Abstract
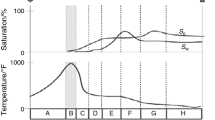
The present study deals with fingerprinting the oxidation behavior of a Brazilian crude (12° API) oil. It focuses on the determination of reaction kinetic parameters through classical thermal analysis techniques such as thermogravimetry (TG), differential thermal analysis (DTG) and scanning calorimetry (DSC). The required experimental data are collected from oil and oil–sand samples. The reaction data are treated following distinct conventional and isoconversional non-isothermal models, using integral and differential approaches based on Arrhenius’ model. The thermoanalytical study is successful in identifying three oxidation temperature ranges: the low-temperature (LTO) range, a transition zone, and the high-temperature (HTO) range. TG and DSC analyses show that the highest variation of mass and the highest level of energy generation occur at the HTO range. At the high end of the LTO range, a mass transfer resistance (skin effect) was evident. Values of activation energy obtained for oil samples are 103 kJ mol−1 for LTO and 278 kJ mol−1 for HTO oxidation reactions by the most straightforward method used—the unity model. By all kinetic models, HTO’s values are higher than those for LTO, observation also valid for the results of oil–sand samples. Results also evidence that the presence of sand contributes to the so-called skin effect.








Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor, 1/s
- ARC:
-
Accelerating rate calorimetry
- ASTM:
-
American Society for Testing and Materials
- BSW:
-
Basic sediment and water
- C,H,N:
-
Carbon, hydrogen and nitrogen
- C 20+ :
-
Icosane plus fraction
- DTA:
-
Differential thermal analysis
- DSC:
-
Scanning calorimetry
- DTG:
-
Differential thermal analysis
- E :
-
Activation energy, kJ mol−1
- E α :
-
Apparent activation energy or activation energy in some conversion, kJ mol−1
- FD:
-
Fuel deposition
- f(α):
-
Differential conversion function, dimensionless
- g(α):
-
Integral conversional function, dimensionless
- H :
-
Enthalpy, J
- HTO:
-
High-temperature oxidation
- ISC:
-
In Situ Combustion
- LTO:
-
Low-temperature oxidation
- m :
-
Actual mass, mg
- m i :
-
Initial mass, mg
- m f :
-
Final mass, mg
- n :
-
Reaction order, dimensionless
- NTG:
-
Negative temperature gradient
- R :
-
Universal gas constant, J/(K-gmol)
- t :
-
Time, s
- T :
-
Absolute temperature, K
- T 0 :
-
Initial temperature, °C
- T m :
-
Peak temperature, °C
- T α :
-
Absolute temperature at given conversion, K
- TG:
-
Thermogravimetry
- x :
-
Remaining mass, mg
- ΔH :
-
Total reaction heat, J
- α:
-
Extent of reaction conversion, dimensionless
- β:
-
Heating rate, °C/min
- Δ:
-
Gradient
References
Adegbesan KO (1882) Kinetics Study of Low Temperature Oxidation of Athabasca Bitumen. PhD thesis, The University of Calgary, Alberta
Budrugeac P, Homentcovschi D, Segal E (2001) Critical analysis of the isoconversional methods for evaluating the activation energy. J Therm Anal Calorim 63:457–463
Burger JG, Sahuquet BC (1972) Chemical aspects of in-situ combustion—heat of combustion and kinetics. Soc Pet Eng J 12(5):410–422
Burnham AK, Braun RL (1999) Global kinetics analysis of complex materials. Energy Fuels 13:1–22
Coats AW, Redfern JP (1964) Kinetics parameters from thermogravimetric data. Nature 201:68–69
Crane LW, Dynes PJ, Kaelble DH (1973) Analysis of curing kinetics in polymer composites. Polym Lett 11:533–540
Crnkovic PM, Leiva CRM, Santos AM, Milioli FE (2007) Kinetics study of the oxidative degradation of Brazilian fuel oils. Energy Fuels 21(6):3415–3419
Fassihi MR, Bringham WE, Ramey HJ (1984) Reaction kinetics of in-situ combustion: part 2—modeling. Soc Pet Eng J 24(4):408–416
Freeman SE, Carroll B (1958) The application of thermoanalytical techniques to reaction kinetics. The thermogravimetric evaluation of the kinetics of the decomposition of calcium oxalate monohydrate. J Phys Chem 62:394–397
