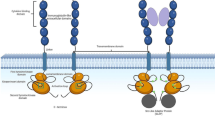
Abstract
The outcomes associated with pediatric acute myeloid leukemia (AML) have improved over the last few decades, with the implementation of intensive chemotherapy, hematopoietic stem cell transplant, and improved supportive care. However, even with intensive therapy and the use of HSCT, both of which carry significant risks of short- and long-term side effects, approximately 30% of children are not able to be cured. The characterization of AML in pediatrics has evolved over time and it currently involves use of a variety of diagnostic tools, including flow cytometry and comprehensive genomic sequencing. Given the adverse effects of chemotherapy and the need for additional therapeutic options to improve outcomes in these patients, the genomic and molecular architecture is being utilized to inform selection of targeted therapies in pediatric AML. This review provides a summary of current, targeted therapy options in pediatric AML.
Similar content being viewed by others
References
Rasche M, Zimmermann M, Borschel L, Bourquin JP, Dworzak M, Klingebiel T, et al. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia. 2018;32(10):2167–77. https://doi.org/10.1038/s41375-018-0071-7.
de Rooij JD, Zwaan CM, van den Heuvel-Eibrink M. Pediatric AML: from biology to clinical management. J Clin Med. 2015;4(1):127–49. https://doi.org/10.3390/jcm4010127.
Lalonde E, Wertheim G, Li MM. Clinical impact of genomic information in pediatric leukemia. Front Pediatr. 2017;5:263. https://doi.org/10.3389/fped.2017.00263.
Lonetti A, Pession A, Masetti R. Targeted therapies for pediatric AML: gaps and perspective. Front Pediatr. 2019;7:463. https://doi.org/10.3389/fped.2019.00463.
Leung W, Hudson MM, Strickland DK, Phipps S, Srivastava DK, Ribeiro RC, et al. Late effects of treatment in survivors of childhood acute myeloid leukemia. J Clin Oncol. 2000;18(18):3273–9. https://doi.org/10.1200/jco.2000.18.18.3273.
Creutzig U, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Lehrnbecher T. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol. 2004;22(21):4384–93. https://doi.org/10.1200/JCO.2004.01.191.
Rubnitz JE, Lensing S, Zhou Y, Sandlund JT, Razzouk BI, Ribeiro RC, et al. Death during induction therapy and first remission of acute leukemia in childhood: the St. Jude experience. Cancer. 2004;101(7):1677–84. https://doi.org/10.1002/cncr.20532.
Ishida H, Iguchi A, Aoe M, Nishiuchi R, Matsubara T, Keino D, et al. Panel-based next-generation sequencing facilitates the characterization of childhood acute myeloid leukemia in clinical settings. Biomed Rep. 2020;13(5):46. https://doi.org/10.3892/br.2020.1353.
Chen J, Glasser CL. New and emerging targeted therapies for pediatric acute myeloid leukemia (AML). Children (Basel). 2020. https://doi.org/10.3390/children7020012.
Bolouri H, Farrar JE, Triche T Jr, Ries RE, Lim EL, Alonzo TA, et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat Med. 2018;24(1):103–12. https://doi.org/10.1038/nm.4439.
Ilyas AM, Ahmad S, Faheem M, Naseer MI, Kumosani TA, Al-Qahtani MH, et al. Next generation sequencing of acute myeloid leukemia: influencing prognosis. BMC Genom. 2015;16(Suppl 1):S5. https://doi.org/10.1186/1471-2164-16-S1-S5.
Levine RL, Valk PJM. Next-generation sequencing in the diagnosis and minimal residual disease assessment of acute myeloid leukemia. Haematologica. 2019;104(5):868–71. https://doi.org/10.3324/haematol.2018.205955.
Cancer Genome Atlas Research N, Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–74. https://doi.org/10.1056/NEJMoa1301689.
Balgobind BV, Zwaan CM, Pieters R, Van den Heuvel-Eibrink MM. The heterogeneity of pediatric MLL-rearranged acute myeloid leukemia. Leukemia. 2011;25(8):1239–48. https://doi.org/10.1038/leu.2011.90.
Mercher T, Schwaller J. Pediatric acute myeloid leukemia (AML): from genes to models toward targeted therapeutic intervention. Front Pediatr. 2019;7:401. https://doi.org/10.3389/fped.2019.00401.
Tarlock K, Zhong S, He Y, Ries R, Severson E, Bailey M, et al. Distinct age-associated molecular profiles in acute myeloid leukemia defined by comprehensive clinical genomic profiling. Oncotarget. 2018;9(41):26417–30. https://doi.org/10.18632/oncotarget.25443.
Burd A, Levine RL, Ruppert AS, Mims AS, Borate U, Stein EM, et al. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med. 2020;26(12):1852–8. https://doi.org/10.1038/s41591-020-1089-8.
Pearson ADJ, Zwaan CM, Kolb EA, Karres D, Guillot J, Kim SY, et al. Paediatric Strategy Forum for medicinal product development for acute myeloid leukaemia in children and adolescents: ACCELERATE in collaboration with the European Medicines Agency with participation of the Food and Drug Administration. Eur J Cancer (Oxford, England: 1990). 2020;136:116–29. https://doi.org/10.1016/j.ejca.2020.04.038.
