Abstract
Granulocyte colony-stimulating factor (G-CSF) biologics, such as pegfilgrastim, are a standard of care in supportive cancer treatment that are administered once per chemotherapy cycle to reduce the incidence of febrile neutropenia. The high cost of these biologics in the United States can be a limiting factor to accessing care; however, lower-cost pegfilgrastim biosimilars have been available for several years for patients requiring prophylaxis of febrile neutropenia. Different options for pegfilgrastim administration are also now available to accommodate specific patient preferences. As patients may want to minimize the risk of both neutropenia and SARS-CoV-2 infection, same-day administration is a pertinent option during the present COVID-19 pandemic. Therefore, individualized, patient-centered approaches and risk-management strategies should be considered when selecting the treatment and administration method for prophylaxis of febrile neutropenia. Three methods of administration would minimize hospital or clinic visits while also providing the prophylactic effect of G-CSF: same-day administration after chemotherapy, use of the US Food and Drug Administration–approved on-body injector delivering pegfilgrastim approximately 27 h after chemotherapy, or self-administration by the patient or caregiver > 24 h after chemotherapy. Choice of the specific administration option should be based on the patient’s specific needs, while also considering mitigating factors, such as the economic burden associated with biologic medications and the risk of COVID-19. Pegfilgrastim biosimilars can minimize the additional financial burden on patients and the health care system during this pandemic and beyond.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Biologics are cornerstones of treatment for patients with cancer, but the high cost can limit treatment access and negatively impact the health care system. |
In the United States, six pegfilgrastim biosimilars have been approved for the prophylaxis of febrile neutropenia. |
Though next-day pegfilgrastim is the FDA-approved administration method, same-day administration can be considered to minimize clinic visits in the context of patient preference and the COVID-19 pandemic. |
Three pegfilgrastim administration options are available; selection should consider the individual patients’ needs and circumstances. |
Introduction
Febrile neutropenia, defined as a temperature of > 38.3 °C or two consecutive readings of > 38.0 °C and absolute neutrophil count of < 0.5 × 109/L, is a serious complication of myelosuppressive chemotherapy with potentially fatal outcomes [1]. Febrile neutropenia can result in treatment delays and dose reductions, thereby limiting the efficacy of anticancer treatments and affecting patient survival rates [1, 2]. Febrile neutropenia also confers a substantial clinical and economic burden. Each year in the United States, more than 60,000 patients are hospitalized for neutropenia and more than 4000 patients die of febrile neutropenia. In 2012, prior to the introduction of filgrastim or pegfilgrastim biosimilars, neutropenia-related hospitalizations accounted for 5.2% of all cancer-related hospitalizations, with a mean hospital stay of up to 9.6 days and a total cost of $2.7 billion [3]. Granulocyte colony-stimulating factor (G-CSF) is recommended by international guidelines to reduce the incidence of febrile neutropenia in patients receiving myelosuppressive chemotherapy [1, 4, 5].
Filgrastim was the first myeloid growth factor approved for the prevention of febrile neutropenia in patients receiving myelosuppressive chemotherapy [6]. For the prophylaxis of febrile neutropenia, filgrastim is administered daily starting the day after chemotherapy until post-nadir recovery of absolute neutrophil count [6]. Filgrastim is indicated for up to 2 weeks of daily administration, but health database reviews report around 5–6 days as the most common duration of treatment for filgrastim as well as its commonly used biosimilar [7, 8]. Pegfilgrastim, a pegylated, long-acting form of filgrastim, was first approved in 2002 [9]. In contrast to filgrastim (nonpegylated G-CSF), pegfilgrastim is not prematurely eliminated from the circulation by the kidneys but is self-regulated by binding to the G-CSF receptor and is subsequently internalized by neutrophils and neutrophil precursor cells [10, 11]. Because of this prolonged activity, pegfilgrastim is required only once per cycle and is usually injected ≥ 24 h after chemotherapy [9]. Pegfilgrastim is the most commonly used G-CSF in the United States, with previous reports indicating its use in > 90% of patients [12, 13]. A recent meta-analysis suggested no statistically significant differences in outcomes between short-acting filgrastim and long-acting pegfilgrastim if their dosing followed recommended guidelines [14]. In clinical practice, however, short-acting filgrastim is commonly underdosed and bears the risk of lower adherence, as it can require daily administration for up to 2 weeks [15]. In the most recent iteration (v1.2022), the National Comprehensive Cancer Network (NCCN) hematopoietic growth factor guidelines recommend next-day administration of pegfilgrastim (i.e., US Food and Drug Administration [FDA]–recommended dosing); however, in acknowledgment of the growing body of evidence, the guidelines indicate that same-day administration may be used [4]. Any use of same-day administration must be weighed against the potential for increased risk of febrile neutropenia [16]. Because of the COVID-19 pandemic, the option of same-day administration is especially pertinent to minimize additional clinic visits and risks of SARS-CoV-2 exposure.
