Abstract
mHealth, such as apps running on consumer smart devices is becoming increasingly popular and has the potential to profoundly affect healthcare and health outcomes. However, it may be disruptive and results achieved are not always reaching the goals. Allergic Rhinitis and its Impact on Asthma (ARIA) has evolved from a guideline using the best evidence-based approach to care pathways suited to real-life using mobile technology in allergic rhinitis (AR) and asthma multimorbidity. Patients largely use over-the-counter medications dispensed in pharmacies. Shared decision making centered around the patient and based on self-management should be the norm. Mobile Airways Sentinel networK (MASK), the Phase 3 ARIA initiative, is based on the freely available MASK app (the Allergy Diary, Android and iOS platforms). MASK is available in 16 languages and deployed in 23 countries. The present paper provides an overview of the methods used in MASK and the key results obtained to date. These include a novel phenotypic characterization of the patients, confirmation of the impact of allergic rhinitis on work productivity and treatment patterns in real life. Most patients appear to self-medicate, are often non-adherent and do not follow guidelines. Moreover, the Allergy Diary is able to distinguish between AR medications. The potential usefulness of MASK will be further explored by POLLAR (Impact of Air Pollution on Asthma and Rhinitis), a new Horizon 2020 project using the Allergy Diary.
Similar content being viewed by others
Background
Allergic rhinitis (AR) is the most common chronic disease worldwide. Evidence-based guidelines have improved knowledge on rhinitis and made a significant impact on AR management. However, many patients remain inadequately controlled and the costs for society are enormous, in particular due to the major impact of AR on school and work productivity [1, 2]. Unmet needs have identified clearly many gaps. These include (1) suboptimal rhinitis and asthma control due to medical, cultural and social barriers [3, 4], (2) poor understanding of endotypes [5], better characterization of phenotypes and multimorbidities [6], better understanding of gender differences [7], (3) assessment of sentinel networks in care pathways for allergen and pollutants exposures, using symptom variation [8], (4) lack of stratification of patients for optimized care pathways [9] and (5) lack of multidisciplinary teams within integrated care pathways, endorsing innovation in real life clinical trials [8] and encouraging patient empowerment [10, 11].
Mobile health (mHealth) is the use of information and communication technology (ICT) for health services and information transfer [12]. mHealth, including apps running on consumer smart devices (i.e., smartphones and tablets), is becoming increasingly popular and has the potential to profoundly impact on healthcare [13]. Novel app-based collaborative systems can have an important role in gathering information quickly and improving coverage and accessibility of prevention and treatment [14]. Implementing mHealth innovations may also have disruptive consequences [15], so it is important to test applicability in each individual situation [16]. A rapid growth of the health apps market has been seen with an estimated 325,000 health apps available in 2017 for most fields of medicine [17]. Benefits and drawbacks have been estimated for a number of disease [18]. The application of mHealth solutions can support the provision of high quality care to patients with AR or asthma, to the satisfaction of both patients and health care professionals, with a reduction in both health care utilization and costs [19]. Appropriately identifying and representing stakeholders’ interests and viewpoints in evaluations of mHealth is a critical part of ensuring continued progress and innovation [20]. Patient, caregiver and clinician evaluations and recommendations play an important role in the development of asthma mHealth tools to support the provision of asthma management [21]. Smart devices and internet-based applications are already used in rhinitis and asthma and may help to address some unmet needs [22]. However, these new tools need to be tested and evaluated for acceptability, usability and cost-effectiveness.
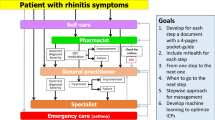
Allergic Rhinitis and its Impact on Asthma (ARIA) has evolved from an evidence-based guideline using the best evidence based approach [1, 23,24,25] to care pathways using mobile technology in AR and asthma multimorbidity [26]. ARIA appears to be close to the patient’s needs but real-life data suggest that few patients follow guideline recommendations and that they often self-medicate. Moreover, patients frequently using OTC medications dispensed in pharmacies [27]. Shared decision making (SDM) centered around the patient for self-management should be used more often.