Meshinchi S, Alonzo TA, Stirewalt DL, Zwaan M, Zimmerman M, Reinhardt D, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108(12):3654–61. https://doi.org/10.1182/blood-2006-03-009233.
Meshinchi S, Woods WG, Stirewalt DL, Sweetser DA, Buckley JD, Tjoa TK, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97(1):89–94.
Conneely SE, Rau RE. The genomics of acute myeloid leukemia in children. Cancer Metastasis Rev. 2020;39(1):189–209. https://doi.org/10.1007/s10555-020-09846-1.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–9. https://doi.org/10.1182/blood.v98.6.1752.
Rubnitz JE. How I treat pediatric acute myeloid leukemia. Blood. 2012;119(25):5980–8. https://doi.org/10.1182/blood-2012-02-392506.
Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol. 2017;18(8):1061–75. https://doi.org/10.1016/S1470-2045(17)30416-3.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–64. https://doi.org/10.1056/NEJMoa1614359.
Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124(23):3441–9. https://doi.org/10.1182/blood-2014-05-578070.
Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485(7397):260–3. https://doi.org/10.1038/nature11016.
Pollard JA, Alonzo TA, Brown PA, Gerbing RB, Fox E, Choi JK, et al. Sorafenib in combination with standard chemotherapy for children with high allelic ratio FLT3/ITD+ AML improves event-free survival and reduces relapse risk: a report from the Children’s Oncology Group Protocol AAML1031. Blood. 2019;134(Supplement_1):292. https://doi.org/10.1182/blood-2019-129557.
Smith CC, Paguirigan A, Jeschke GR, Lin KC, Massi E, Tarver T, et al. Heterogeneous resistance to quizartinib in acute myeloid leukemia revealed by single-cell analysis. Blood. 2017;130(1):48–58. https://doi.org/10.1182/blood-2016-04-711820.
Tarlock K, Hansen ME, Hylkema T, Ries R, Farrar JE, Auvil JG, et al. Discovery and functional validation of novel pediatric specific FLT3 activating mutations in acute myeloid leukemia: results from the COG/NCI target initiative. Blood. 2015;126(23):87. https://doi.org/10.1182/blood.V126.23.87.87.
Frohling S, Scholl C, Levine RL, Loriaux M, Boggon TJ, Bernard OA, et al. Identification of driver and passenger mutations of FLT3 by high-throughput DNA sequence analysis and functional assessment of candidate alleles. Cancer Cell. 2007;12(6):501–13. https://doi.org/10.1016/j.ccr.2007.11.005.
Jones LM, Melgar K, Bolanos L, Hueneman K, Walker MM, Jiang JK, et al. Targeting AML-associated FLT3 mutations with a type I kinase inhibitor. J Clin Invest. 2020;130(4):2017–23. https://doi.org/10.1172/JCI127907.
Sexauer AN, Tasian SK. Targeting FLT3 signaling in childhood acute myeloid leukemia. Front Pediatr. 2017;5:248. https://doi.org/10.3389/fped.2017.00248.
Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–35. https://doi.org/10.1182/blood.v99.12.4326.
Levis M, Perl AE. Gilteritinib: potent targeting of FLT3 mutations in AML. Blood Adv. 2020;4(6):1178–91. https://doi.org/10.1182/bloodadvances.2019000174.
Kennedy VE, Smith CC. FLT3 mutations in acute myeloid leukemia: key concepts and emerging controversies. Front Oncol. 2020;10: 612880. https://doi.org/10.3389/fonc.2020.612880.
Larrosa-Garcia M, Baer MR. FLT3 inhibitors in acute myeloid leukemia: current status and future directions. Mol Cancer Ther. 2017;16(6):991–1001. https://doi.org/10.1158/1535-7163.MCT-16-0876.
Short NJ, Kantarjian H, Ravandi F, Daver N. Emerging treatment paradigms with FLT3 inhibitors in acute myeloid leukemia. Ther Adv Hematol. 2019;10:2040620719827310. https://doi.org/10.1177/2040620719827310.
Cortes JE, Kantarjian HM, Kadia TM, Borthakur G, Konopleva M, Garcia-Manero G, et al. Crenolanib besylate, a type I pan-FLT3 inhibitor, to demonstrate clinical activity in multiply relapsed FLT3-ITD and D835 AML. J Clin Oncol. 2016;34(15_suppl):7008. https://doi.org/10.1200/JCO.2016.34.15_suppl.7008.
Cortes JE, Khaled S, Martinelli G, Perl AE, Ganguly S, Russell N, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):984–97. https://doi.org/10.1016/S1470-2045(19)30150-0.
Wei AH, Kennedy GA, Morris KL, Grigg A, He S, Schwarer A, et al. Results of a phase 2, randomized, double-blind study of sorafenib versus placebo in combination with intensive chemotherapy in previously untreated patients with FLT3-ITD acute myeloid leukemia (ALLG AMLM16). Blood. 2020;136(Supplement 1):36–8. https://doi.org/10.1182/blood-2020-137334.
Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–40. https://doi.org/10.1056/NEJMoa1902688.
Schlenk RF, Taskesen E, van Norden Y, Krauter J, Ganser A, Bullinger L, et al. The value of allogeneic and autologous hematopoietic stem cell transplantation in prognostically favorable acute myeloid leukemia with double mutant CEBPA. Blood. 2013;122(9):1576–82. https://doi.org/10.1182/blood-2013-05-503847.
