Abstract
Background
C-terminal Agrin Fragment (CAF) has emerged as a potent biomarker for identifying sarcopenia. However, the effect of interventions on CAF concentration and the association of CAF with sarcopenia components are unclear.
Objective
To review the association between CAF concentration and muscle mass, muscle strength, and physical performance among individuals with primary and secondary sarcopenia and to synthesize the effect of interventions on the change in the level of CAF concentration.
Methods
A systematic literature search was conducted in six electronic databases, and studies were included if they met the selection criteria decided a priori. The data extraction sheet was prepared, validated, and extracted relevant data.
Results
A total of 5,158 records were found, of which 16 were included. Among studies conducted on individuals with primary sarcopenia, muscle mass was significantly associated with CAF levels, followed by hand grip strength (HGS) and physical performance, with more consistent findings in males. While in secondary sarcopenics, the strongest association was found for HGS and CAF levels, followed by physical performance and muscle mass. CAF concentration was reduced in trials that used functional, dual task, and power training, whereas resistance training and physical activity raised CAF levels. Hormonal therapy did not affect serum CAF concentration.
Conclusion(s)
The association between CAF and sarcopenic assessment parameters varies in primary and secondary sarcopenics. The findings would help practitioners and researchers choose the best training mode/parameters/exercises to reduce CAF levels and, eventually, manage sarcopenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenia, a term Irwin Rosenburg used in the late 1980s, means “loss of flesh” [1]. In the last two decades, a lot of focus has been given to understanding this condition, with various groups like the European Working Group on Sarcopenia in Older People (EWGSOP), Asian Working Group for Sarcopenia (AWGS), and International Working Group for Sarcopenia (IWGS) have come up with the definition which states sarcopenia as a condition characterized by gradual loss of skeletal muscle mass, loss of muscle strength, and reduced physical performance [2,3,4]. The prevalence of sarcopenia ranges from 9 to 12% in community-dwelling older adults, 23–24% among hospitalized people, and 30–50% among residents of long-term care settings, and its incidence increases with age [5, 6]. Considering the complex and multifactorial etiology and association with multiple adverse events, the early identification of sarcopenia is of utmost importance to promote healthy aging [7,8,9,10,11,12].
Despite the criteria set by EWGSOP and AWGS to assist in assessing sarcopenia [2, 3], challenges with acquiring valid performance and the amount of muscle data continue to obstruct the consistent implementation of diagnostic and therapeutic programs [13, 14]. While techniques like dual-energy X-ray absorptiometry (DEXA), magnetic resonance imaging (MRI), computed tomography (CT), and bioimpedance analyzer (BIA) can quantify lean mass but are expensive and difficult to use in different clinical settings like community settings, and primary health centers [13, 15, 16]. Furthermore, researchers debate the objectivity of muscle mass, muscle strength, and physical performance evaluations in older adults, as these are performance-oriented and demand active involvement [14, 17]. As a result, direct methods of assessing muscle mass and performance for the older population are only sometimes practical. Lack of motivation, pain, and depressed mood could interfere with the testing, which might not produce plausible results [17]. Hence, as a sarcopenia screening method, a blood-based biomarker could be a more accessible choice [14].
The search for sarcopenia biomarkers that provide additional information obtained from clinical data has become especially important in recent years. Ascertaining the pathophysiology of the disease is key to the development of sarcopenia biomarkers. The pathogenesis underlying the onset and development of sarcopenia is multifactorial [18,19,20]. The importance of the neurophysiological process in maintaining skeletal muscle health with advancing age has recently gained credence [21, 22]. Reduced reinnervation capacity due to age-related disruption at the neuromuscular junction (NMJ) is recognized as a significant contributor to the development and progression of sarcopenia [23]. In fact, as a person ages, considerable remodeling occurs in NMJ, which is essential for the nerve to muscle cross-talk [21]. As neuromuscular remodeling occurs, the neuronal protease neuro-trypsin proteolytically cleaves and inactivates Agrin, a well-established mediator of NMJ formation and stabilization, dissociating a 22-kDa C-terminal Agrin Fragment (CAF) [24], easily quantifiable in human blood. As a result, the post-synaptic acetylcholine receptor clustering will be compromised, which will cause a gradual buildup of denervated muscle fibers [21]. In light of this, biomarkers of NMJ stability have been suggested as early signs of sarcopenia [25].
The circulatory level of CAF may represent an early indicator of NMJ dismantling and muscle fiber denervation, which signals the onset of sarcopenia [14, 21]. A study on healthy older adults reported that the serum CAF concentration was significantly related to the onset of neuromuscular fatigue [26]. On the other hand, older persons who engage in aerobic activities and balance training reported significantly higher mobility and correspondingly lower CAF levels [27,28,29]. Thus, a change in the concentration of CAF following therapeutic exercise would be a relevant outcome measure along with the assessment of muscle mass, muscle strength, and physical performance, which are performance-oriented.
The wide availability and the low cost of CAF reinforce the potential for use in daily clinical practice by allowing identification or at least contributing to the early identification of patients at risk of sarcopenia in a simple way while requesting a routine blood test [30, 31]. Understanding the CAF association with components of sarcopenia (muscle mass/muscle strength/physical performance) is also of great interest for developing, adapting, or evaluating therapeutic exercise programs and prophylactic treatments. However, a scoping review summarizing and discussing the association between CAF and components of primary and secondary sarcopenia, as well as comparing the effect of different interventions on the level of CAF concentration among sarcopenic older adults, is lacking. The review would be a valuable resource for physicians and researchers who intend to include CAF in research studies and clinical practice as a biomarker of sarcopenia. Thus, this review aims to scope the scientific evidence about the association between the level of CAF and primary sarcopenia, the level of CAF and secondary sarcopenia, and the effect of various interventions (exercise/nutrition/hormonal) on the change in the level of CAF among older adults. We hypothesized that the higher CAF levels would be associated with primary and secondary sarcopenia, and the interventions (exercise/nutrition/hormonal) would reduce the level of CAF among older adults.