Biologic medications, such as pegfilgrastim, are associated with significant financial impact on patients and health care systems. This impact is so pronounced that the term “financial toxicity” is often applied to this situation, implying that the financial burden of biologics dramatically affects patients on both mental and physical levels and limits the medication’s usefulness. In 2019, the United States spent $212 billion on biologic drugs alone, which was 43% of the total medication spending for the year [17].
Biosimilar medications provide a more affordable option to reference product, as they enter the market as lower cost, competitive alternatives to the originator product. This can result in lower out-of-pocket cost for patients, offer significant cost savings to the health care system, and potentially increase drug accessibility for patients [18], all of which are particularly relevant in the time of the COVID-19 pandemic when financial stress is high.
With the growing acceptance of same-day pegfilgrastim administration and the ever-present financial burden of medications for patients, the preferred administration method and potential savings associated with biosimilar use are pertinent topics for discussion. Thus, the objective of this narrative review is to discuss pegfilgrastim and pegfilgrastim biosimilar administration options, focusing on those minimizing clinic visits in the context of patient preference and the COVID-19 pandemic, and to ultimately propose a patient-centric model of pegfilgrastim administration for prophylaxis of febrile neutropenia.
Methods
An initial thorough search of the literature was performed using PubMed and the following search terms: biosimilars AND oncology/human granulocyte colony-stimulating factors/GCSF/Neulasta/pegfilgrastim/chemo-induced neutropenia/supportive care. Articles in English from 2015 to 2021 were included in the nonsystematic review. This initial search produced 862 results. The results of this broad and expansive search were further refined, focusing on references concerning pegfilgrastim and related biosimilars. These results were screened by title and abstract, and full-text articles were reviewed for those of interest and relevance based on the authors’ expertise. As this review is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors, there is no ethics compliance to report.
Overview of Biosimilars
A biosimilar is a biologic medication that is highly similar in structure and function to the originator reference product. It must have no clinically meaningful differences in safety, purity, and potency compared with the originator [19,20,21]. To ensure that these criteria are met, biosimilars undergo an extensive review process prior to market authorization [20, 22]. For approval of a biosimilar candidate, the US FDA requires a totality-of-evidence approach that considers data and information collected from structural and functional characterization, nonclinical assessments, pharmacokinetic and pharmacodynamic analyses, clinical immunogenicity data, and, if deemed necessary, comparative clinical studies [19, 20]. The FDA requires postmarketing surveillance to monitor the safety of biosimilars. Since the introduction of biosimilars in the United States, there has been a 2–4% incremental increase in the overall use of biologics, including biosimilars and their originator products [17]. Over the next 5 years, the use of biosimilars is projected to result in savings exceeding $100 billion in US health care spending [17].
Economics of Biosimilars in Supportive Oncology Care
Although biosimilars for therapeutic monoclonal antibodies, such as rituximab, trastuzumab, and bevacizumab, have only recently been approved in oncology [23], biosimilars have been available for several years in supportive oncology care [15]. In 2015, filgrastim-sndz (ZARXIO®; Sandoz Inc., Princeton, NJ, USA) became the first approved biosimilar product in the United States [23]. Since June 2018, six biosimilars of pegfilgrastim have been approved in the United States [24,25,26,27,28]: pegfilgrastim-apgf (NYVEPRIA®; Pfizer Inc., New York, NY, USA), pegfilgrastim-bmez (ZIEXTENZO®; Sandoz Inc., Princeton, NJ, USA), pegfilgrastim-jmdb (FULPHILA®; Mylan, Rockford, IL, USA), pegfilgrastim-cbqv (UDENYCA®; Coherus BioSciences, Redwood City, CA, USA), pegfilgrastim-pbbk (FYLNETRA™, Amneal Pharmaceuticals LLC, Bridgewater, NJ, USA), and pegfilgrastim-fpgk (STIMUFEND, Fresenius Kabi USA LLC, Lake Zurich, IL, USA).
