Abstract
Background
Statins are the mainstay hypercholesterolemia treatment and reduce the risk of cardiovascular events in patients. However, statin therapy is often interrupted in patients experiencing musculoskeletal pain or myopathy, which are common in this patient group. Currently, the standard tests for diagnosing statin myopathies are difficult to interpret. A pharmacogenomics (PGx) test to diagnose statin-induced myopathy would be highly desirable.
Methods
We developed a Markov state model to assess the cost-effectiveness of a hypothetical PGx test, which aims to identify statin-induced myopathy in high-risk, secondary prevention cardiovascular patients. The alternative strategy hypothesized is that physicians or patients interrupt the statin therapy in the presence of musculoskeletal pain. Our model includes health states specific to the PGx test outcome which assesses the impact of test errors.
Results
Assuming a perfect test, the results indicate that the PGx test strategy dominates when the test costs less than CAN$356, when the strategy is cost neutral. These results are robust to deterministic and probabilistic sensitivity analyses.
Conclusion
Our base-case results show that a PGx test for statin-induced myopathy in a high-risk, secondary prevention of a cardiovascular event population would be a dominant solution for a test cost of CAN$356 or less. Furthermore, the modelling of the complete range of diagnostic test outcomes provide a broader understanding of the economic value of the pharmacogenomics test.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Physicians and pharmacists often discontinue statin therapy in patients with musculoskeletal pain. Even when physicians and pharmacists recommend alternative strategies to maintain the statin therapy, patients may decide to not follow their recommendations. This premature discontinuation results in many patients being deprived of the drug’s beneficial cardiovascular prevention. |
An accurate pharmacogenomics (PGx) test to identify musculoskeletal pain resulting from statin therapy is highly desirable. It would fulfill a need for physicians and pharmacists, but it may also be more useful as tool to convince patients to adhere and persist on statin therapy. |
The results of our simulation show that a PGx test to identify statin-induced myopathy is dominant with a test cost of less than CAN$356. Assuming a public payer willingness to pay of CAN$1000, the PGx test would be cost-effective at a test cost below CAN$906. |
1 Introduction
In Canada, 13.7 million individuals suffer from elevated cholesterol levels (i.e. hypercholesterolemia) [1]. Statins are the mainstay hypercholesterolemia treatment; reducing the risk of a cardiovascular event (CVE) by as much as 25–35% [2]. It is estimated that 3–4 million Canadians are currently prescribed a statin to reduce their cholesterol level [3]. One associated adverse effect of statin therapy is myopathy, a form of musculoskeletal pain, that may lead to the interruption of treatment.
Musculoskeletal pain consists of common symptoms, with a range of origins, from strenuous physical activity to statin-induced myopathy. Currently, statin-induced myopathy is diagnosed using creatine kinase (CK) tests, which have limited diagnostic capacity due to poor internal validity. For instance, musculoskeletal pain detected with CK values could have resulted from heavy exercise rather than statin therapy [4]. In more serious cases, rhabdomyolysis, the extreme condition in which muscle breaks down, potentially leading to severe renal damage or death, could be mistakenly attributed to statin therapy due to CK values, when the source may in fact be variable (e.g. extreme exercise or muscle stress accompanied by dehydration) [5].
In addition to insufficient internal validation of the main test for statin-induced myopathy, the general terminology used to describe muscle toxicities such as myopathy and rhabdomyolysis has been inconsistently represented in the literature. The American College of Cardiology/American Heart Association/National Heart, Lung, and Blood Institute standardized the terminology of muscle toxicity by defining myalgia, myositis, and rhabdomyolysis as statin-induced myopathies. For instance: (1) myalgia is defined as muscle symptoms, such as ache or weakness with normal CK levels; (2) myositis is defined as muscle symptoms with elevated CK levels; and (3) rhabdomyolysis is defined as muscle symptoms with CK elevation (typically >10x the upper limit normal [ULN] value) and creatinine elevation [4]. In this paper, the term statin-induced myopathy will encompass all three levels of muscle toxicity defined above.
Clinical studies have reported suspected statin-induced myopathies in 5–10% of patients [6, 7], and as high as 25% in some observational studies [7–9]. Reported rates of myopathy in clinical trials may underestimate the true incidence, because most clinical trials did not use a standard definition for statin myalgia or, in some cases, patients were screened during the run-in period to eliminate participants with statin intolerance [9]. Researchers are currently developing a pharmacogenomics (PGx) test aimed at diagnosing statin-induced myopathy.