Methods
Scoping review methodology was chosen as it is the most appropriate method to identify the key characteristics related to the topic of investigation: the association between CAF and primary sarcopenia, the association between CAF and secondary sarcopenia, and the effect of interventions (exercise/nutrition/hormonal) on the levels of the CAF [32]. This review followed the Joanna Briggs Institute (JBI) Scoping Review Methodology [33] and Preferred Reporting Items for Systematic reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-ScR) checklist was used for reporting processes [34].
Data sources and search strategy
A systematic literature search was undertaken from April to September 2022 on the following six electronic databases: PubMed, Scopus, Embase, Cumulated Index to Nursing and Allied Health Literature (CINAHL), ProQuest, and Web of Science. Relevant MeSH terms and Boolean phases used for the search: “CAF” OR “agrin” OR “neuromuscular junction degeneration” OR “agrin receptor” OR “blood biomarker” AND “Sarcopenia” OR “physical functional performance” OR “hand strength” OR “muscle, skeletal” AND “older adult” OR “aged” OR “senior citizens” without time restriction and age/ humans as filters were applied (Supplementary material 1).
Eligibility criteria
The studies were included if they met the following criteria: (a) original study, (b) on older adults, (c) including those diagnosed with primary or secondary sarcopenia with specific diseases, e.g., chronic kidney disease, stroke, Parkinson’s, Chronic Obstructive Pulmonary Disease (COPD), and congestive heart failure, (d) any study design, (e) studies looking at the effect of a different intervention (exercise/hormonal/nutrition) on CAF level, and (f) studies analyzing the association between CAF and sarcopenia and/or its outcome measures like muscle mass, muscle strength, and physical performance. The studies were excluded if they failed to meet the inclusion criteria and/or: (a) full text was not available, (b) language was other than English, (c) provided no extractable data, (d) studies not included CAF biomarker, and (e) animal studies.
Selection process
Two reviewers, PK and GN, independently searched the literature. The identified studies were imported to Rayyan (Ref. # 488,637) software. After resolving the duplicates, two reviewers (PK and GN) conducted title and abstract screening separately. If the study was deemed suitable, it progressed to retrieving the full text. Any conflict regarding the study selection was resolved by discussion with two reviewers, KN and SU. PK did a full-text reading, and data extraction was carried out from the relevant studies.
Data charting process and data items
Four included studies were randomly selected and shared with all the reviewers, and the data charting sheet was prepared individually and piloted. Further, the data charting sheet was finalized after a consensus discussion with all the authors. The two authors extracted the data in duplicate for each retrieved article. Any disagreement between the two reviewers on the extracted data from the documents was resolved by simultaneously analyzing the data. For relationship studies between CAF and primary sarcopenia, Inclusion criteria, Exclusion criteria, Sarcopenia diagnostic criteria, Muscle mass, Muscle strength, Physical performance, Statistical analysis, and Association between primary sarcopenia and CAF were extracted; for relationship studies between CAF and secondary sarcopenia, Inclusion, Exclusion, Co-morbidity(ies), Sarcopenia diagnosis criteria, Muscle mass, Muscle strength, Physical performance, Statistical analysis, and Association between secondary sarcopenia and CAF were extracted; for studies which have evaluated the effect of interventions (exercise/nutrition/hormone), Randomization, Intervention, Exercise parameters, Control group, Supervision & Motivation strategies, Outcome measures, Blood collection, CAF Values, Results of relationship and Findings were extracted.
Critical appraisal of individual sources of evidence
Critical appraisal of the individual studies finding out the relationship of CAF and sarcopenia and/or sarcopenia with associated co-morbidities was done by two authors, PK and GN, using JBI critical appraisal checklist for cross-sectional studies [35]. The Consensus on Exercise Reporting Template (CERT) [36] was used to evaluate the completeness of exercise reporting the studies included in this review. The Physiotherapy evidence database scale (PEDro Scale) was used to assess the methodological quality (risk of bias) of the intervention studies included in the review [37].
Results
Search results
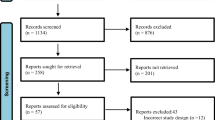
A total of 5,158 studies were identified through database searches. After removing duplicate studies (n = 2337), the titles and abstracts of 2821 studies were screened. A review of the titles and abstracts yielded 50 relevant studies for full-text screening. Finally, 16 studies met all inclusion criteria and were included in this review. A Preferred Reporting Items for Systematic Review and Meta-analysis 2020 (PRISMA 2020) flowchart of the literature search depicts the screening process (Fig. 1) [38].
The results have been taxonomized in three sections: the association between CAF and primary sarcopenia, the association between CAF and secondary sarcopenia, and the effect of interventions (exercise/nutrition/hormonal) on the levels of the CAF.
Association between levels of CAF and primary sarcopenia
A total of four (n = 4) studies have been found assessing the association between the levels of CAF and primary sarcopenia [14, 25, 29, 39]. These studies adopted different designs—one each (n = 1) was cross-sectional [29], multi-center non-randomized trial [25], randomized trial [39], and cohort designs [14]. The criteria used to find out the sarcopenia among the participants varied in the studies, with one (n = 1) study using EWGSOP criteria [25]; two (n = 2) studies utilized the criteria forwarded by EWGSOP2 [14, 29]; while two (n = 2) studies have not reported the criteria used [39]. Looking at the triad of muscle mass, muscle strength, and physical performance to confirm sarcopenia, two (n = 2) studies have measured the physical performance [29, 39], three studies (n = 3) have assessed the grip strength [14, 25, 29], and three (n = 3) studies have assessed the muscle mass [14, 29, 39].