Meshinchi S, Arceci RJ, Sanders JE, Smith FO, Woods WB, Radich JP, et al. Role of allogeneic stem cell transplantation in FLT3/ITD-positive AML. Blood. 2006;108(1):400. https://doi.org/10.1182/blood-2005-12-4938 (author reply -1).
Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Rollig C, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020;38(26):2993–3002. https://doi.org/10.1200/JCO.19.03345.
Xuan L, Wang Y, Huang F, Fan Z, Xu Y, Sun J, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol. 2020;21(9):1201–12. https://doi.org/10.1016/S1470-2045(20)30455-1.
Brunner AM, Li S, Fathi AT, Wadleigh M, Ho VT, Collier K, et al. Haematopoietic cell transplantation with and without sorafenib maintenance for patients with FLT3-ITD acute myeloid leukaemia in first complete remission. Br J Haematol. 2016;175(3):496–504. https://doi.org/10.1111/bjh.14260.
Pratz KW, Rudek MA, Smith BD, Karp J, Gojo I, Dezern A, et al. A prospective study of peritransplant sorafenib for patients with FLT3-ITD acute myeloid leukemia undergoing allogeneic transplantation. Biol Blood Marrow Transplant. 2020;26(2):300–6. https://doi.org/10.1016/j.bbmt.2019.09.023.
Schlenk RF, Weber D, Fiedler W, Salih HR, Wulf G, Salwender H, et al. Midostaurin added to chemotherapy and continued single agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. 2018. https://doi.org/10.1182/blood-2018-08-869453.
Tarlock K, Chang B, Cooper T, Gross T, Gupta S, Neudorf S, et al. Sorafenib treatment following hematopoietic stem cell transplant in pediatric FLT3/ITD acute myeloid leukemia. Pediatr Blood Cancer. 2015;62(6):1048–54. https://doi.org/10.1002/pbc.25437.
Winters AC, Bernt KM. MLL-rearranged leukemias—an update on science and clinical approaches. Front Pediatr. 2017;5:4. https://doi.org/10.3389/fped.2017.00004.
Balgobind BV, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A, et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009;114(12):2489–96. https://doi.org/10.1182/blood-2009-04-215152.
Cooper TM, Ries RE, Alonzo TA, Gerbing RB, Loken MR, Brodersen LE, et al. Revised risk stratification criteria for children with newly diagnosed acute myeloid leukemia: a report from the Children’s Oncology Group. Blood. 2017;130(Supplement 1):407. https://doi.org/10.1182/blood.V130.Suppl_1.407.407.
Dafflon C, Craig VJ, Méreau H, Gräsel J, Schacher Engstler B, Hoffman G, et al. Complementary activities of DOT1L and menin inhibitors in MLL-rearranged leukemia. Leukemia. 2017;31(6):1269–77. https://doi.org/10.1038/leu.2016.327.
Stein EM, Garcia-Manero G, Rizzieri DA, Tibes R, Berdeja JG, Savona MR, et al. The DOT1L inhibitor pinometostat reduces H3K79 methylation and has modest clinical activity in adult acute leukemia. Blood. 2018;131(24):2661–9. https://doi.org/10.1182/blood-2017-12-818948.
Krivtsov AV, Evans K, Gadrey JY, Eschle BK, Hatton C, Uckelmann HJ, et al. A Menin-MLL inhibitor induces specific chromatin changes and eradicates disease in models of MLL-rearranged leukemia. Cancer Cell. 2019;36(6):660-73 e11. https://doi.org/10.1016/j.ccell.2019.11.001.
Klossowski S, Miao H, Kempinska K, Wu T, Purohit T, Kim E, et al. Menin inhibitor MI-3454 induces remission in MLL1-rearranged and NPM1-mutated models of leukemia. J Clin Invest. 2020;130(2):981–97. https://doi.org/10.1172/JCI129126.
McGeehan J. A first-in-class Menin-MLL1 antagonist for the treatment of MLL-r and NPM1 mutant leukemias. https://secureservercdn.net/50.62.194.30/d58.ae1.myftpupload.com/wp-content/uploads/2020/05/SNDX-5613-AACR-2020-PRESENTATION-vF.pdf2020. Accessed 10 Feb 2021.
Andersson AK, Miller DW, Lynch JA, Lemoff AS, Cai Z, Pounds SB, et al. IDH1 and IDH2 mutations in pediatric acute leukemia. Leukemia. 2011;25(10):1570–7. https://doi.org/10.1038/leu.2011.133.
Ho PA, Kutny MA, Alonzo TA, Gerbing RB, Joaquin J, Raimondi SC, et al. Leukemic mutations in the methylation-associated genes DNMT3A and IDH2 are rare events in pediatric AML: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2011;57(2):204–9. https://doi.org/10.1002/pbc.23179.
Zarnegar-Lumley S, Alonzo TA, Othus M, Sun Z, Ries RE, Wang Y-C, et al. Characteristics and prognostic effects of IDH mutations across the age spectrum in AML: a collaborative analysis from COG, SWOG, and ECOG. Blood. 2020;136(Supplement 1):31–2. https://doi.org/10.1182/blood-2020-134211.