We refer to the PGx test as an in vitro diagnostic device to identify a specific patient population (e.g. responders or patients who are susceptible to experience serious adverse events) as part of a personalized medicine strategy aiming to treat patients safely and effectively with a companion targeted therapeutic [10]. In our context, the purpose of the PGx test is to assist physicians in the interpretation of CK values in patients under statin therapy who experience musculoskeletal pain symptoms with low-to-moderate CK values (5 ≤ ULN). The PGx test would fill the unmet need of determining, among patients having musculoskeletal pain, those who suffer from statin-induced myopathy and are thus at risk of developing rhabdomyolysis. The end purpose of the PGx test is, through a negative test result, to determine which patients can maintain statin therapy and avoid further CVE. Thus, the rational for this study is to evaluate the economic value of a hypothetical PGx test to diagnose statin-induced myopathy in patients who are prescribed statin therapy.
2 Methods
2.1 Economic Evaluation
We developed a decision analytic Markov state model in TreeAge Pro software (TreeAge Software, Williamstown, MA, USA) to assess the cost-effectiveness of a hypothetical PGx test to identify statin-induced myopathy in high-risk, secondary prevention cardiovascular (CV) patients experiencing musculoskeletal pain. The model perspective is that of an average statin. The model uses data inputs from previously published studies and public sources (see Tables 1, 2, 3). The model was developed as a Markov cohort with one single patient for each strategy using a 1-month cycle with a time horizon of 20 years. All costs were adjusted to 2014 CAN$. The perspective of the model is that of a public payer in Canada.
2.1.1 PGx Test
Studies suggest that the risk of statin myopathy could be managed when the SLCO1B1 genotype is available especially for patients being prescribed a high-dose simvastatin [11, 12]. However, with the analysis of the data from the SEARCH genome-wide association study, Stewart [13] concluded that there was no direct evidence for the clinical utility of statin prescriptions guided by the SLCO1B1 genotype. In practice, physicians rely on the CK test when diagnosing statin-induced myopathy [11]. To date, no PGx test for statin-induced myopathy exists. However, researchers are developing a PGx test based on blood-based biomarkers identified in a genome-wide genotyping study, for statin-induced myopathy in patients with moderate or no CK elevation (≤5x ULN). The PGx test integrates both personalized CK reference values and a lipidomic biomarker. Therefore, there are no PGx test performance parameters currently published, or available. To address this, we used the false-negative rate (FNR)Footnote 1 and false-positive rate (FPR)Footnote 2 when reporting the model results. The FNR is the proportion of test results in the presence of statin-induced myopathy that would falsely indicate the absence of statin-induced myopathy (false-negative test result). Similarly, the FPR is the proportion of test results in the absence of statin-induced myopathy that would falsely indicate the presence of statin-induced myopathy (false-positive test result). For the base-case scenario, we assume that the PGx test is a perfect test; specifically, that the PGx FNR and FPR are zero. In scenario analyses, we investigate the complete range of possible test performance from 0 to 100% of FNR and FPR. This includes scenarios where the PGx text is subject to misclassification, and assesses the impact of misclassification on the economic evaluation of the PGx strategy.
Furthermore, we assumed that the treating physician will not require a PGx test for patients presenting with rhabdomyolysis. Patients who present with rhabdomyolysis progressed to the true-positive states and discontinue their statin therapy. We assumed that patients experiencing a CVE will return to a statin therapy regardless of the previous PGx test results. The rationale is that high-risk, secondary prevention CV patients will have a greater fear of CVE recurrences than rhabdomyolysis, which has a very low incidence rate (1 per 10,000 person-years) [6, 14] compared to the recurrence of a major CVE (1-year probability of 0.06 major CVE following a myocardial infarction and 0.10 following a stroke) [15].
2.1.2 Model Structure
The model target population are high-risk, secondary prevention, CV patients initiating a statin. The model comprised two alternative strategies, with and without a PGx test to diagnose statin-induced myopathies. The physician diagnosis of statin-induced myopathies, in patients with musculoskeletal pain, will determine whether they continue or discontinue the statin therapy. Without a PGx test, we assumed that when patients experience musculoskeletal pain, their physician permanently interrupts the statin therapy for fear of the patient developing rhabdomyolysis. With a PGx test, only patients experiencing musculoskeletal pain are being tested; thus, public payers only incur the PGx test cost for these patients. We assume that patients and physicians are fully compliant to the PGx test results. That is, physicians will recommend either continuing or permanently discontinuing the statin therapy based on the PGx test results and patients will fully adhere to their physician recommendations. Patients who do not experience any musculoskeletal pain are maintained on statin with perfect adherence.