Mobile Airways Sentinel networK (MASK), the Phase 3 ARIA initiative, has been initiated to reduce the global burden of rhinitis and asthma multimorbidity, giving the patient and the health care professional simple tools to better prevent and manage respiratory allergic diseases. More specifically, MASK is focusing on (1) understanding the disease mechanisms and the effects of air pollution in allergic diseases and asthma, (2) better appraising the burden incurred by medical needs and indirect costs, (3) the implementation of multi-sectoral care pathways integrating self-care, air pollution and patient’s literacy, using emerging technologies with real world data using the AIRWAYS ICPs algorithm [28], (4) proposing individualized and predictive medicine in rhinitis and asthma multimorbidity, (5) proposing the basis for a sentinel network at the global level for pollution and allergy and (6) assessing the societal implications of exposure to air pollution and allergens and its consequences on health inequalities globally.
The freely available MASK app (the Allergy Diary, Android and iOS) [26] is combined with an inter-operable tablet for physicians and other health care professionals (HCPs [29]), using the same extremely simple colloquial language to manage AR (Visual Analogue Scale: VAS) [30, 31]. It is being combined with data on allergen and pollution exposure (POLLAR).
MASK will be scaled up using the EU EIP on AHA strategy [32]. Phase 4 is starting in 2018 and will focus on “change management”. MASK is supported by several EU grants and is a WHO GARD (Global Alliance against Chronic Respiratory Diseases) research demonstration project (Table 1).
Methods
Users
The Allergy Diary is used by people who searched the internet, Apple App store, Google Play or in any other way. The pages of the App are on the Euforea-ARIA website (www.euforea.eu/about-us/aria.html). A few users were clinic patients to whom the app was recommended by their physicians. Users were not requested to complete the diary for a minimum number of days. However, due to anonymization of data, no specific information on the route of access to the app could be gathered [33, 34].
The first question of the App is “I have allergic rhinitis”: Yes/No. We tested the sensitivity and specificity of this question [33]. 93.4% users with a positive answer had nasal symptoms versus 12.1% of users with a negative answer. In the first two versions of the App, allergy was not considered in the user’s questionnaire and AR cannot be differentiated from chronic rhinosinusitis. It is now included in the third version of the App (June 2018) and we will be able to answer more appropriately to this question in the next study. The results of the pilot study were confirmed in over 9000 users.
Settings
MASK is available in 23 countries and 16 languages. To date (01-09-2018) the app has been used by over 24,000 people.
Ethics and privacy of data
The Allergy Diary is CE1 registered. The terms of use were translated into all languages and customized by lawyers according to the legislation of each country, allowing the use of the results for research and commercial purposes. The example of the UK terms of use have been provided in a previous paper [33].
Geolocation
EU data protection rules have changed since the implementation of the General Data Protection Regulation (Art. 4 para. 1 no. 1 GDPR) [35]. Data anonymization is a method of sanitization for privacy. Anonymization renders personal data “in such a manner that the data subject is not or no longer identifiable” [36]. The European Commission’s Article 29 Working Party (WP29) stated already in 2014 with regards to the Directive 95/46/EC [37] that geolocation information is not only personal data but also to be considered as an identifier itself [38, 39]. Processing personal data by means of an app, like e.g. App Diary, besides Directive 95/46/EC [37] also Directive 2002/58/EC [40] as amended by Directive 2009/136/EC [41] applies.
Geolocation was studied for all people who used the Allergy Diary App from December 2015 to November 2017 and who reported medical outcomes. In contradistinction to noise addition (randomization), k-anonymity [42, 43] is an acceptable method for the anonymization of MASK data (generalization) [44] and results can be used for other databases.
Privacy assessment impact
Privacy impact assessments (PIAs), also known as data protection impact assessments (DPIAs) in EU law, is required by GDPR (Article 35 Working Party (WP35). PIA is a systematic process to assess privacy risks to individuals in the collection, use, and disclosure of their personal data. The GDPR introduced PIAs to identify high risks to the privacy rights of individuals when processing their personal data. The assessment shall contain at least:
-
1.
a systematic description of the envisaged processing operations and the purposes of the processing, including, where applicable, the legitimate interest pursued by the controller;
-
2.
an assessment of the necessity and proportionality of the processing operations in relation to the purposes;
-
3.
an assessment of the risks to the rights and freedoms of data subjects and
-
4.
the measures envisaged to address the risks, including safeguards, security measures and mechanisms to ensure the protection of personal data and to demonstrate compliance with this Regulation taking into account the rights and legitimate interests of data subjects and other persons concerned.