Golub D, Iyengar N, Dogra S, Wong T, Bready D, Tang K, et al. Mutant isocitrate dehydrogenase inhibitors as targeted cancer therapeutics. Front Oncol. 2019;9:417. https://doi.org/10.3389/fonc.2019.00417.
Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020;135(7):463–71. https://doi.org/10.1182/blood.2019002140.
Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722–31. https://doi.org/10.1182/blood-2017-04-779405.
Stein EM, DiNardo CD, Fathi AT, Pollyea DA, Stone RM, Altman JK, et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood. 2019;133(7):676–87. https://doi.org/10.1182/blood-2018-08-869008.
Vaseva AV, Yohe ME. Targeting RAS in pediatric cancer: is it becoming a reality? Curr Opin Pediatr. 2020;32(1):48–56. https://doi.org/10.1097/MOP.0000000000000856.
Burgess MR, Hwang E, Firestone AJ, Huang T, Xu J, Zuber J, et al. Preclinical efficacy of MEK inhibition in Nras-mutant AML. Blood. 2014;124(26):3947–55. https://doi.org/10.1182/blood-2014-05-574582.
Borthakur G, Popplewell L, Boyiadzis M, Foran J, Platzbecker U, Vey N, et al. Activity of the oral mitogen-activated protein kinase kinase inhibitor trametinib in RAS-mutant relapsed or refractory myeloid malignancies. Cancer. 2016;122(12):1871–9. https://doi.org/10.1002/cncr.29986.
Maiti A, Naqvi K, Kadia TM, Borthakur G, Takahashi K, Bose P, et al. Phase II trial of MEK inhibitor binimetinib (MEK162) in RAS-mutant acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2019;19(3):142-8 e1. https://doi.org/10.1016/j.clml.2018.12.009.
Lipka DB, Witte T, Toth R, Yang J, Wiesenfarth M, Nollke P, et al. RAS-pathway mutation patterns define epigenetic subclasses in juvenile myelomonocytic leukemia. Nat Commun. 2017;8(1):2126. https://doi.org/10.1038/s41467-017-02177-w.
Niemeyer CM, Flotho C. Juvenile myelomonocytic leukemia: who’s the driver at the wheel? Blood. 2019;133(10):1060–70. https://doi.org/10.1182/blood-2018-11-844688.
Lyubynska N, Lauchle J, Shannon K, Braun BS. Treatment with a MEK inhibitor improves myeloproliferation, anemia and survival in a mouse model of CMML and JMML. Blood. 2009;114(22):966. https://doi.org/10.1182/blood.V114.22.966.966.
Juarez-Salcedo LM, Desai V, Dalia S. Venetoclax: evidence to date and clinical potential. Drugs Context. 2019;8: 212574. https://doi.org/10.7573/dic.212574.
Leverson JD, Sampath D, Souers AJ, Rosenberg SH, Fairbrother WJ, Amiot M, et al. Found in translation: how preclinical research is guiding the clinical development of the BCL2-selective inhibitor venetoclax. Cancer Discov. 2017;7(12):1376–93. https://doi.org/10.1158/2159-8290.CD-17-0797.
Pollyea DA, Amaya M, Strati P, Konopleva MY. Venetoclax for AML: changing the treatment paradigm. Blood Adv. 2019;3(24):4326–35. https://doi.org/10.1182/bloodadvances.2019000937.
Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4(3):362–75. https://doi.org/10.1158/2159-8290.CD-13-0609.
Kivioja JL, Thanasopoulou A, Kumar A, Kontro M, Yadav B, Majumder MM, et al. Dasatinib and navitoclax act synergistically to target NUP98-NSD1(+)/FLT3-ITD(+) acute myeloid leukemia. Leukemia. 2019;33(6):1360–72. https://doi.org/10.1038/s41375-018-0327-2.
Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137–45. https://doi.org/10.1182/blood.2020004856.
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–29. https://doi.org/10.1056/NEJMoa2012971.
Karol SE, Alexander TB, Budhraja A, Pounds SB, Canavera K, Wang L, et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: a phase 1, dose-escalation study. Lancet Oncol. 2020;21(4):551–60. https://doi.org/10.1016/S1470-2045(20)30060-7.
Agresta L, O’Brien MM, Mizuno K, Norris R, Breese EH, Phillips CL, et al. V2 Trial: a phase i study of venetoclax combined with CPX-351 for children, adolescents and young adults with relapsed or refractory acute leukemia. Blood. 2019;134(Supplement_1):3830. https://doi.org/10.1182/blood-2019-121514.
Tarlock K, Alonzo TA, Wang YC, Gerbing RB, Ries R, Loken MR, et al. Functional properties of KIT mutations are associated with differential clinical outcomes and response to targeted therapeutics in CBF acute myeloid leukemia. Clin Cancer Res. 2019;25(16):5038–48. https://doi.org/10.1158/1078-0432.CCR-18-1897.
Pollard JA, Alonzo TA, Gerbing RB, Ho PA, Zeng R, Ravindranath Y, et al. Prevalence and prognostic significance of KIT mutations in pediatric patients with core binding factor AML enrolled on serial pediatric cooperative trials for de novo AML. Blood. 2010;115(12):2372–9. https://doi.org/10.1182/blood-2009-09-241075.