The procedure followed for blood sample collection has been described in the studies. Among the four (n = 4) studies, two (n = 2) have clearly stated and mentioned the median cubital vein as a source to collect the sample [14, 29]. The 5 ml amount of blood drawn has been reported by one (n = 1) study [29]. Two (n = 2) studies mentioned − 80 degree Celsius as the preferred temperature to store the collected blood until future analysis [14, 39]. Three studies (n = 3) have used serum to measure the levels of CAF concentrations [25, 29, 39], and only one (n = 1) used plasma [14]. The studies have used different procedures to analyze the CAF concentration levels. Two (n = 2) studies have utilized the western blot [25, 39], and two (n = 2) have used the commercially available (Enzyme-Linked Immunosorbent Assay) ELISA kit (Abcam #ab216945) [14, 29].
Three studies (n = 3) have reported a significant association between CAF and sarcopenia (p value ranging from < 0.01 to < 0.00001) [14, 25, 29]. Two studies (n = 2) have reported the association between appendicular lean mass (ALM) and the level of CAF [14, 39]. These studies have reported a strong inverse relationship (male: r = − 0.524; female: r = − 0.219) and significant association (p = 0.003) between ALM and = CAF levels, respectively. The association between the hang grip strength (HGS) and CAF has been reported by two (n = 2) studies [14, 39], one stating low HGS displayed significantly higher levels of CAF (p = 0.027) while the other found no association (Table 1). Among the two (n = 2) studies that assessed physical performance, one study (n = 1) reported gait speed not associated with CAF concentration [39]. However, the study by Marcolin et al. 2021 showed that active older dancers (AOD) having low CAF concentration have better performance in TUG (p < 0.001), 10-MWT (p < 0.001) compared to the sedentary older [29].
Association between levels of CAF and secondary sarcopenia
A total of six (n = 6) studies have reported the relationship between levels of CAF and sarcopenia in individuals with co-morbidities [17, 30, 40,41,42,43]. Among the included six studies, two studies (n = 2) are cohort studies [42, 43], and four (n = 4) studies are cross-sectional in design [17, 30, 40, 41]. The co-morbidities among the individuals were stroke (n = 1) [42], COPD (n = 1) [41], heart failure (n = 1) [43], Parkinson’s (n = 1) [40], and hip fracture (n = 1) [17], with one study (n = 1) included individuals with multi-morbidity [30].
The sarcopenia criteria used among the studies vary, with two studies (n = 2) have used EWGSOP as criteria [17, 30], two (n = 2) have used EWGSOP2 [40, 41], one (n = 1) used appendicular skeletal muscle mass [43], and one (n = 1) study has not reported the criteria used to assess the sarcopenia [42]. The muscle mass has been evaluated in all the studies, with four studies (n = 4) have used a BIA [17, 40,41,42], one study (n = 1) used DEXA [43], and one study (n = 1) used mid-arm circumference using inch tape to assess the muscle mass [30]. The HGS has been evaluated in five studies (n = 5), with three studies (n = 3) used a hand-held hydraulic dynamometer [17, 30, 42], and two studies (n = 2) used a digital hand-held dynamometer [40, 41]. Only one study (n = 1) has not assessed muscle strength [43]. The third component, physical performance, of the sarcopenia assessment triad, has been assessed in four studies (n = 4), with two studies (n = 2) used short physical performance battery (SPPB) [40, 41] and one each (n = 1) assessing physical performance using a six-minute walk test (6-MWT) [43] and 4-m walk test [30], respectively.
The details of the method used to draw the blood sample have been reported by four studies (n = 4) [17, 30, 42, 43]. In two studies (n = 2), method used to draw the blood sample has not been reported in detail [40, 41]. Three studies (n = 3) mentioned clearly that the participants were asked to remain overnight fast before their blood samples were taken [17, 30, 42]. Among the included studies, four (n = 4) used the serum [17, 30, 42, 43], while two (n = 2) used the plasma [40, 41]. All six studies (n = 6) have mentioned that the CAF levels were analyzed using commercially available ELISA kits (NTCAF, ELISA, Neurotune, Schlieren-Zurich, Switzerland).
The level of CAF concentration was significantly higher among secondary sarcopenics (p value range from < 0.05 to 0.0001). Two studies (n = 2) reported a significant association between CAF levels and muscle wasting [30, 43]. Four studies (n = 4) reported a significant association between the level of CAF and reduced physical performance, with one study (n = 1) reporting that individuals with less than 400-m distance covered in 6-MWT have a higher concentration of CAF (p = 0.0001) [43], two studies (n = 2) reported the speed < 0.8 m/sec in 4-m walk test have a higher CAF concentration (p value range < 0.003 – < 0.001) [30, 43], and two studies (n = 2) reported higher concentration in an individual with less score in SPPB (9–10 score, p < 0.01; 6–8 score, p < 0.01) [40, 41]. A significant negative association ( p < 0.001) has been reported between CAF and HGS in two (n = 2) studies [30, 40]. Two (n = 2) studies have reported a reduction in CAF levels following rehabilitation which were associated with improvement in hand grip strength (p value range < 0.05 – 0.01) [41, 42] (Table 2).
Effect of interventions (exercise/nutrition/hormonal) on the levels of the CAF
A total of six studies (n = 6) [39, 44,45,46,47,48] have looked at the effect of the intervention on the level of CAF concentration. All six studies were randomized controlled trials with one (n = 1) randomized controlled single-blinded [39], one (n = 1) randomized waitlist control trial [44], one (n = 1) randomized placebo-controlled double-blind, parallel-group [48], one (n = 1) clinical trial [45], one (n = 1) multi-site randomized clinical trial [47], and one (n = 1) randomized controlled trial [46].
Different interventions were used in the included studies to determine the effect on the level of CAF. Among the six (n = 6) studies, only one (n = 1) study used testosterone therapy [48], rest five (n = 5) studies used exercise-based intervention [39, 44,45,46,47]. However, the mode of exercise varied between the studies, with one (n = 1) used resistance training [44], one (n = 1) used functional training (FT) with and without Blood Flow Restriction (BFR) [45], one (n = 1) used dual-task (DT) training with/without BFR [46], one (n = 1) used physical activity [47], and one (n = 1) used power training [39].