When these risks are identified, the GDPR expects that an organization formulates measures to address these risks. Those measures may take the form of technical controls such as encryption or anonymization of data.
The PIA analysis is a self-declarative analysis. In France, the local GDPR representative (Commission Informatique et Liberté, CNIL) has provided a software to guide the reflexion around security of personal data and the exposure risks in case of security fails. This software has been used to assess all the risks to be considered through the app uses. The conclusion was that is “negligeable”.
The field is moving very fast. In France, June, 10 2018, the modified law “LIL” (Loi Informatique et Liberté, 2018-493, https://www.cnil.fr/fr/loi-78-17-du-6-janvier-1978-modifiee) was enacted with a special focus on health-related personal data. Even if the articulation of GDPR and LIL is still unclear, we can anticipate that the app use will remain risk free.
Allergy Diary
The app collects information on AR and asthma symptoms experienced (nasal and ocular) and on disease type (intermittent/persistent) [33] (Table 3). Anonymized and geolocalized users assess daily how symptoms impact their control and AR treatment using the touchscreen functionality on their smart phone to click on five consecutive VAS (i.e. general, nasal and ocular symptoms, asthma and work) (Table 2; Fig. 1). Users input their daily medications using a scroll list that contains all country-specific OTC and prescribed medications available (Fig. 2). The list populated using IMS data and revised by country experts is continuously revised by country experts.

(from Bousquet et al. [26])
Allergy Diary screens relating to Visual Analogue Scale and medications
There is a high degree of correlation between these VAS measurements. The example of VAS global measured and VAS nose is presented in Fig. 2.
Outcomes
Five VAS measurements [VAS-global measured, VAS-nose, VAS-eye, VAS-asthma and VAS-work (Table 4)] and a calculated VAS-global score (VAS-nasal + VAS-ocular divided by 2) were assessed [34]. VAS levels range from zero (not at all bothersome) to 100 (very bothersome). Independency of VAS questions was previously confirmed using the Bland and Altman regression analysis [34, 45].
Transfer of personal data from the App to a print
Patients cannot give access to their electronic data to a HCP due to privacy policies. However, they can easily print the daily control of their disease and the medications that they filled in the Allergy Diary as follows (Fig. 3).

(from Bousquet et al. [46]
Transfer of patient information on a computer and printed information
Additional questionnaires
MASK also includes EQ-5D (EuroQuol) [46,47,48], Work Productivity and Activity Impairment Allergic Specific (WPAI-AS) [49] and Control of AR and Asthma Test (CARAT) [50,51,52,53]. The Epworth Sleepiness Questionnaire [54, 55] is included (June 2018).
Medications
A scroll list is available for all OTC and prescribed medications of the 23 countries. The International Nonproprietary Names classification was used for drug nomenclature [56]. 85 INNs and 505 medications were identified (Fig. 1).
Adherence to treatment
Globally, non-adherence to medications is a major obstacle to the effective delivery of health care. Many mobile phone apps are available to support people to take their medications and to improve medication adherence [57, 58]. However, a recent meta-analysis found that the majority did not have many of the desirable features and were of low quality [57]. However, it is unknown how people use apps, what is considered adherent or non-adherent in terms of app usage, or whether adherence with an app in anyway reflects adherence with medication or control.
In MASK, we did not use adherence questionnaires but first attempted to assess short-term adherence and then to address the long-term issues. [59].
Digitalized ARIA symptom-medication score
Symptom-medication scores are needed to assess the control of allergic diseases. They are currently being developed for MASK and are being compared with existing ones [60].
MASK algorithm and clinical decision support system
Clinical decision support systems (CDSS) are software algorithms that advise health care providers on the diagnosis and management of patients based on the interaction of patient data and medical information, such as prescribed drugs. CDSS should be based on the best evidence and algorithms to aid patients and health care professionals to jointly determine the treatment and its step-up or step-down strategy for an optimal disease control.