Yui S, Kurosawa S, Yamaguchi H, Kanamori H, Ueki T, Uoshima N, et al. D816 mutation of the KIT gene in core binding factor acute myeloid leukemia is associated with poorer prognosis than other KIT gene mutations. Ann Hematol. 2017;96(10):1641–52. https://doi.org/10.1007/s00277-017-3074-y.
Chen X, Dou H, Wang X, Huang Y, Lu L, Bin J, et al. KIT mutations correlate with adverse survival in children with core-binding factor acute myeloid leukemia. Leuk Lymphoma. 2018;59(4):829–36. https://doi.org/10.1080/10428194.2017.1361025.
Schittenhelm MM, Shiraga S, Schroeder A, Corbin AS, Griffith D, Lee FY, et al. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66(1):473–81. https://doi.org/10.1158/0008-5472.CAN-05-2050.
Kampa-Schittenhelm KM, Vogel W, Bonzheim I, Fend F, Horger M, Kanz L, et al. Dasatinib overrides the differentiation blockage in a patient with mutant-KIT D816V positive CBFbeta-MYH11 leukemia. Oncotarget. 2018;9(14):11876–82. https://doi.org/10.18632/oncotarget.24376.
Paschka P, Schlenk RF, Weber D, Benner A, Bullinger L, Heuser M, et al. Adding dasatinib to intensive treatment in core-binding factor acute myeloid leukemia-results of the AMLSG 11–08 trial. Leukemia. 2018;32(7):1621–30. https://doi.org/10.1038/s41375-018-0129-6.
Marcucci G, Geyer S, Laumann K, Zhao W, Bucci D, Uy GL, et al. Combination of dasatinib with chemotherapy in previously untreated core binding factor acute myeloid leukemia: CALGB 10801. Blood Adv. 2020;4(4):696–705. https://doi.org/10.1182/bloodadvances.2019000492.
Cortes JE, Gutzmer R, Kieran MW, Solomon JA. Hedgehog signaling inhibitors in solid and hematological cancers. Cancer Treat Rev. 2019;76:41–50. https://doi.org/10.1016/j.ctrv.2019.04.005.
Cortes JE, Douglas Smith B, Wang ES, Merchant A, Oehler VG, Arellano M, et al. Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: phase 2 study results. Am J Hematol. 2018;93(11):1301–10. https://doi.org/10.1002/ajh.25238.
Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379–89. https://doi.org/10.1038/s41375-018-0312-9.
Xu H, Valerio DG, Eisold ME, Sinha A, Koche RP, Hu W, et al. NUP98 fusion proteins interact with the NSL and MLL1 complexes to drive leukemogenesis. Cancer Cell. 2016;30(6):863–78. https://doi.org/10.1016/j.ccell.2016.10.019.
Struski S, Lagarde S, Bories P, Puiseux C, Prade N, Cuccuini W, et al. NUP98 is rearranged in 3.8% of pediatric AML forming a clinical and molecular homogenous group with a poor prognosis. Leukemia. 2017;31(3):565–72. https://doi.org/10.1038/leu.2016.267.
Maxson JE, Ries R, Wang Y-C, Gerbing RB, Kolb EA, Thompson SL, et al. CSF3R mutations represent a novel therapeutic target in pediatric AML with a high degree of overlap with CEBPA mutations: a report from COG AAML0531 and COG/NCI Target AML Initiative. Blood. 2015;126(23):174. https://doi.org/10.1182/blood.V126.23.174.174.
Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8(3):282–8. https://doi.org/10.1038/nm0302-282.
Cluzeau T, Sebert M, Rahmé R, Cuzzubbo S, Walter-Petrich A, Lehmann J, et al. APR-246 combined with azacitidine (AZA) in TP53 mutated myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) a phase 2 study by the Groupe Francophone Des Myélodysplasies (GFM). Blood. 2019;134(Supplement_1):677. https://doi.org/10.1182/blood-2019-125579.
Johnston DL, Alonzo TA, Gerbing RB, Hirsch B, Heerema NA, Ravindranath Y, et al. Outcome of pediatric patients with acute myeloid leukemia (AML) and −5/5q− abnormalities from five pediatric AML treatment protocols: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2013;60(12):2073–8. https://doi.org/10.1002/pbc.24573.
Steensma DP, Stone RM. Lenalidomide in AML: Del(5q) or who? Blood. 2011;118(3):481–2. https://doi.org/10.1182/blood-2011-05-354324.
Castaigne S, Pautas C, Terre C, Raffoux E, Bordessoule D, Bastie JN, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508–16. https://doi.org/10.1016/S0140-6736(12)60485-1.
Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32(27):3021–32. https://doi.org/10.1200/JCO.2014.55.3628.
Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19(13):3244–54. https://doi.org/10.1200/JCO.2001.19.13.3244.
Lambert J, Pautas C, Terre C, Raffoux E, Turlure P, Caillot D, et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica. 2019;104(1):113–9. https://doi.org/10.3324/haematol.2018.188888.
Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986–96. https://doi.org/10.1016/S1470-2045(14)70281-5.
