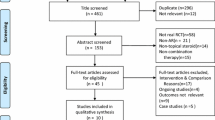
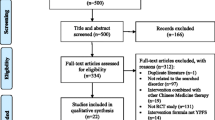
Abstract
Background
The clinical benefit of newer antihistamines (AHs) versus other active treatments has not been assessed in pediatric patients with allergic rhinitis.
Methods
A systematic literature search was performed in MEDLINE, SCOPUS, and the Cochrane Central Register of Controlled Trials from inception through August 2020. Randomized controlled trials (RCTs) comparing newer with older AHs, corticosteroids, or montelukast were included. The Cochrane Risk of Bias Tool was used for quality assessment.
Results
Out of 10,656 citations, 16 RCTs (N = 1653) with a duration from 10 days to 3 months were included. When compared with older-generation AHs, the administration of newer AHs did not confer significant benefit and appeared less effective compared with intranasal corticosteroids. However, newer AHs were more potent in achieving symptom control compared with montelukast. Data regarding quality of life were generally missing. The incidence of adverse events was low in all treatment groups. The included RCTs were characterized by moderate risk of bias.
Conclusions
Newer AHs are effective in symptom control and well tolerated in the pediatric population. However, inadequate reporting, variation in outcome measures, and a paucity of sufficient randomized comparisons precluded us from quantifying the relative efficacy of newer AHs compared with other treatment options.

Similar content being viewed by others
References
Bousquet J, Dahl R, Khaltaev N. Global alliance against chronic respiratory diseases. Allergy. 2007;62(3):216–23. https://doi.org/10.1111/j.1398-9995.2007.01307.x.
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160. https://doi.org/10.1111/j.1398-9995.2007.01620.x.
Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet (London, England). 2006;368(9537):733–43. https://doi.org/10.1016/s0140-6736(06)69283-0.
Leynaert B, Bousquet J, Neukirch C, Liard R, Neukirch F. Perennial rhinitis: an independent risk factor for asthma in nonatopic subjects: results from the European Community Respiratory Health Survey. J Allergy Clin Immunol. 1999;104(2 Pt 1):301–4.
Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;124(3 Suppl):S43–70. https://doi.org/10.1016/j.jaci.2009.05.013.
Kay GG. The effects of antihistamines on cognition and performance. J Allergy Clin Immunol. 2000;105(6 Pt 2):S622–S627627.
Casale TB, Blaiss MS, Gelfand E, Gilmore T, Harvey PD, Hindmarch I, et al. First do no harm: managing antihistamine impairment in patients with allergic rhinitis. J Allergy Clin Immunol. 2003;111(5):S835–S842842.
Brozek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950–8. https://doi.org/10.1016/j.jaci.2017.03.050.
Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126(3):466–76. https://doi.org/10.1016/j.jaci.2010.06.047.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clin Res Ed). 2009;339:b2535. https://doi.org/10.1136/bmj.b2535.
Wallace BC, Small K, Brodley CE, Lau J, Trikalinos TA. Deploying an interactive machine learning system in an evidence-based practice center: abstrackr. In: Proceedings of the 2nd ACM SIGHIT international health informatics symposium; Miami, Florida, USA. 2110464: ACM; 2012, pp. 819–24.
Higgins JPT, Altman DG. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Chichester: Wiley; 2008. p. 187–241.
Engauge Digitizer. https://markummitchell.github.io/engauge-digitizer/. Accessed 1 June 2019.
Baraldi E, Azzolin NM, Carra S, Dario C, Marchesini L, Zacchello F. Effect of topical steroids on nasal nitric oxide production in children with perennial allergic rhinitis: a pilot study. Respir Med. 1998;92(3):558–61.
Bender BG, Milgrom H. Comparison of the effects of fluticasone propionate aqueous nasal spray and loratadine on daytime alertness and performance in children with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2004;92(3):344–9. https://doi.org/10.1016/s1081-1206(10)61573-6.
Wartna JB, Bohnen AM, Elshout G, Pijnenburg MW, Pols DH, Gerth van Wijk RR, et al. Symptomatic treatment of pollen-related allergic rhinoconjunctivitis in children: randomized controlled trial. Allergy. 2017;72(4):636–44. https://doi.org/10.1111/all.13056.
Malizia V, Fasola S, Ferrante G, Cilluffo G, Gagliardo R, Landi M, et al. Comparative effect of beclomethasone dipropionate and cetirizine on acoustic rhinometry parameters in children with perennial allergic rhinitis: a randomized controlled trial. J Investig Allergol Clin Immunol. 2018;28(6):392–400. https://doi.org/10.18176/jiaci.0263.
Benedictis FMD, Forenza N, Armenio L, Boner AL, Giorgi PL, Giudice MMD, et al. Efficacy and safety of cetirizine and oxatomide in young children with perennial allergic rhinitis: a 10-day, multicenter, double-blinded, randomized, parallel-group study. Pediatr Asthma Allergy Immunol. 1997;11(2):119–28. https://doi.org/10.1089/pai.1997.11.119.