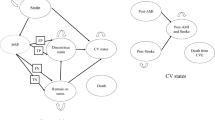
Figure 1 shows the Markov state model structure. The model has one initial statin state; one transitory state, musculoskeletal pain (MSP); two discontinue-statin states (true- and false-positive); two remain-on-statin states (true- and false-negative); four CV states (post-AMI, post-stroke, “post-AMI and stroke”, and death from CV); and background death. Background death can occur from any states including the CV states whereas CV event death can only occur from any of the CV states.
Patients enter the model upon initiating a statin in secondary prevention. Patients may have a CVE, in which case they may transition to one the three CV states, or remain in the statin state. Patients who experienced both stroke and AMI, progress to the “post-AMI and -stroke” state. Only patients experiencing musculoskeletal pain go through the screening process, which is represented by the transitory state MSP. In MSP, patients are redirected to discontinue-statin states for true- and false-positive or remain-on-statin states for true- and false-negative states. Essentially, these four paths differ in terms of treatment (discontinue or remain on the statin therapy) and whether the musculoskeletal pain is a result of statin-induced myopathy. With the PGx strategy, patients will be redirected to these four paths based on the assumed test parameters. Under a perfect PGx test, patients will either move to the true-positive, discontinue-statin for patients with statin-induced myopathy, and all other patients will progress to the true-negative, remain-on-statin states. Without a PGx strategy, patients will progress to discontinue-statin states; when patients have statin-induced myopathy they progress to the true-positive, discontinue-statin state; all others progress to the false-positive, discontinue-statin state.
2.1.3 Transition Probabilities, Hazard Ratio, Relative Risk and Rates
Table 1 presents the monthly transition probabilities, hazard ratio, relative risk, and rates for the base-case scenario; the values used in the deterministic, and probabilistic sensitivity analyses, and the assumed distribution used in the probabilistic sensitivity analysis. The model values were varied with a ±25% for low and high values when the deterministic sensitivity analyses boundaries were not provided in the literature.
We assume that the 5-year major CVE probability is 50% and that major CV recurrent events have a 2-year probability of 30%. Statins protection is captured with the relative risk reduction from major CVE in Pedersen et al. [16].
The model mortality is derived from the digitized overall survival (OS) reported in the Scandinavian Simvastatin Survival Study (4S), Pedersen et al. [16]; using the DigitizeIt software (DigitizeIt, Germany), and Statistics Canada published life tables [17]. The 4S OS curve is applied to patients treated with a statin, whereas the placebo OS is applied to patients who discontinued statin therapy. The monthly and annual probabilities of deaths following a stroke or an AMI were assumed to be equal. Indeed, a study from Law et al. [18] showed that 85% of patients dying within the first year following an AMI, died either before hospital admission or during the hospital admission.
A Gamma function was used to simulate the timing of musculoskeletal pain. The function was calibrated to achieve a 3-year musculoskeletal pain probability of 40%. The whole curve was moved by ±25% in the deterministic sensitivity analysis. We assumed that 25% of patients presenting with musculoskeletal pain had statin-induced myopathy.
2.1.4 Costs
The Canadian cost data presented in Table 2 were obtained from previously published costs studies, cost-effectiveness studies, and governmental public sources. The cost data were inflated to 2014 CAN$ using the all-components consumer price index table from Statistics Canada [19]. The low and high scenarios are set, respectively, to 75 and 200% to account for the skewness observed in health-care costs’ data. For physician visits, the low and high values are based on the minimal and maximal values for a physician visit from the RAMQ physician’s code book [20]. The statin cost is based on the average cost of a 30-day statin prescription list price in Québec, with ±25% for the high and low values [21].