Resistance exercise and functional training (FT), which included the resistance exercise components, were administered in two (n = 2) studies [44, 45]. The frequency reported was 2–3 days/week, the intensity of 10–16 on a Borg scale/5–6 on a 10-point OMNI-Rating of perceived exertion scale, the total session time ranges from 60 to 90 min, sets and repetition ranges from 3 sets of 6–15 repetitions, and total duration of the study, 6 weeks. The physical activity program (walking, strengthening, and flexibility exercises) was used in one study (n = 1) with moderate intensity of 13–15 on a 20-point Borg scale with a total duration of 12 months [47]. The dual task (DT) training with and without BFR was administered in one study, with 3 days/week of frequency, respectively [46]. The DT was performed on the intensity of 45% heart rate reserve (HRR) for 8 weeks. In one of the studies, power training was given and compared to strength training among the participants who were supplemented with vitamin D [39]. The frequency was 2/week for 12 weeks, with repetition varied from 15 reps in the first week to 6 reps in the final week, while the training intensity was maintained as per the Borg rating of perceived exertion (RPE) ranging from 10 to 16 on a scale of 6–20 Borg RPE scale. Among the included six studies, only one (n = 1) used testosterone therapy in which testosterone doses were increased/decreased by 5 g depending on the concentration of the total testosterone till the end of the six months of treatment [48].
The body composition as an outcome measure was assessed in three studies (n = 3) [39, 44, 46], using DEXA in two (n = 2) studies [39, 46] and ultrasound imaging in one (n = 1) of the studies [44]. Muscle strength has been assessed in four (n = 4) of the studies as leg and chest press, and two (n = 2) used 10-repetition maximum (10-RM) as a measure of strength assessment [44, 45]. Three of the studies (n = 3) have assessed physical performance, with one study (n = 1) using a 400-m walk test and SPPB [47], a 12-step stair-climb test in one (n = 1) study [48], and 6-MWT in one (n = 1) study [46]. Two of the studies (n = 2) have assessed mobility as part of physical performance using the timed-up and go test (TUG) [45, 46], while only one (n = 1) study has not assessed the physical performance [44].
Participants were asked to undertake overnight fast in four (n = 4) studies [44,45,46, 48]. The median vein has been reported in three (n = 3) studies as a source from where blood was drawn [44,45,46]. The drawn blood sample after processing into serum in five studies (n = 5) [39, 44, 45, 47, 48] and plasma in only one study (n = 1) [46] was stored at − 80 degree Celsius in one of the studies [39], while at − 20 degree Celsius in two (n = 2) studies [45, 46]. Only one study (n = 1) used western blotting to analyze the levels of CAF [39], while three (n = 3) studies [44, 47, 48] and two (n = 2) studies [45, 46] utilized commercially available ELISA kit Neurotune and Zell bio ZB, respectively.
The results varied among the included studies, which looked at how the interventions affected the changes in CAF concentrations. In two studies (n = 2) which have delivered 6 weeks of resistance exercise, one in the form of functional training with blood flow restriction [45] and the other only resistance training [44], a significant decrease in serum CAF levels was observed in the resistance exercise group compared to the control (F (2,26) = 7.12, p = 0.003, ηP2 = 0.35) [45], while others showing increase in CAF by 10.4% (3.59 ± 1.45 to 4.00 ± 1.20 pg/mL) [44] in older adults. One (n = 1) study compared the strength and power training showed a significant reduction in CAF concentration in the group that underwent power training for 12 weeks [39]. Nutrition as a treatment has not been provided as a single intervention; instead, it was administered to the participants before the strength and power training. Physical activity intervention, including walking, flexibility, and balance training for 12 months, did not significantly reduce the serum CAF levels in older adults compared to health education [47]. Hormonal therapy has been utilized in only one (n = 1) study [48]; despite improvements in muscle strength and stair climbing power, no significant difference is noticed in the change in serum CAF concentration between testosterone and placebo group (effect size = -50.3 pM; 95% CI = -162.1 to 61.5 pm; p = 0.374).
Among the six (n = 6) included studies, five (n = 5) studies also reported the relationship between the CAF concentration and different outcome measures in addition to the effect. One (n = 1) study reported an inverse relationship between CAF and appendicular lean mass (ALM) (r = − 0.524 male; r = − 0.219 female). There was a positive correlation between the CAF levels and cross-sectional area (CSA) of vastus lateralis (r = 0.542) in one (n = 1) of the study. Regarding physical performance, the CAF level was significantly correlated with the gait speed (r = − 0.151) in one (n = 1) study. At the same time, there was no significant correlation between CAF and SPPB (r = − 0.086) in the same study. There were contrasting results in two (n = 2) studies, with one (n = 1) study found no significant relationship between CAF and muscle strength, while the one (n = 1) other study showed significant inverse correlations between the levels of CAF and knee extension strength (r = − 0.45), chest press (r = − 0.53). Only one (n = 1) study reported the correlation between CAF and muscle quality, which was inverse and statistically significant in the Dual-task Blood Flow Restriction (DTBFR) group (r = − 0.81).
The two studies (n = 2) have reported the CAF values pre- and post-intervention. The pre–post change in the CAF values was not similar among the individuals with lower and higher baseline CAF values. In one (n = 1) study, at time point 1 (T1), the low CAF value was 3.14 ± 0.84, which after 12 weeks of training was found to be 3.40 ± 0.84, whereas among individuals with high CAF at time point 1 (T1) 5.69 ± 0.88, the level changed to 4.78 ± 1.77 after 12 weeks of training. The effect of resistance training was compared to the control group in one (n = 1) of the study; the pre-intervention CAF level in the exercise group was 3.59 ± 1.45, and in the control group, it was 3.77 ± 1.06; post-intervention CAF level in the exercise group was 4.00 ± 1.20 and in the control group 3.79 ± 1.02 (Table 3).
