Abstract
Background
Statin (HMG-CoA reductase inhibitor) therapy is the mainstay dyslipidemia treatment and reduces the risk of a cardiovascular (CV) event (CVE) by up to 35%. However, adherence to statin therapy is poor. One reason patients discontinue statin therapy is musculoskeletal pain and the associated risk of rhabdomyolysis. Research is ongoing to develop a pharmacogenomics (PGx) test for statin-induced myopathy as an alternative to the current diagnosis method, which relies on creatine kinase levels. The potential economic value of a PGx test for statin-induced myopathy is unknown.
Methods
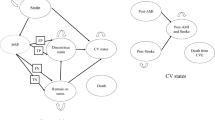
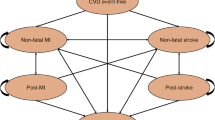
We developed a lifetime discrete event simulation (DES) model for patients 65 years of age initiating a statin after a first CVE consisting of either an acute myocardial infarction (AMI) or a stroke. The model evaluates the potential economic value of a hypothetical PGx test for diagnosing statin-induced myopathy. We have assessed the model over the spectrum of test sensitivity and specificity parameters.
Results
Our model showed that a strategy with a perfect PGx test had an incremental cost-utility ratio of 4273 Canadian dollars ($Can) per quality-adjusted life year (QALY). The probabilistic sensitivity analysis shows that when the payer willingness-to-pay per QALY reaches $Can12,000, the PGx strategy is favored in 90% of the model simulations.
Conclusion
We found that a strategy favoring patients staying on statin therapy is cost effective even if patients maintained on statin are at risk of rhabdomyolysis. Our results are explained by the fact that statins are highly effective in reducing the CV risk in patients at high CV risk, and this benefit largely outweighs the risk of rhabdomyolysis.




Similar content being viewed by others
Notes
In the Canadian Health Measures Survey, dyslipidemia was defined as having unhealthy blood concentrations of low-density lipoprotein cholesterol (LDL-C) (≥ 3.5 mmol/L), a total cholesterol (TC):high-density lipoprotein cholesterol (HDL-C) ratio ≥ 5.0, or self-reported use of a lipid-modifying medication.
References
Statistics Canada. Cholesterol levels of adults, 2012 to 2013. Statistics Canada, Ottawa, Canada. http://www.statcan.gc.ca/pub/82-625-x/2014001/article/14122-eng.htm. Accessed 6 Oct 2017.
Anderson TJ, Gregoire J, Hegele RA, Couture P, Mancini GB, McPherson R, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29(2):151–67. https://doi.org/10.1016/j.cjca.2012.11.032.
Morgan S, Smolina K, Mooney D, Raymond C, Bowen ML, Gorczynski C, et al. The Canadian Rx atlas, 3rd edition (The Canadian prescription drug atlas). Vancouver: Centre for Health Services and Policy Research; 2013.
Talameh JA, Kitzmiller JP. Pharmacogenetics of statin-induced myopathy: a focused review of the clinical translation of pharmacokinetic genetic variants. J Pharmacogenom Pharmacoproteomics. 2014;5(2):128. https://doi.org/10.4172/2153-0645.1000128.
Pasternak RC, Smith SC Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40(3):567–72.
Di Stasi SL, MacLeod TD, Winters JD, Binder-Macleod SA. Effects of statins on skeletal muscle: a perspective for physical therapists. Phys Ther. 2010;90(10):1530–42. https://doi.org/10.2522/ptj.20090251.
Rallidis LS, Fountoulaki K, Anastasiou-Nana M. Managing the underestimated risk of statin-associated myopathy. Int J Cardiol. 2012;159(3):169–76. https://doi.org/10.1016/j.ijcard.2011.07.048.
Mampuya WM, Frid D, Rocco M, Huang J, Brennan DM, Hazen SL, et al. Treatment strategies in patients with statin intolerance: the Cleveland Clinic experience. Am Heart J. 2013;166(3):597–603. https://doi.org/10.1016/j.ahj.2013.06.004.