The selection of pharmacotherapy for AR patients depends on several factors, including age, prominent symptoms, symptom severity, AR control, patient preferences and cost. Allergen exposure, pollution and resulting symptoms vary, needing treatment adjustment. In AR, The MASK CDSS is incorporated into an interoperable tablet [29] for HCPs (ARIA Allergy Diary Companion) [10, 26]. This is based on an algorithm to aid clinicians to select pharmacotherapy for AR patients and to stratify their disease severity [26] (Fig. 4). It uses a simple step-up/step-down individualized approach to AR pharmacotherapy and may hold the potential for optimal control of symptoms, while minimizing side-effects and costs. However, its use varies depending on the availability of medications in the different countries and on resources. The algorithm is now digitalized and available in English (Fig. 5).

(from Bousquet et al. [28])
Clinical decision support systems consensus for allergic rhinitis
MASK follows the CHRODIS criteria of “Good Practice”
The European Commission is co-funding a large collaborative project named JA-CHRODIS in the context of the 2nd EU Health Programme 2008–2013 [61]. JA-CHRODIS has developed a check-list of 27 items for the evaluation of Good Practices (GP) (http://chrodis.eu/our-work/04-knowledge-platform/). According to the JA-CHRODIS, a Good Practice has been proven to work well and produce good results, and is therefore recommended as a model to be scaled up. The JA-CHRODIS criteria are grouped into nine categories:
-
Equity.
-
Practice.
-
Ethical considerations.
-
Evaluation.
-
Empowerment and participation.
-
Target population.
-
Sustainability.
-
Governance.
-
Scalability
As part of SUNFRAIL, MASK tested the 27 item criteria of CHRODIS and was found to be an example of Good Practice [62].
Pilot study of mobile phone technology in AR
A pilot study in 3260 users found that Allergy Diary users were able to properly provide baseline simple phenotypic characteristics. Troublesome symptoms were found mainly in the users with the largest number of symptoms. Around 50% of users with troublesome rhinitis and/or ocular symptoms suffered work impairment. Sleep was impaired by troublesome symptoms and nasal obstruction (Fig. 6). results suggest novel concepts and research questions in AR that may not be identified using classical methods [33].

(from Bousquet et al. [33])
Impact of allergic rhinitis depending on the number of symptoms
Validation of the MASK Visual Analogue Scale on cell phones
VAS included in the Allergy Diary was found to be a validated tool to assess control in AR patients following COSMIN guidelines [63] in 1225 users and 14,612 days: internal consistency (Cronbach’s α-coefficient > 0.84 and test–retest > 0.7), reliability (intra-class correlation coefficients), sensitivity and acceptability [64]. In addition, e-VAS had a good reproducibility when users (n = 521) answered the e-VAS twice in less than 3 h.
Transfer of innovation of AR and asthma multimorbidity in the elderly: Reference Site Twinning (EIP on AHA)
The EIP on AHA includes 74 Reference Sites. The aim of this TWINNING was to transfer innovation from the MASK App to other reference sites. The phenotypic characteristics of rhinitis and asthma multimorbidity in adults and the elderly are compared using validated mHealth tools (i.e. the Allergy Diary and CARAT) in 23 Reference Sites or regions across Europe and Argentina, Australia, Brazil and Mexico [46]. This will improve understanding, assessment of burden, diagnosis and management of rhinitis in the elderly by comparison with an adult population. The pilot study has been completed in Germany and the project is fully operative using two protocols (Table 3).
Results
Work productivity
AR impairs social life, work and school productivity. Indirect costs associated with lost work productivity are the principal contributor to the total AR costs and result mainly from impaired work performance by presenteeism [2]. The severity of AR symptoms was the most consistent disease-related factor associated with impact of AR on work productivity, although ocular symptoms and sleep disturbances may independently affect work productivity. Overall, the pharmacologic treatment of AR showed a beneficial effect on work productivity.
A cross-sectional study using Allergy diary in 1136 users (5659 days) assessed the impact on work productivity of uncontrolled AR assessed by VAS [34]. In users with uncontrolled rhinitis (VAS global measured ≥ 50), approximately 90% had some work impairment and over 50% had severe work impairment (VAS-work ≥ 50). There was a significant correlation between VAS-global calculated and VAS-work (Rho = 0.83, p < 0.00001, Spearman rank test). The study has been extended to almost 17,000 days and similar results were observed (Fig. 7).
The baseline study found that bothersome symptoms, nasal obstruction and ocular symptoms were involved in work productivity impact [33] (Fig. 8).

