Abstract
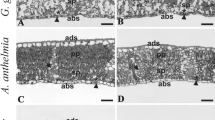
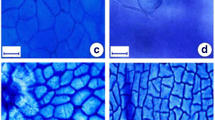
For this work we hypothesize the existence of proportional increase in the thickness of the mesophyll cells and dimensions of the fiber cells of stem and the content of the cell wall polymers (cellulose, hemicelluloses and lignin) with the ontogeny of brazilwood. We also postulate that the capacity of this heliophile in inhabit lower stratum of forest densely shaded until the canopy highly illuminated is due to high plasticity of specific leaf area and stomatal density. Juvenile individuals stood out for higher specific leaf area, lower stomatal density, size of vessel elements and lignin contents as much in leaf and in stem. Young individuals presented, in general, intermediate values. The adult individuals, whose crowns reached the canopy, stood out for greater thickening of the spongy parenchyma, stomatal density, water content and hemicelluloses and lignins of leaf and stem. The cellulose content of the leaf and stem did not vary between different stages of ontogeny. From all the structural variables, the dimensions of the vessel elements and the proportion of hemicelluloses of leaves showed greater plasticity. Qualitative analysis of the cell wall suggested that the hemicelluloses are the type xylan with the possibility of presence of xyloglucan. Differently than what was hypothesized, we concluded that the capacity of Caesalpinia echinata Lam. in inhabit lower stratum of forest densely shaded until the canopy highly illuminated is due to high plasticity of density and diameter vessel and hemicelluloses of leaves and not specific leaf area and stomatal density.


Similar content being viewed by others
References
Angyalossy V, Amano E, Alves ES (2005) Madeiras utilizadas na fabricação de arcos para instrumentos de corda: aspectos anatômicos. Acta Bot Bras 19(4):819–834
ASTM E 1757–01 (2001) Standard test method for determination of carbohydrates in biomass by high performance liquid chromatography. American Society for Testing and Materials, USA [S.I.]
Aranda I, Pardo F, Gil L, Pardos JA (2004) Anatomical basis of the change in leaf mass per area and nitrogen investment with relative irradiance within the canopy of eight temperate tree species. Acta Oecol 25(3):187–195
Bernacci LC, Martins FR, Santos FAM (2008) Estrutura de estádios ontogenéticos em população nativa da palmeira Syagrus romanzoffiana (Cham.) Glassman (Arecaceae). Acta Bot Bras 22:119–130
Bragatto J (2007) Avaliação da composição química da parede celular de plantas de tabaco (Nicotiana tabacum) que superexpressam o gene ugdh de soja, que codifica a enzima UDP-glicose desidrogenase (EC 1.1.1.22). Dissertation, Escola Superior de Agricultura Luiz de Queiroz, Piracicaba
Brendel O, Losetta PPMG, Stewart D (2000) A rapid on simple method to isolate pure alpha celulose. Phytochem Annal 17:7–10
Brüx C, David AB, Shezifi DS, Leon M, Niefind K, Shoham Y (2006) The structure of an inverting GH43 β-xylosidase from Geobacillus stearothermophilus with its substrate reveals the role of the three catalytic residues. J Mol Biol 359:97–109
Budowski A (1965) Distribution of tropical rain forest species in the light of successional progress. Turrialba 15:40–42
Carlquist S (2001) Comparative wood anatomy. Springer, Berlin
Carpita NC, Kanabus J (1987) Extraction of starch by dimethyl sulfoxide and quantitation by enzymatic assay. Anal Biochem 161(1):132–139
Carpita NC, McCann M (2000) The cell wall. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plantas. American Society of Plant Biologists, Rockville, pp 52–108
Dickison WC (2000) Integrative plant anatomy. Harcourt Academic Press, San Diego
Dos Santos WD, Ferrarese ML, Nakamura CV, Mourão KSM, Mangolin CA, Ferrarese-Filho O (2008) Soybean (Glycine max) root lignification induced by ferulic acid. The possible mode of action. J Chem Ecol 34:1230–1241
EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária (2000) Levantamento generalizado e semidetalhado de solos da Aracruz Celulose S.A. no Espírito Santo e no extremo sul do estado da Bahia e sua aplicação aos plantios de eucaliptos. Centro Nacional de Pesquisa de Solos, Ministério da Agricultura e do Abastecimento, Rio de Janeiro, Brazil
Esteban LG (2003) Madera y su anatomia. AMVE Ediciones, Madri
Fini A, Ferrini F, Frangi P, Amoroso G, Giordano C (2010) Growth, leaf gas exchange and leaf anatomy of three ornamental shrubs grown under different light intensities. Eur J Hortic Sci 75(3):111–117
Gama VN (2013) Análises morofisiológicas de plantas de pau-brasil (Caesalpinia echinata Lam.) cultivadas em pleno sol e em sombreamento natural. Dissertation, Federal University of Espírito Santo, Vitória
Gurevitch J, Scheiner SM, Fox GA (2009) Ecologia vegetal. Artmed, Porto Alegre
Hacke U, Sperry JS (2001) Functional and ecological xylem anatomy. Perspect Plant Ecol 4(2):97–115
Houter NC, Pons TL (2012) Ontogenetic changes in leaf traits of tropical rainforest trees differing in juvenile light requirement. Oecologia 169:33–45
Hunt R (1982) Plant growth curves: the functional approach to plant growth analysis. Edward Arnold Publishers, London
Ishida A, Yazaki K, Hoe AL (2005) Ontogenetic transition of leaf physiology and anatomy from seedlings to mature trees of a rain forest pioneer tree, Macaranga gigantea. Tree Physiol 25(2):513–522