Abd TT, Jacobson TA. Statin-induced myopathy: a review and update. Expert Opin Drug Saf. 2011;10(3):373–87. https://doi.org/10.1517/14740338.2011.540568.
Ganga HV, Slim HB, Thompson PD. A systematic review of statin-induced muscle problems in clinical trials. Am Heart J. 2014;168(1):6–15. https://doi.org/10.1016/j.ahj.2014.03.019.
Keltz E, Khan FY, Mann G. Rhabdomyolysis. The role of diagnostic and prognostic factors. Muscles Ligaments Tendons J. 2013;3(4):303–12.
Chai C, James H. Working out ’til you’re sick? Doctors warn of rhabdo, a deadly condition linked to over exercising. Global news, Canada, Montreal. 2014. http://globalnews.ca/news/1082282/doctors-warn-of-rhabdo-a-deadly-condition-linked-to-over-exercising/. Accessed 22 Nov 2017.
Mancini GBJ, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Consensus Working Group Update (2016). Can J Cardiol. 2016;32(7):S35–65. https://doi.org/10.1016/j.cjca.2016.01.003.
Swankhuizen M, Regier L. Statin intolerance ‐ management considerations. RxFiles, Saskatoon, Canada. 2013. http://www.rxfiles.ca/rxfiles/uploads/documents/Lipid-Statin-Intolerance.pdf. Accessed 24 Oct 2017.
Hovingh GK, Gandra SR, McKendrick J, Dent R, Wieffer H, Catapano AL, et al. Identification and management of patients with statin-associated symptoms in clinical practice: a clinician survey. Atherosclerosis. 2016;245:111–7. https://doi.org/10.1016/j.atherosclerosis.2015.12.015.
Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, Dawes M, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32(11):1263–82. https://doi.org/10.1016/j.cjca.2016.07.510.
Jansen ME, Rigter T, Rodenburg W, Fleur TMC, Houwink EJF, Weda M, et al. Review of the reported measures of clinical validity and clinical utility as arguments for the implementation of pharmacogenetic testing: a case study of statin-induced muscle toxicity. Front Pharmacol. 2017;8:555. https://doi.org/10.3389/fphar.2017.00555.
Pharmacogenomics Research Network. Pharmacogenomics of Statin Therapy Center. 2017. http://www.pgrn.org/pgx-of-statin-therapy.html. Accessed 9 Oct 2017.
Canada G. Personalized medicine strategies for molecular diagnostics and targeted therapeutics of cardiovascular disease. Ottawa: Génome Canada; 2018. https://www.genomecanada.ca/en/personalized-medicine-strategies-molecular-diagnostics-and-targeted-therapeutics-cardiovascular. Accessed 17 Jan 2018.
Québec G. Personalized medicine strategies for molecular diagnostics and targeted therapeutics of cardiovascular disease. Génome Québec, Montreal. 2018. http://www.genomequebec.com/159-en/project/personalized-medicine-strategies-for-molecular-diagnostics-and-targeted-therapeutics-of-cardiovascular-diseases.html. Accessed 17 Jan 2018.
Mitchell D, Guertin JR, Iliza AC, Fanton-Aita F, LeLorier J. Economic evaluation of a pharmacogenomics test for statin-induced myopathy in cardiovascular high-risk patients initiating a statin. Mol Diagn Ther. 2017;21(1):95–105. https://doi.org/10.1007/s40291-016-0238-8.
Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. 2004;5(3):81–7. https://doi.org/10.1016/j.atherosclerosissup.2004.08.027.
Caro JJ, Möller J, Karnon J, Stahl J, Ishak J. Discrete event simulation for health technology assessment. Boca Raton: CRC Press; 2016.
Standfield L, Comans T, Scuffham P. Markov modeling and discrete event simulation in health care: a systematic comparison. Int J Technol Assess Health Care. 2014;30(2):165–72. https://doi.org/10.1017/s0266462314000117.
Ambapkar SN, Shetty N, Dwivedy A, Malve HO. Statin-induced rhabdomyolysis in patient with renal failure and underlying undiagnosed hypothyroidism. Indian J Crit Care Med. 2016;20(5):305–7. https://doi.org/10.4103/0972-5229.182210.