(from Bousquet et al. [33])
Impact of symptoms on work, school and daily activities
The Allergy Diary includes the WPAI:AS in six EU countries. All consecutive users who completed the VAS-work from June 1 to July 31, 2016 were included in the study [66]. A highly significant correlation was found between Questions 4 (impairment of work) and 9 (impairment of activities) in 698 users (Rho = 0.85).
All these studies combine to confirm the impact of uncontrolled AR on work productivity.
Novel phenotypes of allergic diseases
Multimorbidity in allergic airway diseases is well known [6], but no data exist regarding the daily dynamics of symptoms. The Allergy Diary assessed the presence and control of daily allergic multimorbidity (asthma, conjunctivitis, rhinitis) and its impact on work productivity in 4025 users and 32,585 days monitored in 19 countries from May 25, 2015 to May 26, 2016. VAS levels < 20/100 were categorized as “Low” burden and VAS levels ≥ 50/100 as “High” burden. VAS global measured levels assessing the global control of the allergic disease were significantly associated with daily allergic multimorbidity. Eight hypothesis-driven patterns were defined based on “Low” and “High” VAS levels. There were < 0.2% days of Rhinitis Low and Asthma High or Conjunctivitis High patterns. There were 5.9% days with a Rhinitis High—Asthma Low pattern. There were 1.7% days with a Rhinitis High—Asthma High—Conjunctivitis Low pattern. A novel Rhinitis High—Asthma High—Conjunctivitis High pattern was identified in 2.9% days and had the greatest impact on uncontrolled VAS global measured and impaired work productivity (Fig. 9). The mobile technology enabled investigation in a novel approach of the intra-individual variability of allergic multimorbidity using days. It identified an unrecognized extreme pattern of uncontrolled multimorbidity [59].

(from Bousquet et al. [60])
VAS levels in severe rhinitis depending on multimorbidity
Treatment of allergic rhinitis using mobile technology with real world data
Large observational implementation studies are needed to triangulate the findings from randomized control trials (RCTs) as they reflect “real world” everyday practice. We attempted to provide additional and complementary insights into the real-life AR treatment using mobile technology. The Allergy Diary was filled in by 2871 users who reported 17,091 days of VAS in 2015 and 2016. Medications were reported for 9634 days. The assessment of days appeared to be more informative than the course of the treatment as, in real life, patients rarely use treatment on a daily basis; rather, they appear to increase treatment use with the loss of symptom control and to stop it when symptoms disappear. The Allergy Diary allowed the differentiation between treatments within or between classes (intranasal corticosteroid use containing medications and oral H1-antihistamines). The control of days differed between no (best control), single or multiple treatments (worst control) (Fig. 10). The study confirms the usefulness of the Allergy Diary in accessing and assessing everyday use and practice in AR [59].

(from Bousquet et al. [59])
Treatments received in MAS
Adherence to medications was studied in almost 7000 users reporting medications. 1770 users reported over 7 days of VAS between January 1, 2016 and August 31, 2016 and a major lack of adherence to treatment was observed for all medications (Menditto et al., in preparation).
MASK in the pharmacy
Multidisciplinary integrated care is necessary to reduce the burden of chronic diseases. A significant proportion of patients with AR self-manage their condition and often the pharmacist is the first HCP that a person with nasal symptoms contacts [66, 67]. Pharmacists are trusted in the community and are easily accessible. As such, pharmacists are an important part of the multidisciplinary healthcare team, acting at different steps of rhinitis care pathways.
Pharmacists are important in many areas of intervention in AR:
-
Recognizing (identification).
-
Risk assessment/stratification.
-
OTC treatment.
-
Manage refils.
-
Patient education.
-
Referral to a physician.
-
Administration of topical treatment technique and adherence to treatment.
Simple algorithms and tools are essential in the routine implementation of these steps. A first approach was made by ARIA in the pharmacy [68] and is currently being updated using MASK.
POLLAR (Impact of air POLLution on Asthma and Rhinitis)
AR and asthma are impacted by allergens and air pollution. However, interactions between air pollution, sleep [55, 69] and allergic diseases are insufficiently understood. POLLAR aims at combining emerging technologies [search engine TLR2 (technology readiness level); pollution sampler TLR6, App TLR9] with machine learning to (1) understand effects of air pollution in AR and its impact on sleep, work, asthma, (2) propose novel care pathways integrating pollution and patient’s literacy, (3) study sleep, (4) improve work productivity, (5) propose the basis for a sentinel network at the EU level for pollution and allergy and (6) assess the societal implications of the interaction.