2.1.5 Health Utilities
Table 3 presents the health state utility values used in the model for the base case, the deterministic sensitivity analysis, and the distribution used in the probabilistic sensitivity analysis. For asymptomatic elderly, post-AMI events, post-stroke events, and expected disutility for myopathy, the health-utility values used in the model are converted to monthly utility values. However, for CVE disutility of major events (i.e. AMI, stroke, and rhabdomyolysis) we assumed that the total disutility is incurred within the cycle where the event occurs in the model. We assumed that the disutility value of myopathy is similar to that of going from mild to moderate fibromyalgia [22]. For rhabdomyolysis, we assumed that the disutility is equivalent to that of a stroke. The deterministic sensitivity analysis low and high values for asymptomatic elderly, post-AMI, and post-stroke patients are based on data from van Kempen et al. [23], whereas the values for disutilities are set to ±25%.
2.2 Base-Case Analysis
In the base-case analysis with a PGx test, we assume a “perfect world” which is defined as: (1) the PGx test is perfect (FNR = 0% and FPR = 0%); (2) physicians will require PGx tests for all high-risk secondary prevention CV patients on statin therapy presenting with musculoskeletal pain, and will recommend to either continue or interrupt statins based on the PGx test results; and (3) patients will adhere to their physician recommendations regardless of whether or not they still suffer musculoskeletal pain.
For the strategy without a PGx test, we assume that physicians, and patients, are risk-averse in the presence of musculoskeletal pain, and interrupt the statin therapy in fear of rhabdomyolysis. This situation is equivalent to that of a PGx test with FNR = 0% and FPR = 100%. This would also be the case when patients ignore physicians’ recommendations to try alternative statin treatment patterns (e.g. switch molecules, dose reduction, stop and re-challenge, etc.).
2.3 Sensitivity Analysis
We carried out sensitivity analyses to assess the model parameters uncertainty. Deterministic sensitivity analysis and probabilistic sensitivity analysis parameter values are specified in Tables 1, 2, and 3. The results of the deterministic sensitivity analysis are presented in a tornado diagram while probabilistic sensitivity analysis results are summarized in a cost-effectiveness acceptability curve (CEAC).
2.4 Scenario Analysis
In scenario analyses, we allow the FNR and FPR parameters to vary from 0 to 100%, therefore allowing the analysis of the model sensitivity to the full extent of PGx test parameter values. The purpose of this analysis is to determine the economically acceptable range of PGx test parameter combinations. The scenario analysis is an important aspect of the economic evaluation for three reasons. First, the model evaluates a hypothetical situation, thus we do not know the “real-life” test parameters. Second, evaluating the complete range of test parameters provides comprehensive picture for public payers. Third, if the economic evaluation is done sufficiently early, it allows test developers to understand the optimal combination of test parameters from an economic perspective.
3 Results
3.1 Base-Case Analysis
The base-case results are presented in Table 4. The results indicate that the “with PGx test” strategy dominates “without PGx test” strategy when the PGx test costs less than CAN$250. In fact, the “with PGx” test strategy remains the dominant strategy as long as the PGx test costs less than CAN$356, where the strategy is cost neutral. At a willingness to pay (WTP) of CAN$1000, our results show that the “with PGx” strategy would be cost effective as long as the test costs less than CAN$906.
3.2 Sensitivity Analyses
In order to assess the robustness of the model base incremental cost-effectiveness ratio (ICER) of −CAN$194, we performed deterministic sensitivity analyses (Fig. 2). The three most important factors are the CVE risk reduction from statins, the cost of AMI, and the cost of statins. The range of ICERs obtained varies from −CAN$2835 to CAN$4321 per QALY. The maximal ICER value in the deterministic sensitivity analysis (CAN$4321 per QALY) is obtained with the high parameter value of the CV relative risk-reduction of 0.825, which was set to ±0.25%. The maximal ICER obtained is well below all accepted WTP thresholds.
Tornado diagram comparing the strategy “with PGx test” to “without PGx test”. The diagram shows 15 scenario variations. The most important factors are the risk reduction of CVE from statin, followed by the cost of AMI events, and the cost of statins. Although the unit cost of the PGx test, the sensitivity, and the specificity appear in the figure, their ranks are, respectively, 14, 15, and 23 among all parameters varied. AMI acute myocardial infarction, CV cardiovascular, CVE cardiovascular event, PGx pharmacogenomics
Figure 3 shows the CEAC comparing the two strategies. We ran 10,000 simulations for the probabilistic sensitivity analysis. The probabilistic sensitivity analysis model simulations favor the “without PGx test” when the payer’s WTP is below CAN$750 per QALY. With a WTP of CAN$0 per QALY, the model shows that the “with PGx test” strategy is favored by 43% of the model simulations. When the payer’s WTP exceeds CAN$750 per QALY, over 50% of the model simulations favor the “with PGx test” strategy and this number reaches 90% when the payer’s WTP = CAN$6,150 per QALY.