Discussion
This review aimed to scope the scientific evidence about the relationship between the level of CAF and primary sarcopenia, CAF and secondary sarcopenia, and the effect of various interventions (exercise/nutrition/hormonal) on the change in the level of CAF. Many studies have been published fulfilling the eligibility criteria for the review, with an apparent surge in research about CAF concentration and its subsequent role in causing sarcopenia. The results support the hypothesis made for the higher level of CAF in primary and secondary sarcopenia. However, the results for the hypothesis that the interventions (exercise/nutrition/hormonal) will reduce the level of CAF among older adults are mixed and inconclusive.
CAF levels among individuals with primary sarcopenia
There is variability between the included studies looking at the relationship of CAF and sarcopenia in healthy individuals, given that the studies did not use uniform diagnostic criteria for sarcopenia. Studies have reported that the individuals in the sarcopenic group have a higher concentration of circulating CAF than the non-sarcopenic group. It has been reported in the cross-sectional cohort study that CAF has a sensitivity of 61.3% and specificity of 51.7% for sarcopenia [14]. However, among the component triad of sarcopenia assessment, reduced muscle mass is found to be significantly associated with higher CAF concentration followed by reduced grip strength with more consistent findings in males. The review findings highlight the subgroup of patients with sarcopenia having higher CAF concentration with a primary mechanism of muscle wasting. Observations from the study show that CAF levels are associated with appendicular lean mass and are significantly elevated in participants with muscle wasting [49]. The sensitivity and the specificity of CAF for the low appendicular lean mass (43.3% and 70%) and low muscle strength (56.7% and 52.1%) have been reported [14]. This review also highlights the influence of gender on CAF levels, with males, irrespective of the presence of sarcopenia or not, having higher CAF concentrations compared to females. While differences in muscle architecture and adaptation mechanisms are well-established between sexes [50, 51], knowledge surrounding such disparities in biomarkers remains elusive. The person’s age also affects the level of the CAF, with higher age associated with a higher tertile of CAF [52].
Regarding the quality of the studies, four (n = 4) studies have clearly defined the inclusion criteria of the sample, described the study subject and setting in detail, measured the exposure in a valid and reliable way, used the standard criteria for measurement of the condition, measured the outcome in a valid and reliable way, and have used appropriate statistical analysis. Three studies (n = 3) have identified confounding factors and stated the strategy to deal with the same, while only one (n = 1) study has not reported the confounding factors and the strategy used to deal with them. The methodological quality of studies included in this section is of a good standard and shows the rigor of the researchers presenting reliable results (Supplementary material 2).
CAF levels among individuals with secondary sarcopenia
There exists heterogeneity among the studies finding the association between the CAF concentration and secondary sarcopenia as the diagnostic criteria for sarcopenia and the population studies differed; hence, pooling the result was difficult. The population was heterogeneous with various co-morbidities, hip fracture, stroke, COPD, CHF, Parkinson’s disease, and multi-morbidities. The studies included in this review have reported that the CAF concentration levels were higher in comorbid sarcopenic individuals. Studies among individuals with CHF and multi-morbidity have reported that muscle wasting is associated with significantly elevated CAF concentration. In line with the findings of this review, studies have reported the loss of muscle mass within two months of hip fracture repair [53]. Also, in a mouse model of neuro-trypsin over-expression, the full phenotype of muscle wasting has been reported [54]. In this context, it should be noted that agrin-dependent sarcopenia can be distinguished from aging-associated muscle wasting [55]. Studies conducted on individuals with stroke, COPD, PD, and multi-morbidity in this review reported that CAF showed the strongest correlation with HGS, also the CAF concentration was significantly higher in individuals with low hand grip strength. Similar findings were reported by a study done in older women with hip fractures, that a decline in muscle strength predicted poor mobility recovery [56]. The physical performance has been measured in the studies included in this review using different tests like 6-MWT, gait speed test, and SPPB. As a third important component in assessing sarcopenia, studies included in this review reported physical performance significantly associated with higher concentrations of CAF. Similar findings have been reported in a study on mobility-limited older adults with slow-walking speed [27, 28, 47]. This review has identified a gender difference in the CAF concentration, as evident from the higher level of CAF concentration in males with comorbidity compared to females. Though reduced ALM, HGS, and physical performance are associated with higher CAF levels, the strongest association is found to be of reduced HGS, followed by physical performance and ALM in secondary sarcopenics. Musculoskeletal disease like disuse atrophy is significantly associated with higher levels of CAF [57]. The increased levels of CAF have also been reported in studies conducted on diabetic [52] and diabetic nephropathy patients [58]. A recent study examining the potential causes of cachexia in colorectal and pancreatic cancer patients discovered that pre-cachectic and cachectic patients had higher CAF concentrations than age-matched controls [59].
Regarding the quality of the studies, four (n = 4) studies have clearly defined the inclusion criteria of the sample, described the study subject and setting in detail, measured the exposure in a valid and reliable way, used the standard criteria for measurement of the condition, measured the outcome in a valid and reliable way, and have used appropriate statistical analysis. The confounding factors have been identified in two (n = 2) studies; in three (n = 3) studies, it is unclear, while one (n = 1) study did not identify confounding factors. However, the strategies to deal with the confounding factors have been reported in the statistical analysis in five (n = 5) of the studies, while in only one (n = 1) study, strategies have not been reported (Supplementary material 3).
Changes in levels of CAF in response to interventions
In this review, studies have been included which have used the interventions to evaluate whether there could be a reversal of the degradation of the NMJ by looking at the change in the corresponding CAF levels. It was interesting to note that all the studies have provided the intervention details, allowing replication in future research. However, variability in the exercise prescription in terms of the frequency ranging from 2 to 3 times per week, intensity ranging from 10 to 16 on the Borg scale/5–6 on a 10-point OMNI-rating of perceived exertion scale, duration per session ranging from 60 to 90 min, and also the overall duration of the intervention ranging from 6 weeks to 12 months has been noticed. Currently, there is a lack of consensus among practitioners about the dosage ranging from 3 to 4 sets of 6–15 repetitions, as well as the mode of intervention (resistance training/power training/aerobic training) that is required to get the best results in terms of reduction in the level of CAF and improvement in the triad of sarcopenia outcomes of muscle mass, muscle strength, and physical performance. Two of the studies in this review have reported the non-traditional exercise, a BFR, which is a non-conventional mode of training [45, 46].