Smolina K, Wright FL, Rayner M, Goldacre MJ. Long-term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5(4):532–40. https://doi.org/10.1161/circoutcomes.111.964700.
Asanin MR, Vasiljevic ZM, Matic MD, Mrdovic IB, Perunicic JP, Matic DP, et al. The long-term risk of stroke in patients with acute myocardial infarction complicated with new-onset atrial fibrillation. Clin Cardiol. 2009;32(8):467–70. https://doi.org/10.1002/clc.20603.
Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MS. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology. 2006;66(5):641–6. https://doi.org/10.1212/01.wnl.0000201253.93811.f6.
Smolina K, Wright FL, Rayner M, Goldacre MJ. Incidence and 30-day case fatality for acute myocardial infarction in England in 2010: national-linked database study. Eur J Public Health. 2012;22(6):848–53. https://doi.org/10.1093/eurpub/ckr196.
Canadian Institute for Health Information. 30-Day stroke in-hospital mortality. In: Health Indicators Interactive Tool. Ottawa: Canadian Institute for Health Information. 2017. http://indicatorlibrary.cihi.ca/display/HSPIL/30-Day+Stroke+In-Hospital+Mortality. Accessed 15 Sep 2017.
Jones SB, Sen S, Lakshminarayan K, Rosamond WD. Poststroke outcomes vary by pathogenic stroke subtype in the Atherosclerosis Risk in Communities Study. Stroke. 2013;44(8):2307–10. https://doi.org/10.1161/strokeaha.113.000830.
Aarnio K, Haapaniemi E, Melkas S, Kaste M, Tatlisumak T, Putaala J. Long-term mortality after first-ever and recurrent stroke in young adults. Stroke. 2014;45(9):2670–6. https://doi.org/10.1161/strokeaha.114.005648.
Statistics Canada. Life tables, Canada, provinces and territories—2010 to 2012. Ottawa (Ontario); Statistics Canada; 2016. Cat No.: 84-537-XIE.
Statistique Canada. Indice des prix à la consommation, aperçu historique (1997 à 2016). 2017. http://www.statcan.gc.ca/tables-tableaux/sum-som/l02/cst01/econ46a-fra.htm. Accessed 15 Mar 2017.
Mihaylova B, Briggs A, O’Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20(8):897–916. https://doi.org/10.1002/hec.1653.
Régie de l’assurance-maladie du Québec. Manuel des médecins omnipraticiens: rémunération à l’acte. Québec: RAMQ; 2017.
Régie de l’assurance-maladie du Québec. Liste des médicaments: dernière mise à jour le 3 mai 2017. Québec: RAMQ; 2017.
Sullivan PW, Lawrence WF, Ghushchyan V. A national catalog of preference-based scores for chronic conditions in the United States. Med Care. 2005;43(7):736–49.
Hauber AB, McCirnk L, Garcia-Cebrian A, Maas G, Das Gupta R, Le T. Population health-state utilities for fibromyalgia in the United Kingdom. Value Health. 2008;11(6):A353.
Gorodetskaya I, Zenios S, McCulloch CE, Bostrom A, Hsu CY, Bindman AB, et al. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005;68(6):2801–8. https://doi.org/10.1111/j.1523-1755.2005.00752.x.
Giacomini M. How good is good enough? Standards in policy decisions to cover new health technologies. Healthc Policy. 2007;3(2):91–101.
Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292(21):2585–90. https://doi.org/10.1001/jama.292.21.2585.
Erickson KF, Japa S, Owens DK, Chertow GM, Garber AM, Goldhaber-Fiebert JD. Cost-effectiveness of statins for primary cardiovascular prevention in chronic kidney disease. J Am Coll Cardiol. 2013;61(12):1250–8. https://doi.org/10.1016/j.jacc.2012.12.034.
Cardinal H, Monfared AA, Dorais M, Lelorier J. The concept of the ‘percent wasted patients’ in preventive health strategies. Pharmacoepidemiol Drug Saf. 2006;15(1):57–61. https://doi.org/10.1002/pds.1148.