POLLAR will use the freely existing application for AR monitoring (Allergy Diary, 14,000 users, TLR8) combined with a new tool allowing queries on allergen and pollen (TLR2) and existing pollution data. Machine learning will be used to assess the relationship between air pollution and AR comparing polluted and non-polluted areas in 6 EU countries. Data generated in 2018 will be confirmed in 2019 and extended by the individual assessment of pollution (Canarin®, portable sensor, TLR6) in AR and sleep apnea patients used as a control group having impaired sleep. The geographic information system GIS will map the results.
Google Trends (GT) searches trends of specific queries in Google and reflects the real-life epidemiology of AR. We compared GT terms related to allergy and rhinitis in all European Union countries, Norway and Switzerland from January 1, 2011 to December, 20 2016. An annual and clear seasonality of queries was found in most countries but the terms ‘hay fever’, ‘allergy’ and ‘pollen’—show cultural differences [70]. Using longitudinal data in different countries and multiple terms, we identified an awareness-related spike of searches (December 2016) [70]. In asthma, GTs can identify spikes of mortality as was found in Australia and Kuwait in 2016. However, the usual peaks of asthma during allergen exposure or virus infections cannot be easily monitored [71].
Global applicability of MASK and POLLAR, and their benefits
Although MASK has been devised to optimize care pathways in rhinitis and asthma multimorbidity, its applicability is far more extensive (Table 4).
For MASK, several steps have been achieved.
Conclusion
MASK is a novel approach to obtain real-life data concerning rhinitis and asthma multimorbidity and to help patients and physicians for a better SDM. It can be used for multiple purposes in a friendly manner in order to improve the control of allergic diseases in a cost-effective approach.
Change history
09 October 2019
Following publication of the original article [1], the authors reported that one of the collaborators’ names was spelled incorrectly. In this Correction the incorrect and correct author name are shown. In the author list of this Correction article, only the corresponding author and institutional author are presented.
Abbreviations
- AHA:
-
active and healthy ageing
- AIRWAYS ICPs:
-
integrated care pathways for airway diseases
- AR:
-
allergic rhinitis
- ARIA:
-
Allergic Rhinitis and Its Impact on Asthma
- CARAT:
-
Control of Allergic Rhinitis and Asthma Test
- CDSS:
-
clinical decision support system
- CNIL:
-
Commission Informatique et Liberté
- CRD:
-
Chronic Respiratory Disease
- DG CONNECT:
-
Directorate General for Communications Networks, Content & Technology
- DG Santé:
-
Directorate General for Health and Food Safety
- DG:
-
Directorate General
- EFA:
-
European Federation of Allergy and Airways Diseases Patients’ Associations
- EIP on AHA:
-
European Innovation Partnership on AHA
- EIP:
-
European Innovation Partnership
- EQ-5D:
-
Euroquol
- GARD:
-
WHO Global Alliance against Chronic Respiratory Diseases
- GDPR:
-
General Data Protection Regulation
- GIS:
-
geographic information system
- GP:
-
Good Practice
- GT:
-
Google Trends
- HCP:
-
health care professional
- ICP:
-
integrated care pathway
- IMS:
-
Institute of Medical Science
- JA-CHRODIS:
-
Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle
- MACVIA-LR:
-
contre les MAladies Chroniques pour un VIeillissement Actif (Fighting chronic diseases for AHA)
- MASK:
-
Mobile Airways Sentinel networK
- MeDALL:
-
Mechanisms of the Development of ALLergy (FP7)
- mHealth:
-
mobile health
- NCD:
-
non-communicable disease
- OTC:
-
over the counter
- PIA:
-
privacy Impact Assessment
- POLLAR:
-
Impact of air POLLution on Asthma and Rhinitis
- QOL:
-
quality of life
- SCUAD:
-
severe chronic upper airway disease
- TRL:
-
technology readiness level
- TWINNING:
-
transfer of innovation of mobile technology
- VAS:
-
Visual Analogue Scale
- WHO:
-
World Health Organization
- WPAI-AS:
-
Work Productivity and Activity Questionnaire
References
Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160.
