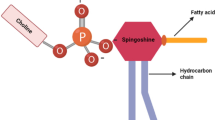
Abstract
The ever-increasing prevalence of metabolic diseases such as dyslipidemia and diabetes in the western world continues to be of great public health concern. Biologically active sphingolipids, such as sphingosine 1-phosphate (S1P) and ceramide, are important regulators of lipid metabolism. S1P not only directly functions as an active intracellular mediator, but also activates multiple signaling pathways via five transmembrane G protein-coupled receptors (GPCRs), S1PR1-5. S1P is exclusively formed by sphingosine kinases (SphKs). Two isoforms of SphKs, SphK1 and SphK2, have been identified. Recent identification of the conjugated bile acid-induced activation of S1PR2 as a key regulator of SphK2 opened new directions for both the sphingolipid and bile acid research fields. The role of SphKs/S1P-mediated signaling pathways in health and various human diseases has been extensively reviewed elsewhere. This review focuses on recent findings related to SphKs/S1P-mediated signaling pathways in regulating hepatic lipid metabolism.

Similar content being viewed by others
Abbreviations
- ABC:
-
ATP-binding cassette
- AKT:
-
Protein kinase B
- ApoE:
-
Apolipoprotein E
- ATF4:
-
Activating transcription factor 4
- EDG1:
-
Endothelial differentiation gene 1
- ER:
-
Endoplasmic reticulum
- ERK:
-
Extracellular signal-regulated kinase
- FAS:
-
Fatty acid synthase
- FXR:
-
Farnesoid x receptor
- GPCRs:
-
G protein-coupled receptors
- HDAC:
-
Histone deacetylases
- HDL:
-
High density lipoprotein
- HPH:
-
Hypoxia-mediated pulmonary hypertension
- LDLR:
-
Low-density lipoprotein receptor
- MAPK:
-
Mitogen-activated protein kinase
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- NPC:
-
Niemann-Pick type C
- ob/ob:
-
Leptin-deficient
- PPARγ:
-
Proliferator-activated receptor gamma
- S1P:
-
Sphingosine 1-phosphate
- SHP:
-
Small heterodimer partner
- Sph:
-
Sphingosine
- SphKs:
-
Sphingosine kinases
- Spns2:
-
Spinster homolog 2
- S1PR:
-
Sphingosine 1-phosphate receptor
- SREBP-1c:
-
Sterol regulating element-binding protein 1
- TCA:
-
Taurocholic acid
- TGR5:
-
G protein-coupled bile acid receptor
References
Chen Y, Liu Y, Sullards MC, Merrill AH Jr. An introduction to sphingolipid metabolism and analysis by new technologies. NeuroMolecular Med. 2010 Dec;12(4):306–19.
Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–50.
Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11(6):403–15.
Ng ML, Wadham C, Sukocheva OA. The role of sphingolipid signalling in diabetes associated pathologies (review). Int J Mol Med. 2017;39(2):243–52.
Al Fadel F, Fayyaz S, Japtok L, Kleuser B. Involvement of sphingosine 1-phosphate in palmitate-induced non-alcoholic fatty liver disease. Cell Physiol Biochem: Int J Exp Cell Physiol, Biochem Pharmacol. 2016;40(6):1637–45.
Nagahashi M, Yuza K, Hirose Y, Nakajima M, Ramanathan R, Hait NC, et al. The roles of bile acids and sphingosine-1-phosphate signaling in the hepatobiliary diseases. J Lipid Res. 2016;57(9):1636–43.
Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9(11):883–97.
Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta. 2003;1632(1–3):16–30.
Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol. 2017;13(2):79–91.
Hanada K, Kumagai K, Tomishige N, Yamaji T. CERT-mediated trafficking of ceramide. Biochim Biophys Acta. 2009 Jul;1791(7):684–91.
Rao RP, Acharya JK. Sphingolipids and membrane biology as determined from genetic models. Prostaglandins & other lipid mediators. 2008;85(1–2):1–16.
Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147(3):545–58.
Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275(26):19513–20.
Leclercq TM, Pitson SM. Cellular signalling by sphingosine kinase and sphingosine 1-phosphate. IUBMB Life. 2006;58(8):467–72.
Melendez AJ, Carlos-Dias E, Gosink M, Allen JM, Takacs L. Human sphingosine kinase: molecular cloning, functional characterization and tissue distribution. Gene. 2000;251(1):19–26.
Maceyka M, Milstien S, Spiegel S. Sphingosine-1-phosphate: the Swiss army knife of sphingolipid signaling. J Lipid Res. 2009;50(Suppl):S272–6.
Pitson SM, Pebay A. Regulation of stem cell pluripotency and neural differentiation by lysophospholipids. Neurosignals. 2009;17(4):242–54.
Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10(7):489–503.
Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278(47):46832–9.
Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325(5945):1254–7.
Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J: Off Publ Fed Am Soc Exp Biol. 2011;25(2):600–12.
Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148(5):988–1000.
Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, et al. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278(41):40330–6.
Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22(1):50–60.
Stoffel W, Assmann G. Metabolism of sphingosine bases. XV. Enzymatic degradation of 4t-sphingenine 1-phosphate (sphingosine 1-phosphate) to 2t-hexadecen-1-al and ethanolamine phosphate. Hoppe Seylers Z Physiol Chem. 1970;351(8):1041–9.
Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103(44):16394–9.
English D, Welch Z, Kovala AT, Harvey K, Volpert OV, Brindley DN, et al. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J: Off Publ Fed Am Soc Exp Biol. 2000;14(14):2255–65.
Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323(5913):524–7.
Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122(4):1416–26.
Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol. 2010;688:141–55.
Okamoto H, Takuwa N, Gonda K, Okazaki H, Chang K, Yatomi Y, et al. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. J Biol Chem. 1998;273(42):27104–10.
Pyne NJ, McNaughton M, Boomkamp S, MacRitchie N, Evangelisti C, Martelli AM, et al. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Adv Biol Regul. 2016;60:151–9.
Karimian G, Buist-Homan M, Schmidt M, Tietge UJ, de Boer JF, Klappe K, et al. Sphingosine kinase-1 inhibition protects primary rat hepatocytes against bile salt-induced apoptosis. Biochim Biophys Acta. 2013;1832(12):1922–9.
Kihara Y, Maceyka M, Spiegel S, Chun J. Lysophospholipid receptor nomenclature review: IUPHAR review 8. Br J Pharmacol. 2014;171(15):3575–94.
Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N. Sphingosine-1-phosphate signaling in physiology and diseases. Biofactors. 2012;38(5):329–37.
Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582(1–3):222–7.
Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106(8):951–61.
Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–60.
MacLennan AJ, Benner SJ, Andringa A, Chaves AH, Rosing JL, Vesey R, et al. The S1P2 sphingosine 1-phosphate receptor is essential for auditory and vestibular function. Hear Res. 2006;220(1–2):38–48.
MacLennan AJ, Carney PR, Zhu WJ, Chaves AH, Garcia J, Grimes JR, et al. An essential role for the H218/AGR16/Edg-5/LP(B2) sphingosine 1-phosphate receptor in neuronal excitability. Eur J Neurosci. 2001;14(2):203–9.
Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, et al. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J Biol Chem. 2001;276(36):33697–704.
Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J, et al. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci U S A. 2005;102(26):9270–5.
Wang W, Graeler MH, Goetzl EJ. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB J: Off Publ Fed Am Soc Exp Biol. 2005;19(12):1731–3.
Terai K, Soga T, Takahashi M, Kamohara M, Ohno K, Yatsugi S, et al. Edg-8 receptors are preferentially expressed in oligodendrocyte lineage cells of the rat CNS. Neuroscience. 2003;116(4):1053–62.
Pyne NJ, Long JS, Lee SC, Loveridge C, Gillies L, Pyne S. New aspects of sphingosine 1-phosphate signaling in mammalian cells. Adv Enzym Regul. 2009;49(1):214–21.
Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, et al. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335(6070):851–5.
Park SJ, Im DS. Sphingosine 1-phosphate receptor modulators and drug discovery. Biomol Ther. 2017;25(1):80–90.
Aoki M, Aoki H, Ramanathan R, Hait NC, Takabe K. Sphingosine-1-phosphate signaling in immune cells and inflammation: roles and therapeutic potential. Mediat Inflamm. 2016;2016:8606878.
Rodriguez-Cuenca S, Pellegrinelli V, Campbell M, Oresic M, Vidal-Puig A. Sphingolipids and glycerophospholipids—the “ying and yang” of lipotoxicity in metabolic diseases. Prog Lipid Res. 2017;66:14–29.
Chen J, Wang W, Qi Y, Kaczorowski D, McCaughan GW, Gamble JR, et al. Deletion of sphingosine kinase 1 ameliorates hepatic steatosis in diet-induced obese mice: role of PPARgamma. Biochim Biophys Acta. 2016;1861(2):138–47.
Nagahashi M, Takabe K, Liu R, Peng K, Wang X, Wang Y, et al. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology. 2015;61(4):1216–26.
Lee SY, Hong IK, Kim BR, Shim SM, Sung Lee J, Lee HY, et al. Activation of sphingosine kinase 2 by endoplasmic reticulum stress ameliorates hepatic steatosis and insulin resistance in mice. Hepatology. 2015;62(1):135–46.
Kowalski GM, Kloehn J, Burch ML, Selathurai A, Hamley S, Bayol SA, et al. Overexpression of sphingosine kinase 1 in liver reduces triglyceride content in mice fed a low but not high-fat diet. Biochim Biophys Acta. 2015;1851(2):210–9.