Pollard JA, Loken M, Gerbing RB, Raimondi SC, Hirsch BA, Aplenc R, et al. CD33 expression and its association with gemtuzumab ozogamicin response: results from the randomized phase III Children’s Oncology Group Trial AAML0531. J Clin Oncol. 2016;34(7):747–55. https://doi.org/10.1200/JCO.2015.62.6846.
Pollard JA, Alonzo TA, Loken M, Gerbing RB, Ho PA, Bernstein ID, et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood. 2012;119(16):3705–11. https://doi.org/10.1182/blood-2011-12-398370.
Tarlock K, Alonzo TA, Gerbing RB, Raimondi SC, Hirsch BA, Sung L, et al. Gemtuzumab ozogamicin reduces relapse risk in FLT3/ITD acute myeloid leukemia: a report from the Children’s Oncology Group. Clin Cancer Res. 2016;22(8):1951–7. https://doi.org/10.1158/1078-0432.CCR-15-1349.
Fournier E, Duployez N, Ducourneau B, Raffoux E, Turlure P, Caillot D, et al. Mutational profile and benefit of gemtuzumab ozogamicin in acute myeloid leukemia. Blood. 2020;135(8):542–6. https://doi.org/10.1182/blood.2019003471.
Pollard J, Alonzo TA, Gerbing RB, Raimondi SC, Hirsch BA, Sung L, et al. Treatment of 11q23/MLL + AML with gemtuzumab ozogamicin: results from the randomized phase III Children’s Oncology Group Trial AAML0531. Blood. 2015;126(23):799. https://doi.org/10.1182/blood.V126.23.799.799.
Stein EM, Walter RB, Erba HP, Fathi AT, Advani AS, Lancet JE, et al. A phase 1 trial of vadastuximab talirine as monotherapy in patients with CD33-positive acute myeloid leukemia. Blood. 2018;131(4):387–96. https://doi.org/10.1182/blood-2017-06-789800.
Fathi AT, Erba HP, Lancet JE, Stein EM, Ravandi F, Faderl S, et al. A phase 1 trial of vadastuximab talirine combined with hypomethylating agents in patients with CD33-positive AML. Blood. 2018;132(11):1125–33. https://doi.org/10.1182/blood-2018-03-841171.
Aigner M, Feulner J, Schaffer S, Kischel R, Kufer P, Schneider K, et al. T lymphocytes can be effectively recruited for ex vivo and in vivo lysis of AML blasts by a novel CD33/CD3-bispecific BiTE antibody construct. Leukemia. 2013;27(5):1107–15. https://doi.org/10.1038/leu.2012.341.
Friedrich M, Henn A, Raum T, Bajtus M, Matthes K, Hendrich L, et al. Preclinical characterization of AMG 330, a CD3/CD33-bispecific T-cell-engaging antibody with potential for treatment of acute myelogenous leukemia. Mol Cancer Ther. 2014;13(6):1549–57. https://doi.org/10.1158/1535-7163.MCT-13-0956.
Ravandi F, Stein AS, Kantarjian HM, Walter RB, Paschka P, Jongen-Lavrencic M, Ossenkoppele GJ, Yang Z, Mehta B, Subklewe M. A phase 1 first-in-human study of AMG 330, an anti-CD33 bispecific T-Cell Engager (BiTE®) antibody construct, in relapsed/refractory acute myeloid leukemia (R/R AML). Blood. 2018;132(Supplement 1):25.
Liu Y, Bewersdorf JP, Stahl M, Zeidan AM. Immunotherapy in acute myeloid leukemia and myelodysplastic syndromes: the dawn of a new era? Blood Rev. 2019;34:67–83. https://doi.org/10.1016/j.blre.2018.12.001.
Ravandi F, Walter RB, Subklewe M, Buecklein V, Jongen-Lavrencic M, Paschka P, Ossenkoppele GJ, Kantarjian HM, Hindoyan A, Agarwal SK, Dai T, Khaldoyanidi S, Stein AS. Updated results from phase I dose-escalation study of AMG 330, a bispecific T-cell engager molecule, in patients with relapsed/refractory acute myeloid leukemia (R/R AML). J Clin Oncol. 2020;38(15):7508.
Raza A, Jurcic JG, Roboz GJ, Maris M, Stephenson JJ, Wood BL, et al. Complete remissions observed in acute myeloid leukemia following prolonged exposure to lintuzumab: a phase 1 trial. Leuk Lymphoma. 2009;50(8):1336–44. https://doi.org/10.1080/10428190903050013.
Lamble AJ, Eidenschink Brodersen L, Alonzo TA, Wang J, Gerbing RB, Pardo L, et al. Correlation of CD123 expression level with disease characteristics and outcomes in pediatric acute myeloid leukemia: a report from the Children’s Oncology Group. Blood. 2019;134(Supplement_1):459. https://doi.org/10.1182/blood-2019-124587.
Ehninger A, Kramer M, Rollig C, Thiede C, Bornhauser M, von Bonin M, et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014;4: e218. https://doi.org/10.1038/bcj.2014.39.
Testa U, Pelosi E, Frankel A. CD 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomark Res. 2014;2(1):4. https://doi.org/10.1186/2050-7771-2-4.
Pemmaraju N, Lane AA, Sweet KL, Stein AS, Vasu S, Blum W, et al. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2019;380(17):1628–37. https://doi.org/10.1056/NEJMoa1815105.