Use of these biosimilar products can result in significant savings, as biosimilars typically cost less than their reference product. The cost savings from biosimilar pegfilgrastim can potentially be used to expand treatment access to more patients. For example, economic modeling using the average sales price of originator and biosimilar pegfilgrastim in a population of 20,000 patients showed that cost savings of $326,744 (10% conversion from originator on-body injector [OBI] to biosimilar prefilled syringe for 1 cycle) to $22,286,640 (100% conversion from originator prefilled syringe to biosimilar prefilled syringe for six cycles) could be realized [29, 30]. These savings from converting 20,000 patients could be used to provide 1054 additional doses of biosimilar pegfilgrastim if all patients receive one cycle or 6322 additional doses if patients receive six cycles [29]. Savings could also be reallocated to other cancer treatments, such as antineoplastic or novel biologic-based treatments [31].
Cost savings can also have a significant impact on the financial stress experienced by patients with cancer, which is a prominent issue for these individuals. A systematic review found that up to 48% of cancer survivors experience financial toxicity [32], which can then cause survivors to forgo future medical treatments because of long-term, continued financial concerns [33]. Reduced medical costs can also directly impact patient quality of life, as financial burden has been found to be the strongest predictor of poor quality of life in patients with cancer [34]. Specific data are not available on the relationship between the impact of pegfilgrastim biosimilars on patients’ financial stress and quality of life. However, the availability of a G-CSF biosimilar in Europe (i.e., Germany, the United Kingdom, and The Netherlands) correlated with a 10–20% increase in G-CSF use, which was suggested to be related to increased patient access owing to affordability [35]. This evidence suggests a positive relationship between lower costs of biosimilars and treatment access, although a direct examination would be valuable to investigate whether decreased costs with biosimilars positively impact patients’ financial toxicity and quality of life.
Although biosimilars provide an encouraging potential for economic benefit, they can be hindered by several factors. One is drug rebate walls, where competitors offer financial rebates to buyers that act as a barrier to the new market entry of biosimilars [36]. Confusion around interchangeability between an originator and biosimilar can also be a hurdle. To gain interchangeable status, a switching study with the biosimilar must be performed to prove switching is not associated with decreased efficacy or increased safety risk; some health care professionals may incorrectly perceive this FDA requirement to imply that a biosimilar is not clinically the same as the reference product and, as a result, be hesitant to prescribe a biosimilar product [36]. To further complicate interchangeability/switching, policies vary between the FDA and European Medicines Agency. Medicare reimbursement for biosimilars also presents a challenge. Generic drugs are billed under the same billing code as the brand-name version, with the average sales price representing a weighted average of the molecules. This differs from biosimilars, where each biosimilar receives its own billing code and is paid based on its own average sales price; this can limit price competition between the biosimilar and reference product [37]. Despite these potential economic barriers, a 2021 report suggests that, in the absence of biosimilar competition, the average sales price of reference pegfilgrastim would have been expected to increase by 96.2%; thus, the introduction of biosimilar pegfilgrastim products has lowered the estimated price of reference pegfilgrastim [38].
Administration Options for Pegfilgrastim
The FDA-approved, and NCCN-supported, indication for pegfilgrastim administration for febrile neutropenia prophylaxis is next-day administration at the clinic [9]. Based on recently published evidence, the NCCN also supports same-day administration [4], which is particularly important for minimizing clinic visits during the COVID-19 pandemic. Same-day administration may be preferable for patients who find returning to the clinic burdensome, especially for those who live far from the clinic or want to minimize visits because of the emotional and physical exhaustion following chemotherapy [39].