Poti F, Ceglarek U, Burkhardt R, Simoni M, Nofer JR. SKI-II--a sphingosine kinase 1 inhibitor—exacerbates atherosclerosis in low-density lipoprotein receptor-deficient (LDL-R−/−) mice on high cholesterol diet. Atherosclerosis. 2015;240(1):212–5.
Poti F, Simoni M, Nofer JR. Atheroprotective role of high-density lipoprotein (HDL)-associated sphingosine-1-phosphate (S1P). Cardiovasc Res. 2014;103(3):395–404.
Wang F, Okamoto Y, Inoki I, Yoshioka K, Du W, Qi X, et al. Sphingosine-1-phosphate receptor-2 deficiency leads to inhibition of macrophage proinflammatory activities and atherosclerosis in apoE-deficient mice. J Clin Invest. 2010;120(11):3979–95.
Luk FS, Kim RY, Li K, Ching D, Wong DK, Joshi SK, et al. Immunosuppression with FTY720 reverses cardiac dysfunction in hypomorphic ApoE mice deficient in SR-BI expression that survive myocardial infarction caused by coronary atherosclerosis. J Cardiovasc Pharmacol. 2016;67(1):47–56.
Wang G, Kim RY, Imhof I, Honbo N, Luk FS, Li K, et al. The immunosuppressant FTY720 prolongs survival in a mouse model of diet-induced coronary atherosclerosis and myocardial infarction. J Cardiovasc Pharmacol. 2014;63(2):132–43.
Huang K, Li SQ, Wang WJ, Liu LS, Jiang YG, Feng PN, et al. Oral FTY720 administration induces immune tolerance and inhibits early development of atherosclerosis in apolipoprotein E-deficient mice. Int J Immunopathol Pharmacol. 2012;25(2):397–406.
Poti F, Costa S, Bergonzini V, Galletti M, Pignatti E, Weber C, et al. Effect of sphingosine 1-phosphate (S1P) receptor agonists FTY720 and CYM5442 on atherosclerosis development in LDL receptor deficient (LDL-R(−)/(−)) mice. Vasc Pharmacol. 2012;57(1):56–64.
Nofer JR, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, et al. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115(4):501–8.
Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, et al. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190(9):1032–43.
Newton J, Hait NC, Maceyka M, Colaco A, Maczis M, Wassif CA, et al. FTY720/fingolimod increases NPC1 and NPC2 expression and reduces cholesterol and sphingolipid accumulation in Niemann-Pick type C mutant fibroblasts. FASEB J: Off Publ Fed Am Soc Exp Biol. 2017;12
Li L, Liu DW, Yan HY, Wang ZY, Zhao SH, Wang B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev: Off J Int Assoc Study Obes. 2016;17(6):510–9.
Blachnio-Zabielska AU, Pulka M, Baranowski M, Nikolajuk A, Zabielski P, Gorska M, et al. Ceramide metabolism is affected by obesity and diabetes in human adipose tissue. J Cell Physiol. 2012;227(2):550–7.
Galadari S, Rahman A, Pallichankandy S, Galadari A, Thayyullathil F. Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis. 2013;12:98.
Kristensen D, Prats C, Larsen S, Ara I, Dela F, Helge JW. Ceramide content is higher in type I compared to type II fibers in obesity and type 2 diabetes mellitus. Acta Diabetol. 2013;50(5):705–12.
Hashimoto T, Igarashi J, Kosaka H. Sphingosine kinase is induced in mouse 3T3-L1 cells and promotes adipogenesis. J Lipid Res. 2009;50(4):602–10.
Bruce CR, Risis S, Babb JR, Yang C, Kowalski GM, Selathurai A, et al. Overexpression of sphingosine kinase 1 prevents ceramide accumulation and ameliorates muscle insulin resistance in high-fat diet-fed mice. Diabetes. 2012;61(12):3148–55.
Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Prim. 2015;1:15080.
Geng T, Sutter A, Harland MD, Law BA, Ross JS, Lewin D, et al. SphK1 mediates hepatic inflammation in a mouse model of NASH induced by high saturated fat feeding and initiates proinflammatory signaling in hepatocytes. J Lipid Res. 2015;56(12):2359–71.
Neubauer HA, Pitson SM. Roles, regulation and inhibitors of sphingosine kinase 2. FEBS J. 2013;280(21):5317–36.
Xu T, Li L, Huang C, Peng Y, Li J. Sphingosine kinase 2: a controversial role in arthritis. Rheumatol Int. 2014;34(7):1015–6.
Lewis CS, Voelkel-Johnson C, Smith CD. Suppression of c-Myc and RRM2 expression in pancreatic cancer cells by the sphingosine kinase-2 inhibitor ABC294640. Oncotarget. 2016;7(37):60181–92.