Mani R, Goswami S, Gopalakrishnan B, Ramaswamy R, Wasmuth R, Tran M, et al. The interleukin-3 receptor CD123 targeted SL-401 mediates potent cytotoxic activity against CD34(+)CD123(+) cells from acute myeloid leukemia/myelodysplastic syndrome patients and healthy donors. Haematologica. 2018;103(8):1288–97. https://doi.org/10.3324/haematol.2018.188193.
Frankel A, Liu JS, Rizzieri D, Hogge D. Phase I clinical study of diphtheria toxin-interleukin 3 fusion protein in patients with acute myeloid leukemia and myelodysplasia. Leuk Lymphoma. 2008;49(3):543–53. https://doi.org/10.1080/10428190701799035.
Lane AL, Sweet KL, Wang ES, Donnellan W, Walter RB, Mantzaris I, Maris MB, Bixby DL, Rizzieri DA, Faderl S, Malinowski M, Pemmaraju N, Wysowskyj H, Shemesh S, Chen J, Lindsay R, Brooks C, Goswami T, Stone RM, Kantarjian HM, Jabbour EJ, Konopleva M. Results from ongoing phase 1/2 trial of SL-401 as consolidation therapy in patients with acute myeloid leukemia (AML) in remission with minimal residual disease (MRD). Blood. 2017;130(Supplement 1):2583.
Al-Hussaini M, Rettig MP, Ritchey JK, Karpova D, Uy GL, Eissenberg LG, et al. Targeting CD123 in acute myeloid leukemia using a T-cell-directed dual-affinity retargeting platform. Blood. 2016;127(1):122–31. https://doi.org/10.1182/blood-2014-05-575704.
Kovtun Y, Jones GE, Adams S, Harvey L, Audette CA, Wilhelm A, et al. A CD123-targeting antibody-drug conjugate, IMGN632, designed to eradicate AML while sparing normal bone marrow cells. Blood Adv. 2018;2(8):848–58. https://doi.org/10.1182/bloodadvances.2018017517.
Daver NG, Erba HP, Papadantonakis N, DeAngelo DJ, Wang ES, Konopleva MY, Sloss CM, Culm-Merdek K, Zweidler-McKay PA, Kantarjian HK. A phase I, first-in-human study evaluating the safety and preliminary antileukemia activity of IMGN632, a novel CD123-targeting antibody-drug conjugate, in patients with relapsed/refractory acute myeloid leukemia and other CD123-positive hematologic malignancies. Blood. 2018;132(Supplement 1):27.
Uy GL, Aldoss I, Foster MC, Sayre PH, Wieduwilt MJ, Advani AS, et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood. 2021;137(6):751–62. https://doi.org/10.1182/blood.2020007732.
Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia. 2015;29(8):1637–47. https://doi.org/10.1038/leu.2015.52.
Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123(15):2343–54. https://doi.org/10.1182/blood-2013-09-529537.
Budde L, Song JY, Kim Y, Blanchard S, Wagner J, Stein AS, Weng L, Del-Real M, Hernandez R, Marcucci E, Shepphird JK, Wang X, Wood B, Marcucci G, Brown CE, Forman SJ. Remissions of acute myeloid leukemia and blastic plasmacytoid dendritic cell neoplasm following treatment with CD123-specific CAR T cells: a first-in-human clinical trial. Blood. 2017;130(Supplement 1):811.
Yao S, Jianlin C, Yarong L, Botao L, Qinghan W, Hongliang F, et al. Donor-derived CD123-targeted CAR T cell serves as a RIC regimen for haploidentical transplantation in a patient with FUS-ERG+ AML. Front Oncol. 2019;9:1358. https://doi.org/10.3389/fonc.2019.01358.
Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo B, et al. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther. 2015;23(1):184–91. https://doi.org/10.1038/mt.2014.164.
Liu F, Cao Y, Pinz K, Ma Y, Wada M, Chen K, Ma G, Shen J, Tse CO, Su Y, Xiong Y, He G, Li Y, Ma Y. First-in-human CLL1-CD33 compound CAR T cell therapy induces complete remission in patients with refractory acute myeloid leukemia: update on phase 1 clinical trial. Blood. 2018;132(1):901.
Tambaro FP, Singh H, Jones E, Rytting M, Mahadeo KM, Thompson P, et al. Autologous CD33-CAR-T cells for treatment of relapsed/refractory acute myelogenous leukemia. Leukemia. 2021. https://doi.org/10.1038/s41375-021-01232-2.
Wang J, Chen S, Xiao W, Li W, Wang L, Yang S, et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J Hematol Oncol. 2018;11(1):7. https://doi.org/10.1186/s13045-017-0553-5.
Baumeister SH, Murad J, Werner L, Daley H, Trebeden-Negre H, Gicobi JK, et al. Phase I trial of autologous CAR T cells targeting NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer Immunol Res. 2019;7(1):100–12. https://doi.org/10.1158/2326-6066.CIR-18-0307.
Spear P, Barber A, Rynda-Apple A, Sentman CL. NKG2D CAR T-cell therapy inhibits the growth of NKG2D ligand heterogeneous tumors. Immunol Cell Biol. 2013;91(6):435–40. https://doi.org/10.1038/icb.2013.17.
