Abstract
Background
Sarcopenia and osteoporosis increase the risk of bone fracture in the elderly due to the loss of muscle mass and the decrease in bone mineral density. Myostatin and Bone Morphogenetic Proteins (BMPs) are important molecules involved in muscle mass homeostasis.
Aim
In this study, we investigated the role of BMP4 and myostatin in the pathophysiogenesis of sarcopenia related to osteoporosis and osteoarthritis.
Methods
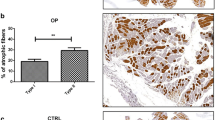
Muscle atrophy, BMP4 and myostatin expression were evaluated in 27 biopsies of osteoarthritic (OA) women and 27 biopsies from osteoporotic (OP) group by immunohistochemical reaction. Muscle stem cell niches were investigated by transmission electron microscopy analysis.
Results
Myostatin and BMP4 expression was evaluated by counting the number of positive fibers on 25 high-power field. We found that OA muscle biopsies showed a significantly higher number of BMP4-positive fibers (37.35 ± 5.63) as compared with muscle of OP patients (9.60 ± 1.57). Unlike BMP4 expression, the number of myostatin-positive fibers in OP patients (33.95 ± 4.10) was significantly higher compared to OA group (13.86 ± 1.68). The ultrastructural analysis of BMP4-positive tissues displayed the presence of a high rate of satellite cells both single or as syncytium giving proof of muscle regeneration capability.
Discussion
Our results indicated that sarcopenia and osteoporosis shared an impairment of metabolic activity. Conversely, the molecular mechanisms of OA seem to inhibit the onset of an age-related sarcopenia.
Conclusion
The characterization of molecular mechanisms underlying the bone–muscle crosstalk could open new therapeutic perspectives in elderly diseases.




Similar content being viewed by others
References
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127:990S–991S
Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50:889–896
Olde Rikkert MG, Rigaud AS, van Hoeyweghen RJ et al (2003) Geriatric syndromes: medical misnomer or progress in geriatrics? Neth J Med 61:83–87
Dupont-Versteegden EE (2005) Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol 40:473–481
Adhihetty PJ, O’Leary MF, Hood DA (2008) Mitochondria in skeletal muscle: adaptable rheostats of apoptotic susceptibility. Exerc Sport Sci Rev 36:116–121
Tarantino U, Piccirilli E, Fantini M et al (2015) Sarcopenia and fragility fractures: molecular and clinical evidence of the bone–muscle interaction. J Bone Joint Surg Am 97:429–437
Marzetti E, Calvani R, Cesari M et al (2013) Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol 45:2288–2301
Tarantino U, Baldi J, Celi M et al (2013) Osteoporosis and sarcopenia: the connections. Aging Clin Exp Res. 25:S93–S95
Oliveira A, Vaz C (2015) The role of sarcopenia in the risk of osteoporotic hip fracture. Clin Rheumatol. doi:10.1007/s10067-015-2943-9
Iolascon G, Giamattei MT, Moretti A et al (2013) Sarcopenia in women with vertebral fragility fractures. Aging Clin Exp Res. 25:S129–S131
Iolascon G, Resmini G, Tarantino U (2013) Mechanobiology of bone. Aging Clin ExpRes. 25:S3–S7
Scimeca M, Bonanno E, Piccirilli E et al (2015) Satellite cells CD44 positive drive muscle regeneration in osteoarthritis patients. Stem Cells Int. doi:10.1155/2015/469459
Laule S, Bornemann A (2001) Ultrastructural findings at the satellite cell-myofiber border in normal and diseased human muscle biopsy specimens. Acta Neuropathol 101:435–439
Dumont NA, Wang YX, Rudnicki MA (2015) Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 142:1572–1581
Sousa-Victor P, García-Prat L, Serrano AL (2015) Muscle stem cell aging: regulation and rejuvenation. Trends Endocrinol Metab 26:287–296
García-Prat L, Sousa-Victor P, Muñoz-Cánoves P (2013) Functional dysregulation of stem cells during aging: a focus on skeletal muscle stem cells. FEBS J 280:4051–4062