Cost-effectiveness acceptability curve comparing the management of statin-induced myopathy with and without a PGx test. The curves show the percentage of simulations that favor one strategy over the other. The curves crossover when payer’s WTP is CAN$750 per QALY. When the payer’s WTP reaches CAN$6150 per QALY, 90% of the model simulations favor the strategy “with PGx test”. PGx pharmacogenomics, QALY quality-adjusted life year, WTP willingness to pay
3.3 Scenario Analyses
Because of the uncertain sensitivity of a future PGx test for statin-induced myopathy, we investigated the whole range of possible values of FNR and FPR. Figure 4 shows the matrix of results for the scenario analyses. The top left corner corresponds to the “perfect test” (FNR = FPR = 0%), and the bottom right corner represents the “worst test” (FNR = FPR = 100%). The combination of FNR and FPR parameter values where the “with PGx test” dominates is represented the by the light grey region (FNR = 80% and FPR = 0%) and (FNR = 0% and FPR = 20%). Thus, we can argue that a PGx would be a dominant strategy for all practical purpose as for a diagnostic test to be considered a valid tool would require minimal misclassification (i.e. FNR and FPR below 20%).
Matrix of ICER results when varying the PGx test FPR and FNR from 0 to 100%. The perfect PGx test is located at the top left corner of the matrix “Perfect Test” (FPR and FNR are 0%) while the “Worst Test” is located at the bottom right corner (FPR and FNR are 100%). The light shaded region shows the combination of test parameters yielding a dominant a PGx strategy. The white cells indicate the region where the PGx test is cost effective (i.e. ICER well below accepted WTP threshold). The black cell indicates the assumed strategy “without PGx test” and thus cannot be evaluated because both strategies yield exactly the same QALYs. In fact, in that situation, the “without PGx test” dominates because with PGx test is systematically more expensive and yields the same level of QALY. FNR false-negative rate, FPR false-positive rate, ICER incremental cost-effectiveness ratio, PGx pharmacogenomics, QALY quality-adjusted life year, WTP willingness to pay
The results in Fig. 4 show that even for a PGx test that would totally misclassify patients (i.e. FNR = FPR = 100%), the ICER is very low, CAN$5987 per QALY. To understand this result, we need to consider the PGx test performance compared to the hypothesized alternative. First, in the “without PGx test”, every patient presenting with MSP will interrupt the statin therapy; hence, patients without statin-induced myopathy will be misclassified (i.e. false-positive). However, with the worst test possible, both patients with and without statin-induced myopathy are misclassified (i.e. false-negative and false-positive). Therefore, the difference between the two scenarios are the patients with statin-induced myopathy. In the “without PGx test”, these patients are properly classified, the statin therapy is interrupted, but they are at greater risk of a CVE. However, in the “with PGx test”, these patients are misclassified as not having statin-induced myopathy; henceforth, the statin therapy is maintained regardless of whether patients still experience MSP. Although, patients’ quality-of-life is penalized with myopathy-associated disutility, these patients benefit from the prevention of future CVE, which counterbalances the misclassification. For these reasons, the ICER of a PGx test that would totally misclassify patients does not increase to an extreme value.
To assess the impact of FPR and FPR on the maximal value of a PGx test, we have analyzed the change in the maximal price value of the PGx test when the payer’s WTP is CAN$1000 per QALY. Table 5 presents the results of this analysis. The results show that 10% change in FNR reduces the maximal PGx price by less than 1% whereas a change in 10% of FPR reduces the maximal PGx price by 10%.
4 Discussion
SCLO1B1 genotyping has been proposed for managing the risk of statin-induced myopathy, especially in patients using a high-dose of simvastatin [11, 12] whereas the purpose of the PGx test in development is to assist physicians and pharmacists to diagnose statin-induced myopathy in patients with moderate or no CK elevation (5 ≤ ULN).
We found that the “with PGx test” strategy to confirm statin-induced myopathies dominates the “without PGx test” strategy in our hypothesized framework where the test costs up to CAN$356. In scenario analyses, we found that for a PGx test cost of CAN$250, the strategy “with PGx test” dominates the “without PGx test” for many FNR and FPR combinations. To our knowledge, there are no previously published papers on the economic value of a PGx test of statin-induced myopathy.