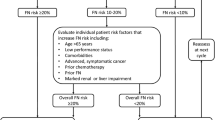
There are three approaches to pegfilgrastim administration that minimize clinic visits: same-day administration of pegfilgrastim after chemotherapy, use of the FDA-approved OBI that delivers pegfilgrastim ~ 27 h after application, or self-administration of pegfilgrastim by the patient or caregiver > 24 h after chemotherapy. Consideration of each option should be based on the individual patient-specific needs and comfort level (Fig. 1).
Self-administration of pegfilgrastim reduces the number of clinic visits and may improve quality of life for patients and their families [40]. However, correct self-injection techniques are crucial for safe and effective self-administration. Although self-injection has been taught successfully across some patient populations (e.g., insulin injection in patients with diabetes) [41], it remains important for advanced practitioners to revisit these techniques across follow-up appointments to minimize risks associated with incorrect self-injection [41, 42]. Furthermore, several barriers to self-injection have been identified and include aversions to injections, fear, anxiety, needle phobia, anticipated pain, and impaired manual dexterity [43]. For pegfilgrastim, patient age and comorbidities often limit the ability to self-administer, resulting in the requirement (and additional burden) for a caregiver who would need to be trained in safe injection techniques.
The pegfilgrastim OBI, an FDA-approved delivery device that is applied the same day as chemotherapy and delivers the standard dose of pegfilgrastim ~ 27 h after application [44], is an alternative for patients who are unable to self-administer G-CSF or who may be unable to return to the hospital the next day for other reasons. Similar OBI-delivery devices that provide biosimilar pegfilgrastim at a lower cost are not currently available, but a pegfilgrastim-cbqv OBI is in development [45]. Complicating reliable febrile neutropenia prophylaxis, OBI failure rates of 1.7–6.9% have been reported and not all patient populations accept the OBI device [46,47,48,49,50,51]. Patient education may be required to ensure the effectiveness of pegfilgrastim OBI and to handle device failure [52]. OBIs and prefilled syringes with originator pegfilgrastim (Neulasta) are currently available at the same price, but both are more expensive than biosimilar products [29]. Compared with originator pegfilgrastim (single-dose syringe or OBI), the average wholesale acquisition cost is approximately 33–37% higher and the average sales price is 5–6% higher than the price of biosimilar pegfilgrastim products [53] (Table 1), which are currently only available as prefilled syringes.
Same-day injection of pegfilgrastim (off-label) at the end of chemotherapy is another administration option that minimizes the risk associated with an additional outpatient clinic visit. Same-day administration may be a preferrable option for patients who are unable to self-inject pegfilgrastim and who are not comfortable having a device (i.e., an OBI) attached to their skin. Concern over same-day administration is rooted in observations that administration of nonpegylated, shorter-acting filgrastim may exacerbate neutropenia in certain therapeutic settings [54, 55]. For longer-acting pegfilgrastim, a retrospective evaluation found that patients receiving prophylactic pegfilgrastim on the same day as chemotherapy or 4–5 days after chemotherapy had a significantly higher incidence of febrile neutropenia compared with patients receiving pegfilgrastim on days 1–3 following chemotherapy [56]. However, same-day administration of pegfilgrastim is not uncommon in clinical practice, and an increasing number of studies across various tumor types have not detected differences in outcomes compared with next-day administration [16, 57,58,59]. In a large meta-analysis, the incidence of grade 4 neutropenia was equal between patients receiving same-day or standard next-day pegfilgrastim [57]. In another meta-analysis of 23 studies, rates of febrile neutropenia reduction were low with same- or next-day administration, and no increase in risk of grade 3/4 chemotherapy-induced neutropenia was observed [58]. A retrospective study in patients with gastrointestinal cancers receiving FOLFOX or FOLFIRI concluded that same-day administration of pegfilgrastim was a safe, effective alternative in this patient population [59]. A similar retrospective study in patients with lung cancer also reported low rates of febrile neutropenia and grade 3/4 neutropenia with same-day pegfilgrastim administration [60]. A recent review showed that the efficacy of same-day pegfilgrastim appears to be dependent on the chemotherapy regimen administered [16]. Overall, as acknowledged in the NCCN guidelines [4], the growing body of available data show that same-day pegfilgrastim can be considered for the prophylaxis of chemotherapy-induced neutropenia and febrile neutropenia. Any decision on same-day administration should take into account the prescribed chemotherapy regimen, patient-specific risk factors, and outpatient visit–associated risks [16].