Wang Y, Xu Y, Li S, Liu J, Xing Y, Xing H, et al. Targeting FLT3 in acute myeloid leukemia using ligand-based chimeric antigen receptor-engineered T cells. J Hematol Oncol. 2018;11(1):60. https://doi.org/10.1186/s13045-018-0603-7.
Gomes-Silva D, Atilla E, Atilla PA, Mo F, Tashiro H, Srinivasan M, et al. CD7 CAR T cells for the therapy of acute myeloid leukemia. Mol Ther. 2019;27(1):272–80. https://doi.org/10.1016/j.ymthe.2018.10.001.
Chapuis AG, Egan DN, Bar M, Schmitt TM, McAfee MS, Paulson KG, et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med. 2019;25(7):1064–72. https://doi.org/10.1038/s41591-019-0472-9.
Dossa RG, Cunningham T, Sommermeyer D, Medina-Rodriguez I, Biernacki MA, Foster K, et al. Development of T-cell immunotherapy for hematopoietic stem cell transplantation recipients at risk of leukemia relapse. Blood. 2018;131(1):108–20. https://doi.org/10.1182/blood-2017-07-791608.
Bleakley M, Riddell SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat Rev Cancer. 2004;4(5):371–80. https://doi.org/10.1038/nrc1365.
Steger B, Milosevic S, Doessinger G, Reuther S, Liepert A, Braeu M, et al. CD4(+)and CD8(+)T-cell reactions against leukemia-associated- or minor-histocompatibility-antigens in AML-patients after allogeneic SCT. Immunobiology. 2014;219(4):247–60. https://doi.org/10.1016/j.imbio.2013.10.008.
Montagna D, Maccario R, Locatelli F, Montini E, Pagani S, Bonetti F, et al. Emergence of antitumor cytolytic T cells is associated with maintenance of hematologic remission in children with acute myeloid leukemia. Blood. 2006;108(12):3843–50. https://doi.org/10.1182/blood-2006-05-021535.
Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–85. https://doi.org/10.1016/j.cell.2009.05.046.
Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–99. https://doi.org/10.1016/j.cell.2009.05.045.
Chao MP, Takimoto CH, Feng DD, McKenna K, Gip P, Liu J, et al. Therapeutic targeting of the macrophage immune checkpoint CD47 in myeloid malignancies. Front Oncol. 2019;9:1380. https://doi.org/10.3389/fonc.2019.01380.
Sallman DA, Asch AS, Kambhampati S, Al Malki MM, Zeidner JF, Donnellan W, Lee DJ, Vyas P, Jeyakumar D, Mannis GN, Tanaka TN, Chai-Ho W, Larson RA, Whiteley AR, Marcucci G, Komrokji RS, Garcia-Manero G, Van Elk J, Lin M, Maute R, Volkmer J-P, Takimoto CH, Chao MP, Daver N. The first-in-class anti-CD47 antibody magrolimab combined with azacitidine is well-tolerated and effective in AML patients: phase 1b results. Blood. 2020;(Supplement):330.
Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375(2):143–53. https://doi.org/10.1056/NEJMoa1601202.
Ghosh A, Barba P, Perales M-A. Checkpoint inhibitors in AML: are we there yet? Br J Haematol. 2020;188(1):159–67. https://doi.org/10.1111/bjh.16358.
Leonti AR, Manselle M, Smith JL, Ries RE, Kolb EA, Meshinchi S. Target-Informed Repurposing Of Immunotherapies in AML—a transcriptome based approach for identifying immediately available therapeutics. Blood. 2020;136(Supplement 1):5–6.
Lu YJ, Chu H, Wheeler LW, Nelson M, Westrick E, Matthaei JF, et al. Preclinical evaluation of bispecific adaptor molecule controlled folate receptor CAR-T cell therapy with special focus on pediatric malignancies. Front Oncol. 2019;9:151. https://doi.org/10.3389/fonc.2019.00151.
Kaeding AJ, Barwe SP, Gopalakrishnapillai A, Ries RE, Alonzo TA, Gerbing RB, et al. Mesothelin is a novel cell surface disease marker and potential therapeutic target in acute myeloid leukemia. Blood Adv. 2021;5(9):2350–61. https://doi.org/10.1182/bloodadvances.2021004424.
van der Lee DI, Reijmers RM, Honders MW, Hagedoorn RS, de Jong RC, Kester MG, et al. Mutated nucleophosmin 1 as immunotherapy target in acute myeloid leukemia. J Clin Invest. 2019;129(2):774–85. https://doi.org/10.1172/JCI97482.
Roerden M, Nelde A, Walz JS. Neoantigens in hematological malignancies-ultimate targets for immunotherapy? Front Immunol. 2019;10:3004. https://doi.org/10.3389/fimmu.2019.03004.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. https://doi.org/10.1056/NEJMoa1407222.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. https://doi.org/10.1056/NEJMoa1215134.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
LMJ has no disclosures. KT has no disclosures. TC: Consultant for Kura Oncology.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
LMJ, KT, and TC wrote and edited the manuscript.
Rights and permissions
About this article
Cite this article
Jones, L.M., Tarlock, K. & Cooper, T. Targeted Therapy in Pediatric AML: An Evolving Landscape. Pediatr Drugs 23, 485–497 (2021). https://doi.org/10.1007/s40272-021-00467-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-021-00467-x