Yang J, Yang C, Zhang S, Mei Z, Shi M, Sun S, et al. ABC294640, a sphingosine kinase 2 inhibitor, enhances the antitumor effects of TRAIL in non-small cell lung cancer. Cancer Biol Ther. 2015;16(8):1194–204.
Wallington-Beddoe CT, Powell JA, Tong D, Pitson SM, Bradstock KF, Bendall LJ. Sphingosine kinase 2 promotes acute lymphoblastic leukemia by enhancing MYC expression. Cancer Res. 2014;74(10):2803–15.
Beljanski V, Lewis CS, Smith CD. Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Cancer Biol Ther. 2011;11(5):524–34.
Ding X, Chaiteerakij R, Moser CD, Shaleh H, Boakye J, Chen G, et al. Antitumor effect of the novel sphingosine kinase 2 inhibitor ABC294640 is enhanced by inhibition of autophagy and by sorafenib in human cholangiocarcinoma cells. Oncotarget. 2016;7(15):20080–92.
Ding L, Yang L, Wang Z, Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B. 2015;5(2):135–44.
Kim KH, Choi S, Zhou Y, Kim EY, Lee JM, Saha PK, et al. Hepatic FXR/SHP axis modulates systemic glucose and fatty acid homeostasis in aged mice. Hepatology. 2017;
Pereira-Fantini PM, Lapthorne S, Joyce SA, Dellios NL, Wilson G, Fouhy F, et al. Altered FXR signalling is associated with bile acid dysmetabolism in short bowel syndrome-associated liver disease. J Hepatol. 2014;61(5):1115–25.
Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67(3):863–7.
Borude P, Edwards G, Walesky C, Li F, Ma X, Kong B, et al. Hepatocyte-specific deletion of farnesoid X receptor delays but does not inhibit liver regeneration after partial hepatectomy in mice. Hepatology. 2012;56(6):2344–52.
Wang L, Han Y, Kim CS, Lee YK, Moore DD. Resistance of SHP-null mice to bile acid-induced liver damage. J Biol Chem. 2003;278(45):44475–81.
Anakk S, Watanabe M, Ochsner SA, McKenna NJ, Finegold MJ, Moore DD. Combined deletion of Fxr and Shp in mice induces Cyp17a1 and results in juvenile onset cholestasis. J Clin Invest. 2011;121(1):86–95.
Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002;298(5):714–9.
Pols TW. TGR5 in inflammation and cardiovascular disease. Biochem Soc Trans. 2014 Apr;42(2):244–9.
Duboc H, Tache Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Digestive and Liver Disease: Official Journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2014;46(4):302–12.
Studer E, Zhou X, Zhao R, Wang Y, Takabe K, Nagahashi M, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55(1):267–76.
Cao R, Cronk ZX, Zha W, Sun L, Wang X, Fang Y, et al. Bile acids regulate hepatic gluconeogenic genes and farnesoid X receptor via G(alpha)i-protein-coupled receptors and the AKT pathway. J Lipid Res. 2010;51(8):2234–44.
Fang Y, Studer E, Mitchell C, Grant S, Pandak WM, Hylemon PB, et al. Conjugated bile acids regulate hepatocyte glycogen synthase activity in vitro and in vivo via Galphai signaling. Mol Pharmacol. 2007;71(4):1122–8.
Liu R, Li X, Qiang X, Luo L, Hylemon PB, Jiang Z, et al. Taurocholate induces cyclooxygenase-2 expression via the sphingosine 1-phosphate receptor 2 in a human cholangiocarcinoma cell line. J Biol Chem. 2015;290(52):30988–1002.
Liu R, Zhao R, Zhou X, Liang X, Campbell DJ, Zhang X, et al. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology. 2014;60(3):908–18.
Wang Y, Aoki H, Yang J, Peng K, Liu R, Li X, et al. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology. 2017;24
Acknowledgements
We would like to acknowledge the funding support by National Institutes of Health Grant R01 DK104893 (to HZ and PBH), R01DK-057543 (to PBH and HZ), VA Merit Award I01BX001390 (to HZ); National Natural Science Foundation of China Grants 81070245 and 81270489 (to H.Z.); and Massey Cancer Center pilot grant (to HZ and PBH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interest. All reported studies with animal subjects performed by the authors have been previously published and complied with all applicable ethical standards. All animal study protocols were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Liver & Xenobiotic Metabolism
Rights and permissions
About this article
Cite this article
Kwong, E.K., Li, X., Hylemon, P.B. et al. Sphingosine Kinases/Sphingosine 1-Phosphate Signaling in Hepatic Lipid Metabolism. Curr Pharmacol Rep 3, 176–183 (2017). https://doi.org/10.1007/s40495-017-0093-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-017-0093-2