Abstract
Purpose of Review
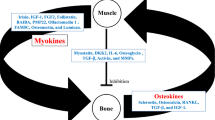
In this review we aim to summarize the latest findings on the network of molecules produced by muscle and bone under physiological and pathological conditions.
Recent Findings
The concomitant onset of osteoporosis and sarcopenia is currently one of the main threats that can increase the risk of falling fractures during aging, generating high health care costs due to hospitalization for bone fracture surgery. With the growing emergence of developing innovative therapies to treat these two age-related conditions that often have common onset, a broader understanding of molecular messengers regulating the communication between muscle and bone tissue became imperative.
Summary
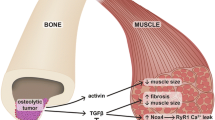
Recently it has been highlighted that two muscle-derived signals, such as the myokines Irisin and L-BAIBA, positively affect bone tissue. In parallel, there are signals derived from bone that affect either positively the skeletal muscle, such as osteocalcin, or negatively, such as RANKL.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–65.
Pedersen BK, Akerström TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–8.
Dunstan D. Diabetes: exercise and T2DM-move muscles more often! Nat Rev Endocrinol. 2011;7:189–90.
Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162–84.
Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175:959–67.
O’Donovan G, Lee IM, Hamer M, Stamatakis E. Association of “weekend warrior” and other leisure time physical activity patterns with risks for all-cause, cardiovascular disease, and Cancer mortality. JAMA Intern Med. 2017;177:335–42.
McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature. 1997;387:83–90.
Hamrick MW, Samaddar T, Pennington C, McCormick J. Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res. 2006;21:477–83.
Hamrick MW, Shi X, Zhang W, Pennington C, Thakore H, Haque M, et al. Loss of myostatin (GDF-8) function increases osteogenic differentiation of bone marrow-derived stem cells but the osteogenic effect is ablated with unloading. Bone. 2007;40:1544–53.
Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA, Pedersen BK. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J Physiol. 2000;528(Pt 1):157–63.
Udagawa N, Takahashi N, Katagiri T, Tamura T, Wada S, Findlay DM, et al. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J Exp Med. 1995;182(5):1461–8.
Hiscock N, Chan MH, Biscci T, Darby IA, Febbraio MA. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J. 2004;18:992–4.
Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M. Prostaglandin E2: from clinical applications to its potential role in bone-muscle crosstalk and myogenic differentiation. Recent Pat Biotechnol. 2012;6:223–9.
Colaianni G, Sanesi L, Storlino G, Brunetti G, Colucci S, Grano M. Irisin and bone: from preclinical studies to the evaluation of its circulating levels in different populations of human subjects. Cells. 2019;8:5–451.
•• Kitase Y, Vallejo JA, Gutheil W, Vemula H, Jähn K, Yi J, et al. β-aminoisobutyric Acid, l-BAIBA, Is a Muscle-Derived Osteocyte Survival Factor. Cell Rep. 2018;22:1531–44 This work identifies a new muscle-derived molecule with anabolic effect on bone mass.
•• Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galán-Díez M, et al. Osteocalcin Signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2016;23(6):1078–92 This is the first study showing that the bone-derived hormone osteocalcin is involved in muscle metabolism and improves muscle functions during exercise.
•• Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S. RANKL Inhibition Improves Muscle Strength and Insulin Sensitivity and Restores Bone Mass. J Clin Invest. 2019;129(8):3214–23 This work provides evidence that RANKL is a negative factor affecting skeletal muscle integrity and function.
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8.
Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A. 2015;112:12157–62.
Allen MR, Bloomfield SA. Hindlimb unloading has a greater effect on cortical compared with cancellous bone in mature female rats. J Appl Physiol. 2003;94:642–50.
Swift JM, Nilsson MI, Hogan HA, Sumner LR, Bloomfield SA. Simulated resistance training during hindlimb unloading abolishes disuse bone loss and maintains muscle strength. J Bone Miner Res. 2010;25:564–74.
•• Colaianni G, Mongelli T, Cuscito C, Pignataro P, Lippo L, Spiro G, et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci Rep. 2017;7:2811 This study demonstrated that intermittent treatment with low dose of recombinant Irisin can prevent the unload-induced onset of osteoporosis and muscular atrophy in mice.