4.1 PGx False-Negative and False-Positive
When evaluating the economic value of a PGx test it is important to model the PGx test parameters. In our model, the scenario analyses show that false-negative and false-positive PGx test results have different impacts on the economic value of the test. To appreciate that point, we need to understand the consequences of a PGx test misclassification. Patients with false-negative test results continue their statin therapy, even though they suffer from statin-induced myopathy. Thus, they suffer from the discomfort, and sometimes danger, of muscle toxicities that we account for with statin-induced myopathy disutility. These patients are at risk of rhabdomyolysis, which can lead to very severe and costly outcomes; however, rhabdomyolysis is a very rare event. Rallidis et al. [6] reported the rate of rhabdomyolysis of 3.2 per 100,000 person-years but most studies report a rate of rhabdomyolysis around 10 per 100,000 person-years [14, 24]. The rate of rhabdomyolysis development is important, because these patients continue their statin therapy, they benefit from the prevention of CVEs, which is less costly than a hospitalized rhabdomyolysis, but also much more likely to occur. In the case of false-positive PGx test results, patients’ myopathy is not related to statin therapy, and these patients are mistakenly interrupting their statin therapy. The consequences are that these patients are no longer benefiting from the protection of statin therapy, which leads to an increase in CVEs with the increased costs, and reduced QALYs, associated with these events. Thus, because of the CVE protection associated with a false-negative test results, it turns out that an increase in FNR has a limited impact compared to an increase in FPR.
These patients will no longer benefit from the statin protection of CVE. For payers, patients inadequately interrupting their statin therapy may represent an economic loss. As explained by Cardinal et al. [25], in preventive health strategies, patients who interrupt their treatment before they incur any benefit represent a resource inefficiency, which they refer to as the concept of “percent wasted patients”. Indeed, as can be seen in the study from Pedersen et al. [16], the statin benefit materializes after 1.5 years of statin treatment when compared to placebo.
Furthermore, the development of an accurate PGx test would be a useful tool for physicians and pharmacists to help maintain patients on continuous statin therapy. Many studies highlighted the poor adherence and persistence to statin therapy. In a claims database, Catalan and LeLorier [26] showed that in a cohort of patients initiating a statin only 33% were still adherent after 1 year. Dorais et al. [27], reported that, among 19,727 patients initiating a statin, 53.3% had discontinued treatment after 1 year. Wouters et al. [28] explored the many reasons for statin non-adherence. Their study results show that among 229 patients, 40–70% doubted the necessity and lacked the knowledge about the statin efficacy, while 20–35% were worried about joint and muscle side effects [28].
4.2 Strength
The model design was not limited by the lack of “real-world” PGx test parameters. We developed the base-case model with a perfect PGx test environment; however, by including the complete range of FNR and FPR in scenario analysis, we gave the model enough flexibility to analyze an imperfect test environment. The concept of an imperfect test encompasses not only test errors, but also non-adherence to test results by physicians and/or patients. Indeed, when physicians or patients do not adhere to the test results, it is comparable to a test misclassification. Our model assesses the impact of FNR and FPR on the economic value of the PGx test. The model shows that FNR and FPR may affect the economic value of the PGx test differently. Evaluating the complete range of test parameters provides essential information to payers on the optimal test parameters.
4.3 Limitations
There is uncertainty surrounding the incidence of statin-induced severe rhabdomyolysis and its associated disutility. An increase in the rate of severe rhabdomyolysis would increase the value of the PGx test. The results we obtained are not generalizable to all patients under statin therapy.
The strategy “without PGx test” may be seen as limiting as we assumed that all physicians and pharmacists will recommend discontinuing statin therapy when patients suffer from musculoskeletal pain. Regardless of their physician or pharmacist recommendation, it is likely that patients will discontinue the drug as adherence and persistence issues with statins which will lead to an identical outcome [26–28]. Although long-term persistence issues are not addressed in the model, we argue that in the context of this model it is not as limiting as it first appears. The reason is that without myopathy, patients will be treated identically in both model strategies. Therefore, this would have no impact on the incremental costs or incremental QALYs
5 Conclusion
Our base-case results show that a PGx test for statin-induced myopathy in a high-risk secondary prevention of CVE population would be a dominant solution for a test cost of CAN$356 or less. Deterministic and probabilistic sensitivity analyses show that a PGx test for statin-induced myopathy is a cost-effective solution for all accepted WTP thresholds. Including the full range of possible PGx test parameters in an economic evaluation is an important aspect when assessing the economic value of PGx tests.