Galwey AK, Brown ME (1999) Thermal decomposition of ionic solids. Elsevier, Amsterdam
Galwey AK (2003) What is meant by the term variable activation energy when applied in the kinetics analyses of solid state decompositions (crystolysis reactions)? Thermochim Acta 397:249–268
Grueiro LF (2001) Estúdio Cinético, Dinamomecánico y Termogravimétrico del Sistema Epoxídico BADGR (n = 0)/m-XDA mediante las Técnicas de Análisis Térmico: DSC, DMA y TGA. Construcción de um Diagrama TTT. PhD thesis, Universidad de Santiago de Compostela, Galiza
Hayashitani M, Bennion DW, Moore RG (1978) Thermal cracking models for Athabasca oil sands oil. SPE 7549. In: 53rd Annual Fall Technical Conference and Exhibition of SPE of AIME, Houston, October 1–3
Iscan AG, Kök MV, Bagci AS (2007) Kinetic analysis of central anatoli oil shale by combustion cell experiments. J Therm Anal Calorim 88:6553–6556
Kök MV, Keskin C (2001) Comparative combustion kinetics for in situ combustion process. Thermochimica Acta 369:143–147
Kök MV, Okandan E (1997) Kinetic analysis of crude oils by a weighted mean activation energy approach. J Therm Anal 46(6):343–348
Kissinger HE (1956) Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand 57:217–221
Li J (2006) New insights into the oxidation behaviours of crude oils. PhD thesis, University of Calgary, Alberta
Moore RG, Ursenbach MG, Laureshen CJ, Mehta SA (1995) Ramped temperature oxidation analysis of athabasca oil sands bitumen. In: 46th Annual Technical Meeting of the Petroleum Society of CIM, Banff, May, pp 14–17
Morgan PA, Robertson SD, Unsworth JF (1986) Combustion studies by thermogravimetric analysis. Fuel 65:1546–1551
Ozawa T (1965) A new methods of thermogravimetric data. Bull Chem Soc Jpn 38:1881–1886
Sarathi P (1999) In-situ combustion handbook principles and practices, Report DOE/PC/91008-0374, OSTI ID3175
Simon P (2004) Isoconversional methods: fundamentals, meaning and application. J Therm Anal Calorim 76:123–132
Tadema HJ (1959) Mechanism of oil production of underground combustion. In: Proceedings of 5th World Pet. Congress, New York, Sec. II, Paper 22, pp 279–287
Vossoughi S, Barlett GW, Wilhite GP (1982) Automation of an in-situ combustion tube and study of the effect on the in-situ combustion process. SPE J 25:656–664, Trans., AIME, 273
Vyazovkin S, Dollimore D (1996) Linear and nonlinear procedures in isoconversional computations of the activation energy of nonisothermal reaction in solids. J Chem Inf Comput Sci 36:42–45
Vyazovkin S (2000) Kinetic concepts of thermally stimulated reactions in solids: a view from historical perspective. Int Rev Phys Chem 19:45–60
Vyazovkin S, Wigtht CA (1997) Kinetics in solids. Annu Rev Phys Chem 48:125–149
Vyazovkin S, Wigtht CA (1999) Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta 340–341:53–68
Vyazovkin S, Sbirrazzuoli N (1997) Confidence intervals for the activation energy estimated by few experiments. Anal Chim Acta 355:175–180
Vyazovkin SA (1996) Unified approach to kinetic processing of nonisothermal data. Int J Chem Kinetic 28:95–101
Wagoner CL, Winegartner EC (1973) Further development of the burning profile. J Eng Power 95:119–123
Yagmur S, Durusoy T (2006) Kinetics of the pyrolysis and combustion of göynük oil shale. J Therm Anal Calorim 86:479–482
Acknowledgments
The authors would like to acknowledge Petrobras-Cenpes and Finep-Ctpetro for the support given to this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Techincal Editor: Demetrio Neto.
Rights and permissions
About this article
Cite this article
Pereira, A.N., Trevisan, O.V. Thermoanalysis and reaction kinetics of heavy oil combustion. J Braz. Soc. Mech. Sci. Eng. 36, 393–401 (2014). https://doi.org/10.1007/s40430-013-0093-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40430-013-0093-z