Lai DS, Lue KH, Hsieh JC, Lin KL, Lee HS. The comparison of the efficacy and safety of cetirizine, oxatomide, ketotifen, and a placebo for the treatment of childhood perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2002;89(6):589–98. https://doi.org/10.1016/s1081-1206(10)62107-2.
Tinkelman DG, Kemp J, Mitchell DQ, Galant SP. Treatment of seasonal allergic rhinitis in children with cetirizine or chlorpheniramine: a multicenter study. Pediatr Asthma Allergy Immunol. 1996;10(1):9–17. https://doi.org/10.1089/pai.1996.10.9.
Boner AL, Miglioranzi P, Richelli C, Marchesi E, Andreoli A. Efficacy and safety of loratadine suspension in the treatment of children with allergic rhinitis. Allergy. 1989;44(6):437–41.
Wu KG, Li TH, Wang TY, Hsu CL, Chen CJ. A comparative study of loratadine syrup and cyproheptadine HCL solution for treating perennial allergic rhinitis in Taiwanese children aged 2–12 years. Int J Immunopathol Pharmacol. 2012;25(1):231–7. https://doi.org/10.1177/039463201202500125.
Wandalsen GF, Miranda C, Ensina LF, Sano F, Amazonas RB, Silva JMD, et al. Association between desloratadine and prednisolone in the treatment of children with acute symptoms of allergic rhinitis: a double-blind, randomized and controlled clinical trial. Braz J Otorhinolaryngol. 2017;83(6):633–9. https://doi.org/10.1016/j.bjorl.2016.08.009.
Hsieh J-C, Lue K-H, Lai D-S, Sun H-L, Lin Y-H. A comparison of cetirizine and montelukast for treating childhood perennial allergic rhinitis. Pediatr Asthma Allergy Immunol. 2004;17(1):59–69. https://doi.org/10.1089/088318704322994958.
Chen ST, Lu KH, Sun HL, Chang WT, Lue KH, Chou MC. Randomized placebo-controlled trial comparing montelukast and cetirizine for treating perennial allergic rhinitis in children aged 2–6 yr. Pediatr Allergy Immunol. 2006;17(1):49–544. https://doi.org/10.1111/j.1399-3038.2005.00351.x.
Segundo GR, Gomes FA, Fernandes KP, Alves R, Silva DA, Taketomi EA. Local cytokines and clinical symptoms in children with allergic rhinitis after different treatments. Biol Targets Ther. 2009;3:469–74.
Sienra-Monge JJ, Gazca-Aguilar A, Del Rio-Navarro B. Double-blind comparison of cetirizine and loratadine in children ages 2 to 6 years with perennial allergic rhinitis. Am J Ther. 1999;6(3):149–55.
Nayak AS, Berger WE, LaForce CF, Urdaneta ER, Patel MK, Franklin KB, et al. Randomized, placebo-controlled study of cetirizine and loratadine in children with seasonal allergic rhinitis. Allergy Asthma Proc. 2017;38(3):222–30. https://doi.org/10.2500/aap.2017.38.4050.
Lee CF, Sun HL, Lu KH, Ku MS, Lue KH. The comparison of cetirizine, levocetirizine and placebo for the treatment of childhood perennial allergic rhinitis. Pediatr Allergy Immunol. 2009;20(5):493–9. https://doi.org/10.1111/j.1399-3038.2008.00816.x.
Yanez A, Rodrigo GJ. Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2002;89(5):479–84. https://doi.org/10.1016/s1081-1206(10)62085-6.
Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ (Clin Res Ed). 1998;317(7173):1624–9.
Juniper EF, Howland WC, Roberts NB, Thompson AK, King DR. Measuring quality of life in children with rhinoconjunctivitis. J Allergy Clin Immunol. 1998;101(2 Pt 1):163–70. https://doi.org/10.1016/s0091-6749(98)70380-x.
Bousquet J, Arnavielhe S, Bedbrook A, Bewick M, Laune D, Mathieu-Dupas E, et al. MASK 2017: ARIA digitally-enabled, integrated, person-centred care for rhinitis and asthma multimorbidity using real-world-evidence. Clin Transl Allergy. 2018;8:45. https://doi.org/10.1186/s13601-018-0227-6.
Bousquet J, Hellings PW, Agache I, Amat F, Annesi-Maesano I, Ansotegui IJ, et al., Allergic Rhinitis, and its Impact on Asthma (ARIA) Phase 4. Change management in allergic rhinitis and asthma multimorbidity using mobile technology. J Allergy Clin Immunol. 2018: https://doi.org/10.1016/j.jaci.2018.08.049.
Funding
There is no funding source.
Author information
Authors and Affiliations
Contributions
Conceptualization of the study and design: LV, MM; screening of papers: LV, ZM, EB; data extraction: LV, MM; general supervision of the research team: MM; manuscript drafting and final approval: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Velentza, L., Maridaki, Z., Blana, E. et al. Antihistamines in the Management of Pediatric Allergic Rhinitis: A Systematic Review. Pediatr Drugs 22, 673–683 (2020). https://doi.org/10.1007/s40272-020-00419-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-020-00419-x