Interestingly, the findings of the included studies varied, with few reporting a decrease in the level of CAF concentration [39, 45, 46], with few reporting no change [47, 48], while surprisingly, one study reported an increase in the CAF concentration [44] following the intervention. The interventions like power training (with prior supplementation with vitamin D), FTBFR, and DTBFR decreased the CAF concentration. Similar results have been found in the studies showing that the active older adults involved in dancing present with lower CAF levels than their physically inactive peers [60]. Also, among two elderly groups undergoing training for 6 months, the group practicing dance presented decreased CAF levels, whereas CAF levels were unchanged in the group indulging in a general fitness program [29]. Reduction in CAF levels has been reported among postmenopausal women following 10 weeks of resistance training [61]. Conversely, it was noted that a 6-week resistance training intervention significantly increased the CAF concentration [44]. A similar increase in CAF levels following 10 weeks of resistance exercise has been reported in perimenopausal women [61]. This would be attributed to the increased exercise-induced stress and micro-trauma at the NMJ, which might be transient and go off with time. In contrast, the one-year physical activity program did not reduce the serum CAF concentration despite improving physical function [47]. Similarly, following the hormonal testosterone therapy, there was no change in the CAF concentration compared to the placebo despite improving muscle strength and stair climbing power [48]. Similar findings have been reported in obese older adults trained for 6 months with either only aerobic, only resistance, or a combination of both [62]. There was variation in levels of CAF following the three interventions indicating that exercise training was able to preserve but not improve NMJ health [62]. The role of doctors/health care professionals trained in geriatrics cannot be underestimated in promoting physical activity in older adults [63,64,65].
The findings showed that power training is more beneficial in reducing CAF concentration than strength. The possible reason could be that power training involves high neuromuscular activity for a short time which will have high reinnervating potential. These findings are from a study that showed that a high amount of neuromuscular activity protects against degeneration at NMJ resulting in sarcopenia [66]. The study that utilized FTBFR with various resistance exercises as part of the FT and the study that utilized only resistance exercises have shown contradictory results. As to improve the loss of strength and muscle mass, a combination of the mechanical and metabolic load is essential, which can be applied effectively using BFR during the training session [67]. These differences in findings among interventions may suggest that CAF responses to exercise may depend on the intensity/duration/volume of the training session [68].
We used CERT to evaluate and assess exercise interventions described in the included studies [36]. Out of the six (n = 6) studies included in this section, five (n = 5) [39, 44,45,46,47] studies delivered exercise as the intervention. The components like a detailed description of how the exercise program progressed, a detailed description of each exercise to enable replication, and a detailed description of exercise intervention have been mentioned in all five (n = 5) studies, while the way the exercise interventions were tailored is not reported in many of the studies (Supplementary material 4).
Three studies (n = 3) scored fair on the PEDro scale [39, 44, 47], two scored (n = 2) good [45, 46], and only one (n = 1) scored optimal [48]. Random allocation was done in all the studies (n = 6), while concealed allocation was done in only three (n = 3) studies [45, 46, 48] (Supplementary material 5).
Strengths and limitations
First, a comprehensive systematic search strategy was performed in six databases, to identify a broad range of studies related to the topic. Second, no time restriction in the search strategy strengthens the search for literature published on the topic. Third, we followed acknowledged method recommendations for scoping reviews and did duplicate study selection and data extraction to raise validity. Also, to the best of our knowledge, this is the first study scoping the literature regarding the association of CAF level and components of sarcopenia (muscle mass/muscle strength/physical performance) and the various interventions used to influence the level of CAF.
This study has a few limitations as well. First and foremost, this review has considered only the published articles in electronic databases. Second, considered only full-text articles as abstracts, and proceedings were excluded. Third, we did not use search strategies with terms other than English, and we may have missed eligible studies in the other languages we intended to include.
Future recommendations
This review has scoped the scientific literature about the intervention component and its effect on CAF concentration. A systematic review with a meta-analysis could be conducted to quantify which exercise program is most beneficial. Also, studies published in non-electronic databases and on gray literature could be carried out as an update to this review. The direction of future studies should be aimed at: (a) determining the most appropriate frequency, intensity, time, and mode of exercise or rehabilitation intervention to reduce CAF in older adults and (b) conducting a randomized controlled trial to look at the effect of a multicomponent intervention on the levels of CAF among sarcopenic older adults residing in long-term care settings and community-dwelling.
Significance of this review
This review has scoped the scientific evidence and taxonomically summarized the results. Even though the causal NMJ mechanism leading to sarcopenia is beyond the scope of this review, a consistent association has been noticed between CAF and the sarcopenia triad. Though reduced ALM, HGS, and physical performance are associated with higher CAF levels, the strongest association is found between CAF and reduced muscle mass, followed by HGS and physical performance among primary sarcopenics. In contrast, the strongest association is found to be for reduced HGS, followed by physical performance and reduced ALM in secondary sarcopenics. Also, evidence points to power and resistance training compared to aerobic exercise in improving CAF levels among primary and secondary sarcopenics. The gender difference should be considered in designing the exercise intervention as the cause of muscle loss with aging in men may be associated with degeneration of the NMJ as measured by CAF. In women, sarcopenia may be more multifaceted. The findings may help physicians and researchers to target the specific component in rehabilitation to improve the health of older adults. In addition, the review has summarized the effect and components of exercise/nutritional/hormonal intervention to target CAF levels. The intervention studies included in the review have been evaluated using the CERT checklist, which would help the researcher and practitioners decide the mode and the parameters to be selected while prescribing the intervention (exercise/nutrition/hormone).