Storlino G, Colaianni G, Sanesi L, Lippo L, Brunetti G, Errede M, et al. Irisin prevents disuse-induced osteocyte apoptosis. J Bone Miner Res. 2019;35:766–75. https://doi.org/10.1002/jbmr.3944.
Vaughan RA, Gannon NP, Mermier CM, Conn CA. Irisin, a unique non-inflammatory myokine in stimulating skeletal muscle metabolism. J Physiol Biochem. 2015 Dec;71(4):679–89.
Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes. 2014 Dec;38(12):1538–44.
Singhal V, Lawson EA, Ackerman KE, Fazeli PK, Clarke H, Lee H, et al. Irisin levels are lower in young amenorrheic athletes compared with eumenorrheic athletes and non-athletes and are associated with bone density and strength estimates. PLoS One. 2014;9:e100218.
Colaianni G, Notarnicola A, Sanesi L, Brunetti G, Lippo L, Celi M, et al. Irisin levels correlate with bone mineral density in soccer players. J Biol Regul Homeost Agents. 2017;31:21–8.
Faienza MF, Brunetti G, Sanesi L, Colaianni G, Celi M, Piacente L, et al. High irisin levels are associated with better glycemic control and bone health in children with type 1 diabetes. Diabetes Res Clin Pract. 2018;141:10–7.
Soininen S, Sidoroff V, Lindi V, Mahonen A, Kröger L, Kröger H, et al. Body fat mass, lean body mass and associated biomarkers as determinants of bone mineral density in children 6–8 years of age—the physical activity and nutrition in children (PANIC) study. Bone. 2018;108:106–14.
Faienza MF, Ventura A, Delvecchio M, Fusillo A, Piacente L, Aceto G, et al. High Sclerostin and Dickkopf-1 (DKK-1) serum levels in children and adolescents with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2017;102:1174–81.
Colaianni G, Faienza MF, Sanesi L, Brunetti G, Pignataro P, Lippo L, et al. Irisin serum levels positively correlate with bone mineral status in a population of healthy children. Pediatr Res. 2019;85:484–8.
Klangjareonchai T, Nimitphong H, Saetung S, Bhirommuang N, Samittarucksa R, Chanprasertyothin S, et al. Circulating sclerostin and Irisin are related and interact with gender to influence adiposity in adults with prediabetes. Int J Endocrinol. 2014;2014:261545.
Palermo A, Strollo R, Maddaloni E, Tuccinardi D, D’Onofrio L, Briganti SI, et al. Irisin is associated with osteoporotic fractures independently of bone mineral density, body composition or daily physical activity. Clin Endocrinol. 2015;82:615–9.
Anastasilakis AD, Polyzos SA, Makras P, Gkiomisi A, Bisbinas I, Katsarou A, et al. Circulating Irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos Int. 2014;25:1633–42.
Park HS, Kim HC, Zhang D, Yeom H, Lim SK. The novel myokine irisin: clinical implications and potential role as a biomarker for sarcopenia in postmenopausal women. Endocrine. 2019;64:341–8.
•• Palermo A, Sanesi L, Colaianni G, Tabacco G, Naciu AM, Cesareo R, et al. A novel interplay between irisin and PTH: From basic studies to clinical evidence in hyperparathyroidism. J Clin Endocrinol Metab. 2019;104(8):3088–96 This work identifies the existence of a negative interplay between PTH and Irisin biology.
Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19:96–108.
Begriche K, Massart J, Abbey-Toby A, Igoudjil A, Lettéron P, Fromenty B. Beta-aminoisobutyric acid prevents diet-induced obesity in mice with partial leptin deficiency. Obesity. 2008;16:2053–67.
Shi CX, Zhao MX, Shu XD, Xiong XQ, Wang JJ, Gao XY, et al. β-aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci Rep. 2016;6:21924.
Jung TW, Hwang HJ, Hong HC, Yoo HJ, Baik SH, Choi KM. BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARδ-dependent pathway in mice. Diabetologia. 2015;58:2096–105.
Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, et al. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2009;21:605–15. https://doi.org/10.1359/jbmr.060107.