Notes
The false-negative rate can be expressed as 1-sensitivity.
The false-positive rate can be expressed as 1-specificity.
References
Statistics Canada, Cholesterol levels of Canadians, 2009 to 2011. 2012. http://www.statcan.gc.ca/pub/82-625-x/2012001/article/11732-eng.htm. Accessed 2013 06 08.
Anderson TJ, Gregoire J, Hegele RA, Couture P, Mancini GB, McPherson R, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29(2):151–67. doi:10.1016/j.cjca.2012.11.032.
Adriana B. Are we overdosing on this ‘wonder drug’? The Globe and Mail. 2012. http://www.theglobeandmail.com/life/health-and-fitness/health/conditions/are-we-overdosing-on-this-wonder-drug/article4101464/. Accessed 29 janvier 2014.
Pasternak RC, Smith SC Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40(3):567–72.
Chai C, James H. Working out ’til you’re sick? Doctors warn of rhabdo, a deadly condition linked to over exercising. Global news, Canada, Montreal. 2014. http://globalnews.ca/news/1082282/doctors-warn-of-rhabdo-a-deadly-condition-linked-to-over-exercising/. Accessed 23 Jan 2016.
Rallidis LS, Fountoulaki K, Anastasiou-Nana M. Managing the underestimated risk of statin-associated myopathy. Int J Cardiol. 2012;159(3):169–76. doi:10.1016/j.ijcard.2011.07.048.
Mampuya WM, Frid D, Rocco M, Huang J, Brennan DM, Hazen SL, et al. Treatment strategies in patients with statin intolerance: the Cleveland clinic experience. Am Heart J. 2013;166(3):597–603. doi:10.1016/j.ahj.2013.06.004.
Abd TT, Jacobson TA. Statin-induced myopathy: a review and update. Expert Opin Drug Saf. 2011;10(3):373–87. doi:10.1517/14740338.2011.540568.
Ganga HV, Slim HB, Thompson PD. A systematic review of statin-induced muscle problems in clinical trials. Am Heart J. 2014;168(1):6–15. doi:10.1016/j.ahj.2014.03.019.
U.S. Food and Drug Administration. Guidance for industry: in vitro companion diagnostic devices. In: U.S. Department of Health and Human Services, editor. Washington, DC2014.
Wilke RA, Ramsey LB, Johnson SG, Maxwell WD, McLeod HL, Voora D, et al. The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin Pharmacol Ther. 2012;92(1):112–7. doi:10.1038/clpt.2012.57.
Kitzmiller JP, Binkley PF, Pandey SR, Suhy AM, Baldassarre D, Hartmann K. Statin pharmacogenomics: pursuing biomarkers for predicting clinical outcomes. Discov Med. 2013;16(86):45–51.
Stewart A. SLCO1B1 polymorphisms and statin-induced myopathy. PLoS Curr. 2013;5:ecurrents.eogt.d21e7f0c58463571bb0d9d3a19b82203. doi:10.1371/currents.eogt.d21e7f0c58463571bb0d9d3a19b82203.
Erickson KF, Japa S, Owens DK, Chertow GM, Garber AM, Goldhaber-Fiebert JD. Cost-effectiveness of statins for primary cardiovascular prevention in chronic kidney disease. J Am Coll Cardiol. 2013;61(12):1250–8. doi:10.1016/j.jacc.2012.12.034.
Wagner M, Goetghebeur M, Merikle E, Pandya A, Chu P, Taylor DC. Cost-effectiveness of intensive lipid lowering therapy with 80 mg of atorvastatin, versus 10 mg of atorvastatin, for secondary prevention of cardiovascular disease in Canada. Can J Clin Pharmacol. 2009;16(2):e331–45.
Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. 2004;5(3):81–7. doi:10.1016/j.atherosclerosissup.2004.08.027.
Statistique Canada. Tables de mortalité, Canada, provinces et territoires, 2009 à 2011. No 84-537-X au catalogue—No 005 ISSN: 1910-3492. Ottawa, 2013.
Law MR, Watt HC, Wald NJ. The underlying risk of death after myocardial infarction in the absence of treatment. Arch Intern Med. 2002;162(21):2405–10.
Statistique Canada. Indice des prix à la consommation, aperçu historique (1994 à 2014). Tableau 326-0021. Ottawa, 2015.