Pegfilgrastim Administration in the Context of COVID-19
Patients with cancer are generally at an increased risk of infection compared with healthy individuals [61]. Frequent hospital visits may further increase the risk of contracting COVID-19 during the current pandemic, especially in immunocompromised older patients with poor functional status [61]. During the pandemic, outpatient visits for patients with cancer should therefore be minimized without compromising adequate patient care [61]. The risk–benefit ratio for therapeutic and supportive oncology care may be altered, as hospital visits to receive treatment and treatment-induced immunosuppression may increase the risk of contracting COVID-19. Although the benefits of same-day pegfilgrastim treatment options have not been exclusively studied in the context of the COVID-19 pandemic, these methods were successfully used before the COVID-19 pandemic and may provide risk-minimization opportunities [16]. Reducing patient visits can also reduce the workload for health care workers, who are currently overburdened and overworked because of COVID-19 [62].
COVID-19 has also resulted in a huge health-related financial burden. The American Hospital Association estimates $202.6 billion in lost revenue for health care systems and hospitals because of the COVID-19 pandemic [63]. Specific data are lacking on the financial benefits of pegfilgrastim biosimilar use during the COVID-19 pandemic; however, as biosimilars are established as lower-cost alternatives, the use of biosimilar pegfilgrastim can mitigate part of the economic impact associated with COVID-19. Beyond pegfilgrastim in supportive oncology care, lower-cost biosimilar alternatives to expensive biologic medicines may provide health care systems and hospitals with an opportunity to balance the significant revenue reduction and cost increase associated with the COVID-19 pandemic [63]. COVID-19 has also significantly impacted the finances of patients, particularly the marginalized and vulnerable populations who may not have comprehensive insurance coverage [64]. Therefore, cost savings to these patients through biosimilar use could improve patients’ financial situation and treatment access.
Conclusions
Several G-CSF same-day administration options are available to avoid or minimize additional health care visits and the associated risk of COVID-19 exposure while also accommodating specific patient needs and preferences. Pegfilgrastim biosimilars can play a key role in minimizing the treatment-associated financial burden on patients, payers, and health care systems during the COVID-19 pandemic and beyond.
References
Klastersky J, de Naurois J, Rolston K, Rapoport B, Maschmeyer G, Aapro M, et al. Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann Oncol. 2016;27(suppl 5):v111–8.
Wang L, Baser O, Kutikova L, Page JH, Barron R. The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2015;23(11):3131–40.
Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552–61.
National Comprehensive Cancer Network. NCCN practice guidelines in oncology. Hematopoietic Growth Factors. Version 1.2022. 2021. Accessed 02/17/2022.
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32.
Amgen Inc. NEUPOGEN® (filgrastim) injection, for subcutaneous or intravenous use. Thousand Oaks: Amgen Inc; 2018.
Gascón P, Aapro M, Ludwig H, Bokemeyer C, Boccadoro M, Turner M, et al. Treatment patterns and outcomes in the prophylaxis of chemotherapy-induced (febrile) neutropenia with biosimilar filgrastim (the MONITOR-GCSF study). Support Care Cancer. 2016;24(2):911–25.
Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402–7.
Amgen Inc. NEULASTA® (pegfilgrastim) injection, for subcutaneous use. Thousand Oaks: Amgen Inc; 2019.
Arvedson T, O’Kelly J, Yang BB. Design rationale and development approach for pegfilgrastim as a long-acting granulocyte colony-stimulating factor. BioDrugs. 2015;29(3):185–98.
Roskos LK, Lum P, Lockbaum P, Schwab G, Yang BB. Pharmacokinetic/pharmacodynamic modeling of pegfilgrastim in healthy subjects. J Clin Pharmacol. 2006;46(7):747–57.
Naeim A, Henk HJ, Becker L, Chia V, Badre S, Li X, et al. Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF). BMC Cancer. 2013;13:1–11.
Weycker D, Bensink M, Lonshteyn A, Doroff R, Chandler D. Use of colony-stimulating factor primary prophylaxis and incidence of febrile neutropenia from 2010 to 2016: a longitudinal assessment. Curr Med Res Opin. 2019;35(6):1073–80.
