AP, Bruce AW. Implantation of lactobacilli Casei var-rhamnosus into the vagina. Lancet. 1994;344:1229.
Acknowledgments
None.
Funding
None.
Availability of data and materials
All data are available for scientific purposes if needed.
Author information
Authors and Affiliations
Contributions
PE, RN, DL: Project development and drafting. PE, RN: Manuscript Editing and Conception. DL, GM: Data Collection, Manuscript writing. PA, BPP: Manuscript Editing and Review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional Review Board approved the study (Clinical Trial Centre, Policlinico Umberto I Hospital, Rome-Italy). Written informed consent was collected from all patients enrolled. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Palma, E., Recine, N., Domenici, L. et al. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: a promising solution against HPV-infection. BMC Infect Dis 18, 13 (2018). https://doi.org/10.1186/s12879-017-2938-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-017-2938-z