Jacobsen AL, Ewers FW, Pratt RB, Paddock IIIWA, Davis SD (2005) Do xylem fibers affect vessel cavitation resistance? Plant Physiol 139:546–556
Jono V, Locosselli GM, Ceccantini G (2013) The influence of tree size and microenvironmental changes on the wood anatomy of Roupala rhombifolia. IAWA J 34(1):88–106
Kraus JE, Arduin M (1997) Manual básico de métodos em morfologia vegetal. EDUR, Seropédica
Laisk A, Eichelmann H, Oja V, Rasulov B, Padu E, Bichele I, Pettai H, Kull O (2005) Adjustment of leaf photosynthesis to shade in a natural canopy: rate parameters. Plant Cell Environ 28(3):375–388
Lambers H, Chapin IIIFS, Pons LT (2008) Plant physiological ecology. Springer, New York
Lars H, Gunnar J, Göran P, Jiebing L, Pierre L, Gunnar H (2000) Do the extracellular enzymes cellobiose dehydrogenase and manganese peroxidase form a pathway in lignin biodegradation? FEBS Lett 477(1–2):79–83
Li G, Ammermann U, Quirós CF (2001) Glucosinolate contents in maca (Lepidium peruvianum chacón) seeds, sprouts, mature plants and several derived commercial products. Econ Bot 55:255–262
Lorenzi H (2002) Árvores brasileiras, 4th edn. Instituto Plantarum, São Paulo
Lüttge U (1997) Physiological ecology of tropical plants. Springer, Berlin
Mendes MM, Gazarini LC, Rodrigues ML (2001) Acclimation of Myrtus communis to contrasting Mediterranean light environments—effects on structure and chemical composition of foliage and plant water relations. Environ Exp Bot 45:165–178
Mengarda LHG, Souza RLF, Campostrini E, Reis FO, Vendrame WA, Cuzzuol RRF (2009) Light as an indicator of ecological succession in brazilwood (Caesalpinia echinata Lam.). Braz J Plant Physiol 21:55–64
Mengarda LHG, Milanez CRD, Silva DM, Aguilar MAG, Cuzzuol GRF (2012) Morphological and physiological adjustments of brazilwood (Caesalpinia echinata Lam.) to direct solar radiation. Braz J Plant Physiol 24(3):161–172
Meschede DK, Velini ED, Carbonari CA, Moraes CP (2012) Teores de lignina e celulose em plantas de cana-de-açúcar em função da aplicação de maturadores. Planta Daninha 30(1):121–127
O’Brien TP, Feder N, Mccully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
Olson ME, Anfodillo T, Rosell JA, Petit G, Crivellaro A, Isnard S, León-Gómez C, Alvarado-Cárdenas LO, Castorena M (2014) Universal hydraulics of the flowering plants: vessel diameter scales with stem length across angiosperm lineages, habits and climates. Ecol Lett 17:988–997
Parida AK, Dias AB, Mittra B (2004) Effects of salt on growth, ion accumulation, photosynthesis and leaf anatomy of the mangrove, Bruguiera parviflora. Trees 18:167–174
Petzold K, Schwikal K, Heinze T (2006) Carboxymethyl xylan—synthesis and detailed structure characterization. Carbohydr Polym 64:292–298
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol 27:595–607
Raes J et al (2003) Genome-wide characterization of the lignifications toolbox in Arabidopsis. Plant Physiol 133(3):1051–1071
Rijkers T, Pons TL, Bongers F (2000) The effect of tree height and light availability on photosynthetic leaf traits of four neotropical species differing in shade tolerance. Funct Ecol 14:77–86
Rocha YT, Simabukuro EA (2008) Estratégias de conservação in situ e ex situ do pau-brasil. In: Figueiredo-Ribeiro RCL, Barbedo CJ, Alves ES, Domingos M, Braga MR (eds) Pau-Brasil da semente à madeira. Instituto de Botânica, São Paulo, pp 102–113
Sanches MC, Mielke MS, de Souza CSD, Vieira AJD, Lopes MMM, da Silva Júnior MB (2009) Morfologia foliar de indivíduos jovens e adultos de Caesalpinia echinata Lam. em uma floresta semidecídua do sul da Bahia. Árvore 33(5):885–893
Santiago LS, Goldstein G, Meinzer FC, Fisher JB, Machado K, Woodruff D, Jones T (2004) Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 140:543–550
Schädel C, Blöchl A, Richter A, Hoch G (2009) Shot-term dynamics of nonstructural carbohydrates and hemicelluloses in young branches of temperate forest trees during bud break. Tree Physiol 29:901–911
Schädel C, Blöchl A, Richter A, Hoch G (2010) Quantification and monosaccharide composition of hemicelluloses from different plant functional types. Plant Physiol Biochem 48:1–8
Taiz L, Zeiger E (2012) Plant physiology, 5th edn. Artmed, Massachusetts
Valladares F, Wright SJ, Lasso E, Kitajima K, Pearcy RW (2000) Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 8:1925–1936
Valladares F, Arrieta S, Aranda I, Lorenzo D, Tena D, Sánchez-Gómez D, Suarez F, Pardos JA (2005) Shade tolerance, photoinhibition sensitivity and phenotypic plasticity of Ilex aquifolium in continental-Mediterranean sites. Tree Physiol 25:1041–1052
Wendling I, Trueman SJ, Xavier A (2014) Maturation and related aspects in clonal forestry—part I: concepts, regulation and consequences of phase change. New For 45:449–471
Funding
Funding was provided by FIBRIA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zani, L.B., Macieira, B.P.B., Corte, V.B. et al. The vessel elements and hemicelluloses as the most plastic structural components of the brazilwood ontogeny (Caesalpinia echinata Lam.) medium morphotype. Braz. J. Bot 40, 793–800 (2017). https://doi.org/10.1007/s40415-017-0375-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-017-0375-2