Cornes P, Gascon P, Chan S, Hameed K, Mitchell CR, Field P, et al. Systematic review and meta-analysis of short- versus long-acting granulocyte colony-stimulating factors for reduction of chemotherapy-induced febrile neutropenia. Adv Ther. 2018;35(11):1816–29.
Cornes P, Gascon P, Vulto AG, Aapro M. Biosimilar pegfilgrastim: improving access and optimising practice to supportive care that enables cure. BioDrugs. 2020;34(3):255–63.
Gerberich AJ, Attilio MR, Svoboda A. Revisiting same-day administration of pegfilgrastim in the age of biosimilars: a review of literature. J Oncol Pharm Pract. 2020;26(8):1970–6.
IQVIA Institute for Human Data Science. Biosimilars in the United States 2020–2024. 2020. https://www.iqvia.com/insights/the-iqvia-institute/reports/biosimilars-in-the-united-states-2020-2024. Accessed June 9, 2022.
Webster J, Smith RE, Wieland D, Verniero J, Scott JA. Cost savings of biosimilar pegfilgrastim in a Medicare OCM population. J Clin Oncol. 2020;38: e19362.
European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. 2014. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-2.pdf. Accessed June 9, 2022.
US Food and Drug Administration. Guidance for Industry. Scientific considerations in demonstrating biosimilarity to a reference product. 2015. https://www.fda.gov/media/82647/download. Accessed June 9, 2022.
European Medicines Agency. Biosimilars in the EU. Information Guide for Healthcare Professionals. 2019. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf. Accessed June 9, 2022.
Wolff-Holz E, Tiitso K, Vleminckx C, Weise M. Evolution of the EU biosimilar framework: past and future. BioDrugs. 2019;33(6):621–34.
US Food and Drug Administration. Biosimilar product information. 2020. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information. Accessed June 9, 2022.
Coherus BioSciences Inc. UDENYCA™ (pegfilgrastim-cbqv) injection, for subcutaneous use. Redwood City: Coherus BioSciences, Inc; 2018.
Mylan GmbH. FULPHILA® (pegfilgrastim-jmdb) injection, for subcutaneous use. Zurich: Mylan GmbH; 2018.
Sandoz Inc. ZIEXTENZO™ (pegfilgrastim-bmez) injection, for subcutaneous use. Princeton: Sandoz, Inc; 2019.
Amneal Pharmaceuticals. FYLNETRA® (pegfilgrastim-pbbk) injection, for subcutaneous use. Bridgewater: Amneal Pharmaceuticals; 2022.
Hospira Inc. NYVEPRIA™ (pegfilgrastim-apgf) injection, for subcutaneous use. Lake Forest: Hospira Inc; 2020.
MacDonald K, McBride A, Alrawashdh N, Abraham I. Cost-efficiency and expanded access of prophylaxis for chemotherapy-induced (febrile) neutropenia: economic simulation analysis for the US of conversion from reference pegfilgrastim to biosimilar pegfilgrastim-cbqv. J Med Econ. 2020;23(12):1466–76.
McBride A, MacDonald K, Abraham I. Simulation modeling of cost-savings from conversion of pegfilgrastim to biosimilar pegfilgrastim-cbqv for the prophylaxis of chemotherapy-induced (febrile) neutropenia (CIN/FN) and expanded access to biosimilar prophylaxis. J Clin Oncol. 2020;38(15_suppl): e19372.
McBride A, MacDonald K, Abraham I. Simulation modeling of budget-neutral expanded access to antineoplastic therapy from cost-savings derived from conversion to biosimilar pegfilgrastim-cbqv for the prophylaxis of chemotherapy-induced (febrile) neutropenia (CIN/FN) in early-stage breast cancer. J Clin Oncol. 2020;38(15_suppl): e19371.
Gordon LG, Merollini KMD, Lowe A, Chan RJ. A systematic review of financial toxicity among cancer survivors: we can’t pay the co-pay. Patient. 2017;10(3):295–309.
Weaver KE, Rowland JH, Bellizzi KM, Aziz NM. Forgoing medical care because of cost: assessing disparities in healthcare access among cancer survivors living in the United States. Cancer. 2010;116(14):3493–504.
Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Practice. 2014;10(5):332–8.
Gascón P, Tesch H, Verpoort K, et al. Clinical experience with Zarzio® in Europe: what have we learned? Support Care Cancer. 2013;21(10):2925–32.
Arad N, Staton E, Hamilton Lopez M, et al. Realizing the benefits of biosimilars: overcoming rebate walls. https://healthpolicy.duke.edu/sites/default/files/2022-03/Biosimilars%20-%20Overcoming%20Rebate%20Walls.pdf. Accessed June 9, 2022.
Rome BN, Sarpatwari A. Promoting biosimilar competition by revising Medicare reimbursement rules. JAMA Netw Open. 2021;4(11): e2134463.
Xcenda and the Biosimilars Forum. Biosimilars Have Significantly Lowered Prices of All Biologics. 2021. https://biosimforum.wpengine.com/wp-content/uploads/Xcenda-ASP-One-Pager.pdf. Accessed June 9, 2022.
Hauber BA, Mange B, Price MA, et al. Administration options for pegfilgrastim prophylaxis: patient and physician preferences from a cross-sectional survey. Support Care Cancer. 2018;26(1):251–60.
Boeru G, Milanov I, De Robertis F, et al. ExtaviJect® 30G device for subcutaneous self-injection of interferon beta-1b for multiple sclerosis: a prospective European study. Med Devices (Auckl). 2013;6:175–84.
Diggle J, Phillips A. How to help patients achieve correct self-injection technique. Pract Nurs. 2014;25(9):451–4.
Memorial Sloan Kettering Cancer Center. Giving yourself an injection of filgrastim (Neupogen®) or Pegfilgrastim (Neulasta®) with a prefilled syringe. 2019. https://www.mskcc.org/cancer-care/patient-education/directions-giving-injection-below-skin-neupogen-filgrastim-neulasta-pegfilgrastim-pre-filled-syringe. Accessed June 9, 2022.
Pagels AA, Hylander B. Anaemia management in chronic kidney disease patients. J Renal Nurs. 2012;4(2):81–5.
Yang BB, Morrow PK, Wu X, Moxness M, Padhi D. Comparison of pharmacokinetics and safety of pegfilgrastim administered by two delivery methods: on-body injector and manual injection with a prefilled syringe. Cancer Chemother Pharmacol. 2015;75(6):1199–206.
Coherus BioSciences Inc. Coherus announces positive results of UDENYCA® on-body injector clinical trial. Redwood City: Coherus BioSciences Inc; 2021.
Joshi RS, Egbuna OI, Cairns AS, et al. Performance of the pegfilgrastim on-body injector as studied with placebo buffer in healthy volunteers. Curr Med Res Opin. 2017;33(2):379–84.
Stuessy P, Sanchez FA, Schober M. Retrospective review of pegfilgrastim on-body injector delivery rates in a large health system. J Clin Oncol. 2017;35(15_suppl): e18273.
Mahler LJ, DiBlasi R, Perez A, Gaspard J, McCauley D. On-body injector: an administration device for pegfilgrastim. Clin J Oncol Nurs. 2017;21(1):121–2.
Townley C, Porter C, McMullen N. Comparing grade 4 neutropenia associated with pegfilgrastim administered via the Onpro Device versus manual injection with a prefilled syringe. JHOP. 2018;8(3):119–25.
McBride A, Krendyukov A, Mathieson N, et al. Febrile neutropenia hospitalization due to pegfilgrastim on-body injector failure compared to single-injection pegfilgrastim and daily injections with reference and biosimilar filgrastim: US cost simulation for lung cancer and non-Hodgkin lymphoma. J Med Econ. 2020;23(1):28–36.
Relias R, Hackenyos DW, Wasif K, Healey P, Smith MH, Saif WM. Ethnic differences in pegfilgrastim Onpro kit (on-body injector) use among cancer patients. J Clin Oncol. 2018;36(15_suppl): e18668.
Jindal A, Kover J, Raduka V, O’Brien TE. Incidence of neutropenic fever at a safety net hospital in cancer chemotherapy patients receiving prophylactic pegfilgrastim manual injection compared to the on-body auto-injector. Blood. 2018;132(Supplement 1):4709.