Sartori R, Sandri M (2015) Bone and morphogenetic protein signalling and muscle mass. Curr Opin Clin Nutr Metab Care. 18:215–220
Carreira AC, Alves GG, Zambuzzi WF et al (2014) Bone Morphogenetic Proteins: structure, biological function and therapeutic applications. Arch Biochem Biophys 1:64–73
Sartori R, Gregorevic P, Sandri M (2014) TGFβ and BMP signaling in skeletal muscle: potential significance for muscle-related disease. Trends Endocrinol Metab 25:464–471
Sartori R, Schirwis E, Blaauw B et al (2013) BMP signaling controls muscle mass. Nat Genet 45:1309–1318
Piccirilli E, Gasbarra E, Baldi J et al (2014) Can muscular impairment be the “key” for femoral fracture? J Gerontol Geriatr Res. doi:10.4172/2167-7182.1000183
Cerocchi I, Ghera S, Gasbarra E et al (2013) Fragility fractures: the clinical pathway. Aging Clin Exp Res 25:S43–S45
Celi M, Rao C, Scialdoni A et al (2013) Bone mineral density evaluation in osteoporosis: why yes and why not? Aging Clin Exp Res 25:S47–S49
Fox CH, Johnson FB, Whiting J et al (1985) Formaldehyde fixation. J Histochem Cytochem. 33:845–853
Brooke MH, Engel WK (1969) The histographic analysis of human muscle biopsies with regard to fiber types: 2. Diseases of the upper and lower motor neuron. Neurology 19:378–393
Dubowitz V, Sewry CA (2007) Muscle biopsy: a practical approach. Elsevier, New York
Scimeca M, Giannini E, Antonacci C et al (2014) Microcalcifications in breast cancer: an active phenomenon mediated by epithelial cells with mesenchymal characteristics. BMC Cancer 14:286
Scimeca M, Orlandi A, Terrenato I et al (2014) Assessment of metal contaminants in non-small cell lung cancer by EDX microanalysis. Eur J Histochem 58:2403
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain based on metal chelation. J Cell Biol 17:208–212
Heidari B, Hosseini R, Javadian Y et al (2015) Factors affecting bone mineral density in postmenopausal women. Arch Osteoporos. 10:217
Lloyd JT, Alley DE, Hawkes WG et al (2014) Body mass index is positively associated with bone mineral density in US older adults. Arch Osteoporos. 9:175
Biolo G, Cederholm T, Muscaritoli M (2014) Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr. 33:737–748
Huang HY, Zhang WT, Jiang WY (2015) RhoGDIβ inhibits bone morphogenetic protein 4 (BMP4)-induced adipocyte lineage commitment and favors smooth muscle-like cell differentiation. J Biol Chem 290:11119–11129
Capuani S, Piccirilli E, Di Pietro G et al (2013) Microstructural differences between osteoporotic and osteoarthritic femoral cancellous bone: an in vitro magnetic resonance micro-imaging investigation. Aging Clin Exp Res. 25:S51–S54
Acknowledgments
Authors acknowledge A. S. I. (Italian Space Agency) and University of Rome “Tor Vergata” (Spatial Biomedicine Center) for funding this study. It was very important to take advantage of the funds made available by the A. S. I. because it encourages our present and further research within the project titled “Multidisciplinary study of the effects of microgravity on bone cells” call number search DC-DTE-2011-033.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no potential conflicts of interest relating to the manuscript, and there were no extramural sources supporting this research. The study is original and the manuscript has not been published yet and is not being considered for publication elsewhere in any language either integrally or partially except as an abstract.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The approval reference number of the Ethical committee ‘Policlinico Tor Vergata’is #85/12.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Tarantino, U., Scimeca, M., Piccirilli, E. et al. Sarcopenia: a histological and immunohistochemical study on age-related muscle impairment. Aging Clin Exp Res 27 (Suppl 1), 51–60 (2015). https://doi.org/10.1007/s40520-015-0427-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-015-0427-z