Régie de l’assurance-médicament du Québec. Manuel des médecins omnipraticiens. Quebec City. 2014. http://www.ramq.gouv.qc.ca/SiteCollectionDocuments/professionnels/manuels/100-facturation-omnipraticiens/maj79_acte_omni.pdf. Accessed 10 April 2014.
Régie de l’assurance-médicament du Québec. Liste des médicaments. Quebec City. 2014. https://www.prod.ramq.gouv.qc.ca/DPI/PO/Commun/PDF/Liste_Med/Liste_Med/liste_med_2014_03_14_fr.pdf. Accessed 10 April 2014.
Hauber AB, Garcia-Cebrian A, Kim Le T, Maas G, Das Gupta R. Population Health-State Utilities for Fibromyalgia in the United Kingdom ISPOR 11th Annual European Meeting; Madrid, 2008.
van Kempen BJ, Spronk S, Koller MT, Elias-Smale SE, Fleischmann KE, Ikram MA, et al. Comparative effectiveness and cost-effectiveness of computed tomography screening for coronary artery calcium in asymptomatic individuals. J Am Coll Cardiol. 2011;58(16):1690–701. doi:10.1016/j.jacc.2011.05.056.
Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292(21):2585–90. doi:10.1001/jama.292.21.2585.
Cardinal H, Monfared AA, Dorais M, Lelorier J. The concept of the ‘percent wasted patients’ in preventive health strategies. Pharmacoepidemiol Drug Saf. 2006;15(1):57–61. doi:10.1002/pds.1148.
Catalan VS, LeLorier J. Predictors of long-term persistence on statins in a subsidized clinical population. Value Health. 2000;3(6):417–26. doi:10.1046/j.1524-4733.2000.36006.x.
Dorais M, Chirovsky D, Ambegaonkar B, Sazonov V, Davies G, Grant S, et al. Utilization patterns of extended-release niacin in Canada: analysis of an administrative claims database. Can J Cardiol. 2010;26(7):e229–35.
Wouters H, Van Dijk L, Geers HCJ, Winters NA, Van Geffen ECG, Stiggelbout AM, et al. Understanding statin non-adherence: knowing which perceptions and experiences matter to different patients. PLoS One. 2016;11(1):e0146272. doi:10.1371/journal.pone.0146272.
Higgins JP, Whitehead A. Borrowing strength from external trials in a meta-analysis. Stat Med. 1996;15(24):2733–49. doi:10.1002/(SICI)1097-0258(19961230)15:24<2733:AID-SIM562>3.0.CO;2-0.
Smolderen KG, Bell A, Lei Y, Cohen EA, Steg PG, Bhatt DL, et al. One-year costs associated with cardiovascular disease in Canada: insights from the REduction of Atherothrombosis for Continued Health (REACH) registry. Can J Cardiol. 2010;26(8):297–305.
Conly J, Clement F, Tonelli M, Hemmelgarn B, Klarenbach S, Lloyd A et al. Cost-effectiveness of the use of low- and high-potency statins in people at low cardiovascular risk. CMAJ. 2011;183(16):E1180–E1188. Date of Publication: 08 Nov 2011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by Genome Canada and Genome Québec. Jason R. Guertin is the recipient of a Pfizer Canada Inc. Post-Doctoral Mentoree Award, a Fonds de recherche du Québec - Santé Postdoctoral Award and of the 2015–2016 Bernie O’Brien Post-Doctoral Fellowship Award. The open access funding was provided by Genome Canada and Genome Québec.
Conflict of interest
Dominic Mitchell, Jason R. Guertin, Ange Christelle Iliza, Fiorella Fanton-Aita, and Jacques LeLorier declare that they have no conflict of interest that are directly relevant to the content of this manuscript.
Author contributions
DM contributed to the conception and design of the study, data acquisition, analysis and interpretation of data, drafting the article, and final approval. JRG, ACI, FF-A, and JL, contributed to the conception and design of the study analysis and interpretation of data, drafting the article, and final approval.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mitchell, D., Guertin, J.R., Iliza, A.C. et al. Economic Evaluation of a Pharmacogenomics Test for Statin-Induced Myopathy in Cardiovascular High-Risk Patients Initiating a Statin. Mol Diagn Ther 21, 95–105 (2017). https://doi.org/10.1007/s40291-016-0238-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-016-0238-8