Catalan VS, LeLorier J. Predictors of long-term persistence on statins in a subsidized clinical population. Value Health. 2000;3(6):417–26. https://doi.org/10.1046/j.1524-4733.2000.36006.x.
Guertin JR, Rahme E, LeLorier J. Use of continuous exposure variables when examining dose-dependent pharmacological effects - application to the association between exposure to higher statin doses and the incidence of diabetes. J Popul Ther Clin Pharmacol. 2017;24(1):5-15. https://doi.org/10.22374/1710-6222.24.1.1.
Dorais M, Chirovsky D, Ambegaonkar B, Sazonov V, Davies G, Grant S, et al. Utilization patterns of extended-release niacin in Canada: analysis of an administrative claims database. Can J Cardiol. 2010;26(7):e229–35.
Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long-term statin therapy? Curr Atheroscler Rep. 2013;15(1):291. https://doi.org/10.1007/s11883-012-0291-7.
Wouters H, Van Dijk L, Geers HC, Winters NA, Van Geffen EC, Stiggelbout AM, et al. Understanding statin non-adherence: knowing which perceptions and experiences matter to different patients. PLoS One. 2016;11(1):e0146272. https://doi.org/10.1371/journal.pone.0146272.
Public Health Agency of Canada. Tracking heart disease and stroke in Canada 2009. Ottawa: Public Health Agency of Canada; 2009.
Ontario Case Costing: OCC Costing Analysis Tool [online database]. Ministry of Health and Long Term Care. 2015. https://hsimi.ca/occp/occpreports/. Accessed 10 Apr 2015.
Smolderen KG, Bell A, Lei Y, Cohen EA, Steg PG, Bhatt DL, et al. One-year costs associated with cardiovascular disease in Canada: insights from the REduction of Atherothrombosis for Continued Health (REACH) registry. Can J Cardiol. 2010;26(8):297–305.
Conly J, Clement F, Tonelli M, Hemmelgarn B, Klarenbach S, Lloyd A, et al. Cost-effectiveness of the use of low- and high-potency statins in people at low cardiovascular risk. CMAJ. 2011;183(16):E1180–8.
Ghali WA, Knudtson ML. Overview of the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease. On behalf of the APPROACH investigators. Can J Cardiol. 2000;16(10):1225–30.
Skrabal MZ, Stading JA, Cannella CA, Monaghan MS. Two cases of rhabdomyolysis associated with high-dose simvastatin. Am J Health Syst Pharm. 2003;60(6):578–81.
CADTH. Guidelines for the economic evaluation of health technologies: Canada, 4th ed. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2017.
Funding
This research was supported by Genome Canada and Genome Québec. Jason R. Guertin is the recipient a of an establishment fund from the Centre de Recherche du CHU de Québec – Université Laval and from the Fondation du CHU de Québec.
Author information
Authors and Affiliations
Contributions
DM contributed to the conception and design of the study, data acquisition, analysis and interpretation of data, drafting the article, and final approval. JRG, AD, MPD, JCT, ACI, FFA, AM, and JL contributed to the conception and design of the study analysis and interpretation of data, drafting the article, and final approval.
Corresponding author
Ethics declarations
Conflict of interest
Dominic Mitchell, Jason R. Guertin, Anick Dubois, Marie-Pierre Dubé, Jean-Claude Tardif, Ange Christelle Iliza, Fiorella Fanton-Aita, Alexis Matteau, and Jacques LeLorier declare that they have no conflicts of interest that are directly relevant to the content of this review.
Open Access
This article is distributed under the terms of the Creative Commons Attribution–NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any non-commercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mitchell, D., Guertin, J.R., Dubois, A. et al. A Discrete Event Simulation Model to Assess the Economic Value of a Hypothetical Pharmacogenomics Test for Statin-Induced Myopathy in Patients Initiating a Statin in Secondary Cardiovascular Prevention. Mol Diagn Ther 22, 241–254 (2018). https://doi.org/10.1007/s40291-018-0323-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-018-0323-2