Amgen Inc. Amgen 2021 biosimilar trend report. 2021. https://www.amgenbiosimilars.com/commitment/-/media/Themes/Amgen/amgenbiosimilars-com/Amgenbiosimilars-com/pdf/USA-CBU-80962_Amgen-2021-Biosimilar-Trends-Report.pdf Accessed June 9, 2022.
Rowinsky EK, Grochow IB, Sartorius SE, et al. Phase I and pharmacologic study of high doses of the topoisomerase I inhibitor topotecan with granulocyte colony-stimulating factor in patients with solid tumors. J Clin Oncol. 1996;14(4):1224–35.
Meropol NJ, Miller LL, Korn EL, et al. Severe myelosuppression resulting from concurrent administration of granulocyte colony-stimulating factor and cytotoxic chemotherapy. J Natl Cancer Inst. 1992;84(15):1201–3.
Weycker D, Bensink M, Lonshteyn A, Doroff R, CHandler D. Risk of chemotherapy-induced febrile neutropenia by day of pegfilgrastim prophylaxis in US clinical practice from 2010 to 2015. Curr Med Res Opin. 2017;33(12):2107–13.
Diri R, Diri R, McBride A, et al. Efficacy of same-day vs. next-day pegfilgrastim for the prevention of chemotherapy-induced (febrile) neutropenia (CIN/FN): a meta-analysis. Blood. 2015;126:4764–4764.
AlRawashdh N, McBride A, Lee C, et al. Outcomes of pegfilgrastim (PFG) administration on the same day vs the day after chemotherapy (CTX) in the prophylaxis of chemotherapy-induced (Febrile) neutropenia (CIN/FN): systematic review and meta-analysis. J Clin Oncol. 2018;36(15_suppl): e14510.
Eckstrom J, Bartels T, Abraham I, et al. A single-arm, retrospective analysis of the incidence of febrile neutropenia using same-day versus next-day pegfilgrastim in patients with gastrointestinal cancers treated with FOLFOX or FOLFIRI. Support Care Cancer. 2019;27(3):873–8.
Alrawashdh N, Vraney J, Choi BM, Almutairi AR, Abraham I, McBride A. Retrospective evaluation of safety and effectiveness of same-day pegfilgrastim in patients with lung cancer. Future Oncol. 2022;18(19):2381–90.
Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25(6):e936–45.
French G, Hulse M, Nguyen D, et al. Impact of hospital strain on excess deaths during the COVID-19 pandemic—United States, July 2020–July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(46):1613–6.
Kaye AD, Okeagu CN, Pham AD, et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: international perspectives. Best Pract Res Clin Anaesthesiol. 2021;35(3):293–306.
Ohsfeldt RL, Choong CKC, Mc Collam PL, Abedtash H, Kelton KA, Burge R. Inpatient hospital costs for COVID-19 patients in the United States. Adv Ther. 2021;38(11):5557–95.
Acknowledgements
Funding
Medical writing support for this manuscript was funded by Coherus BioSciences. The journal’s Rapid Service Fee was funded by the authors.
Medical Writing Assistance
Medical writing assistance was provided by Steffen Biechele, PhD, and Chantel Kowalchuk, PhD, of ApotheCom (San Francisco, CA, USA) and funded by Coherus BioSciences (Redwood City, CA, USA).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript and take responsibility for the integrity of the work as a whole. The first draft of the manuscript was written by Sophia Z. Humphreys. All authors substantially contributed to revising previous versions of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Disclosures
Sophia Z. Humphreys is a speaker for multiple nonbranded educational programs sponsored by Coherus BioSciences and Mylan and was a paid participant for the US Biosimilars Expert Input Forum 2020, which was sponsored by Merck. Robert B. Geller and Paul Walden are employees of Coherus BioSciences.
Compliance With Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
No review article is based on previously conducted studies. No novel data were included.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Humphreys, S.Z., Geller, R.B. & Walden, P. Pegfilgrastim Biosimilars in US Supportive Oncology: A Narrative Review of Administration Options and Economic Considerations to Maximize Patient Benefit. Oncol Ther 10, 351–361 (2022). https://doi.org/10.1007/s40487-022-00207-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-022-00207-2