Conclusions
In this review, we summarize the scientific information about the association of CAF with primary and secondary sarcopenia and also the effects of various interventions on CAF concentration. The CAF seems to be a good biomarker to distinguish between sarcopenic and non-sarcopenic older adults. Reduced ALM and HGS showed the strongest association with primary and secondary sarcopenia, respectively. The selection of training mode/parameters/exercises is critical in reducing the CAF levels and, eventually, managing sarcopenia.
Data availability
The datasets generated during and/ or analysed during the current study are available from the corresponding author on reasonable request.
References
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127:990S-991S
Chen L-K, Woo J, Assantachai P et al (2020) Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 21:300–307
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31
Fielding RA, Vellas B, Evans WJ et al (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12:249–256
Cruz-Jentoft AJ, Landi F, Schneider SM et al (2014) Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 43:748–759
Papadopoulou SK, Tsintavis P, Potsaki G et al (2020) Differences in the prevalence of sarcopenia in community-dwelling, nursing home, and hospitalized individuals. A systematic review and meta-analysis. J Nutr Health Aging 24:83–90. https://doi.org/10.1007/s12603-019-1267-x
Roubenoff R (2000) Sarcopenia and its implications for the elderly. Eur J Clin Nutr 54:S40–S47
Tournadre A, Vial G, Capel F et al (2019) Sarcopenia. Joint Bone Spine 86:309–314
Chang K-V, Hsu T-H, Wu W-T et al (2016) Association between sarcopenia and cognitive impairment: a systematic review and meta-analysis. J Am Med Dir Assoc 17:1164-e7
Bone AE, Hepgul N, Kon S et al (2017) Sarcopenia and frailty in chronic respiratory disease: lessons from gerontology. Chron Respir Dis 14:85–99
Schaap LA, Van Schoor NM, Lips P et al (2018) Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: the longitudinal aging study Amsterdam. J Gerontol Ser A 73:1199–1204
Beaudart C, Locquet M, Reginster J-Y et al (2018) Quality of life in sarcopenia measured with the SarQoL®: impact of the use of different diagnosis definitions. Aging Clin Exp Res 30:307–313
Cesari M, Fielding RA, Pahor M et al (2012) Biomarkers of sarcopenia in clinical trials—recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle 3:181–190
Pratt J, De Vito G, Narici M et al (2021) Plasma C-terminal agrin fragment as an early biomarker for sarcopenia: results from the GenoFit study. J Gerontol Ser A 76:2090–2096
Janssen I, Heymsfield SB, Baumgartner RN et al (2000) Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol 89:465–471
Prado CM, Heymsfield SB (2014) Lean tissue imaging: a new era for nutritional assessment and intervention. J Parenter Enter Nutr 38:940–953
Marzetti E, Calvani R, Lorenzi M et al (2014) Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older hip fractured patients. Exp Gerontol 60:79–82
Morley JE (2017) Hormones and sarcopenia. Curr Pharm Des 23:4484–4492
Pratt J, Boreham C, Ennis S et al (2019) Genetic associations with aging muscle: a systematic review. Cells 9:12
Walston JD (2012) Sarcopenia in older adults. Curr Opin Rheumatol 24:623
Hepple RT, Rice CL (2016) Innervation and neuromuscular control in ageing skeletal muscle. J Physiol 594:1965–1978
Pratt J, De Vito G, Narici M et al (2021) Neuromuscular junction aging: a role for biomarkers and exercise. J Gerontol Ser A 76:576–585
Tintignac LA, Brenner H-R, Rüegg MA (2015) Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev 95:809–852
Stephan A, Mateos JM, Kozlov SV et al (2008) Neurotrypsin cleaves agrin locally at the synapse. FASEB J 22:1861–1873
Hettwer S, Dahinden P, Kucsera S et al (2013) Elevated levels of a C-terminal agrin fragment identifies a new subset of sarcopenia patients. Exp Gerontol 48:69–75
Stout JR, Fragala MS, Hoffman JR et al (2015) C-terminal agrin fragment is inversely related to neuromuscular fatigue in older men. Muscle Nerve 51:132–133
Battaglia G, Bellafiore M, Bianco A et al (2010) Effects of a dynamic balance training protocol on podalic support in older women. Pilot Study Aging Clin Exp Res 22:406–411
Bianco A, Patti A, Bellafiore M et al (2014) Group fitness activities for the elderly: an innovative approach to reduce falls and injuries. Aging Clin Exp Res 26:147–152
Marcolin G, Franchi MV, Monti E et al (2021) Active older dancers have lower C-terminal Agrin fragment concentration, better balance and gait performance than sedentary peers. Exp Gerontol 153:111469
Landi F, Calvani R, Lorenzi M et al (2016) Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older multimorbid community-dwellers: results from the ilSIRENTE study. Exp Gerontol 79:31–36
Kalinkovich A, Livshits G (2015) Sarcopenia-The search for emerging biomarkers. Ageing Res Rev 22:58–71
Munn Z, Peters MD, Stern C et al (2018) Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 18:1–7
Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil H (2020) Chapter 11: Scoping reviews (2020 version). In: Aromataris E, Munn Z (eds) JBI manual for evidence synthesis. JBI. https://synthesismanual.jbi.global. https://doi.org/10.46658/JBIMES-20-12
Tricco AC, Lillie E, Zarin W et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169:467–473
Joanna Briggs Institute (2017) JBI critical appraisal checklist for analytical cross sectional studies
Slade SC, Dionne CE, Underwood M et al (2016) Consensus on exercise reporting template (CERT): explanation and elaboration statement. Br J Sports Med 50:1428–1437
Verhagen AP, De Vet HC, De Bie RA et al (1998) The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 51:1235–1241
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10:1–11
Drey M, Sieber C, Bauer J et al (2013) C-terminal agrin fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp Gerontol 48:76–80