Uno M, Nishimura S, Fukuchi K, Kaneta Y, Oda Y, Komori H, et al. Identification of physiologically active substances as novel ligands for MRGPRD. J Biomed Biotechnol. 2012;2012:816159.
Wallace JM, Rajachar RM, Allen MR, Bloomfield SA, Robey PG, Young MF, et al. Exercise-induced changes in the cortical bone of growing mice are bone- and gender-specific. Bone. 2007;40(4):1120–7.
Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111(8):1221–30.
Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, et al. Bench to bedside: elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11(5):401–19.
McCloskey EV, Johansson H, Oden A, Austin M, Siris E, Wang A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res. 2012;27(7):1480–6.
Huang J, Hsu YH, Mo C, Abreu E, Kiel DP, Bonewald LF, et al. METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-κB signaling pathway. J Bone Miner Res. 2014;29(7):1531–40.
Lewiecki EM. Safety and tolerability of denosumab for the treatment of postmenopausal osteoporosis. Drug Healthc Patient Saf. 2011;3:79–91.
Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J. 2001;15(7):1169–80.
Lee D, Goldberg AL. Muscle wasting in fasting requires activation of NF-κB and inhibition of AKT/mechanistic target of rapamycin (mTOR) by the protein acetylase, GCN5. J Biol Chem. 2015;290(51):30269–79.
Dufresne SS, Dumont NA, Bouchard P, Lavergne É, Penninger JM, Frenette J. Osteoprotegerin protects against muscular dystrophy. Am J Pathol. 2015;185(4):920–6.
Dufresne SS, Dumont NA, Boulanger-Piette A, Fajardo VA, Gamu D, Keke-Guena SA, et al. Muscle RANK is a key regulator of Ca2+ storage, SERCA activity, and function of fast-twitch skeletal muscles. Am J Physiol Cell Physiol. 2016;310(8):C663–72.
Dufresne SS, Boulanger-Piette A, Bossé S, Frenette J. Physiological role of receptor activator nuclear factor-kB (RANK) in denervation-induced muscle atrophy and dysfunction. Receptors Clin Investig. 2016;3(2):e13231–6.
Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047.
Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J, Committee of Scientific Advisors of the International Osteoporosis Foundation. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the international Osteoporosis Foundation. Osteoporos Int. 2000;11(Suppl. 6):S2–17.
Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52.
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69.
Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809.
Wei J, Hanna T, Suda N, Karsenty G, Ducy P. Osteocalcin promotes β-cell proliferation during development and adulthood through Gprc6a. Diabetes. 2014;63:1021–31.
Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–19.
Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50:568–75.
Huang L, Yang L, Luo L, Wu P, Yan S. Osteocalcin improves metabolic profiles, body composition and arterial stiffening in an induced diabetic rat model. Exp Clin Endocrinol Diabetes. 2017;125:234–40.
Mera P, Laue K, Wei J, Berger JM, Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab. 2016;5:1042–7.
Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155:228–41.
Khrimian L, Obri A, Ramos-Brossier M, Rousseaud A, Moriceau S, Nicot A, et al. Gpr158 mediates osteocalcin's regulation of cognition. J Exp Med. 2017;214(10):2859–73.
Berger JM, Singh P, Khrimian L, Morgan DA, Chowdhury S, Arteaga-Solis E, et al. Mediation of the Acute Stress Response by the Skeleton. Cell Metab. 2019;30(5):890–902.e8.
Funding
This work was supported by Ministero dell’Istruzione, dell’Università e della Ricerca, PRIN 2015JSWLTN_003 (Progetto di Ricerca d’Interesse Nazionale, Grant 2015), by ERISTO (ESA) and TecnoMed Puglia grants to MG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper. All authors approved the final version of the submitted manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Graziana Colaianni, Silvia Colucci, and Maria Grano report a patent irisin for care and prevention of osteoporosis issued. Giuseppina Storlino and Lorenzo Sanesi declare no conflict of interest.
Human and Animal Rights and Informed Consent
All studies by the authors involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Muscle and Bone
Rights and permissions
About this article
Cite this article
Colaianni, G., Storlino, G., Sanesi, L. et al. Myokines and Osteokines in the Pathogenesis of Muscle and Bone Diseases. Curr Osteoporos Rep 18, 401–407 (2020). https://doi.org/10.1007/s11914-020-00600-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00600-8