Karim A, Iqbal MS, Muhammad T et al (2022) Evaluation of sarcopenia using biomarkers of the neuromuscular junction in Parkinson’s disease. J Mol Neurosci 72:820–829
Karim A, Muhammad T, Qaisar R (2021) Prediction of sarcopenia using multiple biomarkers of neuromuscular junction degeneration in chronic obstructive pulmonary disease. J Pers Med 11:919
Scherbakov N, Knops M, Ebner N et al (2016) Evaluation of C-terminal agrin fragment as a marker of muscle wasting in patients after acute stroke during early rehabilitation. J Cachexia Sarcopenia Muscle 7:60–67
Steinbeck L, Ebner N, Valentova M et al (2015) Detection of muscle wasting in patients with chronic heart failure using C-terminal agrin fragment: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur J Heart Fail 17:1283–1293
Fragala MS, Jajtner AR, Beyer KS et al (2014) Biomarkers of muscle quality: N-terminal propeptide of type III procollagen and C-terminal agrin fragment responses to resistance exercise training in older adults. J Cachexia Sarcopenia Muscle 5:139–148
Bigdeli S, Dehghaniyan MH, Amani-Shalamzari S et al (2020) Functional training with blood occlusion influences muscle quality indices in older adults. Arch Gerontol Geriatr 90:104110
Kargaran A, Abedinpour A, Saadatmehr Z et al (2021) Effects of dual-task training with blood flow restriction on cognitive functions, muscle quality, and circulatory biomarkers in elderly women. Physiol Behav 239:113500
Bondoc I, Cochrane SK, Church TS et al (2015) Effects of a one-year physical activity program on serum C-terminal agrin fragment (CAF) concentrations among mobility-limited older adults. J Nutr Health Aging 19:922–927
Gagliano-Jucá T, Storer T, Pencina K et al (2018) Testosterone does not affect agrin cleavage in mobility-limited older men despite improvement in physical function. Andrology 6:29–36
Steubl D, Hettwer S, Vrijbloed W et al (2013) C-terminal agrin fragment-a new fast biomarker for kidney function in renal transplant recipients. Am J Nephrol 38:501–508
Anderson LJ, Liu H, Garcia JM (2017) Sex differences in muscle wasting. Gender factors affecting metabolic homeostasis, diabetes and obesity. Springer, Cham, pp 153–197
Horwath O, Moberg M, Larsen FJ et al (2021) Influence of sex and fiber type on the satellite cell pool in human skeletal muscle. Scand J Med Sci Sports 31:303–312
Racha P, Selvam S, Bose B et al (2022) Circulating C-terminal agrin fragment: a potential marker for sarcopenia among type 2 diabetes. Indian J Endocrinol Metab 26:334–340
D’Adamo CR, Hawkes WG, Miller RR et al (2014) Short-term changes in body composition after surgical repair of hip fracture. Age Ageing 43:275–280
Bütikofer L, Zurlinden A, Bolliger MF et al (2011) Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J 25:4378–4393
Morley JE (2012) Sarcopenia in the elderly. Fam Pract 29:i44–i48
Visser M, Harris TB, Fox KM et al (2000) Change in muscle mass and muscle strength after a hip fracture: relationship to mobility recovery. J Gerontol A Biol Sci Med Sci 55:M434–M440
Narici MV, Monti E, Franchi M et al (2020) Early biomarkers of muscle atrophy and of neuromuscular alterations during 10-day bed rest. FASEB J 34:1–1
Devetzis V, Daryadel A, Roumeliotis S et al (2015) C-terminal fragment of agrin (CAF): a novel marker for progression of kidney disease in type 2 diabetics. PLoS ONE 10:e0143524
Sartori R, Hagg A, Zampieri S et al (2021) Perturbed BMP signaling and denervation promote muscle wasting in cancer cachexia. Sci Transl Med 13:eaay9592
Franchi MV, Badiali F, Sarto F et al (2023) Neuromuscular aging: a case for the neuroprotective effects of dancing. Gerontology 69:73–81
Willoughby DS, Beretich KN, Chen M et al (2020) Decreased serum levels of C-terminal agrin in postmenopausal women following resistance training. J Aging Phys Act 28:73–80
Colleluori G, Aguirre L, Phadnis U et al (2019) Aerobic plus resistance exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite weight loss. Cell Metab 30:261–273
Costello E, Leone JE, Ellzy M et al (2013) Older adult perceptions of the physicians’ role in promoting physical activity. Disabil Rehabil 35:1191–1198
Schutzer KA, Graves BS (2004) Barriers and motivations to exercise in older adults. Prev Med 39:1056–1061
Battaglia G, Giustino V, Messina G et al (2020) Walking in natural environments as geriatrician’s recommendation for fall prevention: preliminary outcomes from the “passiata day” model. Sustainability 12:2684
Deschenes MR, Roby MA, Eason MK et al (2010) Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol 45:389–393
Ozaki H, Loenneke JP, Buckner SL et al (2016) Muscle growth across a variety of exercise modalities and intensities: contributions of mechanical and metabolic stimuli. Med Hypotheses 88:22–26
Kumar P, Umakanth S, Girish N (2022) A review of the components of exercise prescription for sarcopenic older adults. Eur Geriatr Med 13:1245–1280. https://doi.org/10.1007/s41999-022-00693-7
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
PK and GN conceived the study. PK, KN, SU, and GN planned the study. PK and GN developed the search strategy. PK and GN performed the screening and data extraction. SU and KN resolved doubts about inclusion. PK took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript. GN is the guarantor of the review.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare no competing interests.
Ethical approval
This article does not include human or animal participants nor violated their rights.
Informed consent
Informed consent was not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, P., Nayak, K., Umakanth, S. et al. Effect of targeted intervention on C-terminal agrin fragment and its association with the components of sarcopenia: a scoping review. Aging Clin Exp Res 35, 1161–1186 (2023). https://doi.org/10.1007/s40520-023-02396-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-023-02396-w