Vandenplas O, Vinnikov D, Blanc PD, Agache I, Bachert C, Bewick M, et al. Impact of rhinitis on work productivity: a systematic review. J Allergy Clin Immunol Pract. 2018;6(4):1274–86.
Bousquet J, Bachert C, Canonica GW, Casale TB, Cruz AA, Lockey RJ, et al. Unmet needs in severe chronic upper airway disease (SCUAD). J Allergy Clin Immunol. 2009;124(3):428–33.
Bousquet J, Mantzouranis E, Cruz AA, Ait-Khaled N, Baena-Cagnani CE, Bleecker ER, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126(5):926–38.
De Greve G, Hellings PW, Fokkens WJ, Pugin B, Steelant B, Seys SF. Endotype-driven treatment in chronic upper airway diseases. Clin Transl Allergy. 2017;7:22.
Cingi C, Gevaert P, Mosges R, Rondon C, Hox V, Rudenko M, et al. Multi-morbidities of allergic rhinitis in adults: European Academy of Allergy and Clinical Immunology task force report. Clin Transl Allergy. 2017;7:17.
Frohlich M, Pinart M, Keller T, Reich A, Cabieses B, Hohmann C, et al. Is there a sex-shift in prevalence of allergic rhinitis and comorbid asthma from childhood to adulthood? A meta-analysis. Clin Transl Allergy. 2017;7:44.
Bousquet J, Addis A, Adcock I, Agache I, Agusti A, Alonso A, et al. Integrated care pathways for airway diseases (AIRWAYS-ICPs). Eur Respir J. 2014;44(2):304–23.
Hellings PW, Fokkens WJ, Bachert C, Akdis CA, Bieber T, Agache I, et al. Positioning the principles of precision medicine in care pathways for allergic rhinitis and chronic rhinosinusitis—an EUFOREA-ARIA-EPOS-AIRWAYS ICP statement. Allergy. 2017;72(9):1297–305.
Bousquet J, Schunemann HJ, Fonseca J, Samolinski B, Bachert C, Canonica GW, et al. MACVIA-ARIA Sentinel NetworK for allergic rhinitis (MASK-rhinitis): the new generation guideline implementation. Allergy. 2015;70(11):1372–92.
Hellings PW, Fokkens WJ, Akdis C, Bachert C, Cingi C, Dietz de Loos D, et al. Uncontrolled allergic rhinitis and chronic rhinosinusitis: where do we stand today? Allergy. 2013;68(1):1–7.
mHealth. New horizons for health through mobile technologies. Global Observatory for eHealth series—Vol. 3 WHO Library Cataloguing-in-Publication Data. 2011; http://www.who.int/goe/publications/goe_mhealth_web.pdf. Accessed 30 Sept 2018.
Ozdalga E, Ozdalga A, Ahuja N. The smartphone in medicine: a review of current and potential use among physicians and students. J Med Internet Res. 2012;14(5):e128.
Freifeld CC, Chunara R, Mekaru SR, Chan EH, Kass-Hout T, Ayala Iacucci A, et al. Participatory epidemiology: use of mobile phones for community-based health reporting. PLoS Med. 2010;7(12):e1000376.
Keijser W, de-Manuel-Keenoy E, d’Angelantonio M, Stafylas P, Hobson P, Apuzzo G, et al. DG Connect funded projects on information and communication technologies (ICT) for old age people: Beyond Silos, CareWell and SmartCare. J Nutr Health Aging. 2016;20(10):1024–33.
Mozaffar H, Cresswell KM, Williams R, Bates DW, Sheikh A. Exploring the roots of unintended safety threats associated with the introduction of hospital ePrescribing systems and candidate avoidance and/or mitigation strategies: a qualitative study. BMJ Qual Saf. 2017;26(9):722–733.
Talboom-Kamp EP, Verdijk NA, Harmans LM, Numans ME, Chavannes NH. An eHealth platform to manage chronic disease in primary care: an innovative approach. Interact J Med Res. 2016;5(1):e5.
Lee L, Sheikh A. Understanding stakeholder interests and perspectives in evaluations of health IT. Stud Health Technol Inf. 2016;222:53–62.
Geryk LL, Roberts CA, Sage AJ, Coyne-Beasley T, Sleath BL, Carpenter DM. Parent and clinician preferences for an asthma app to promote adolescent self-management: a formative study. JMIR Res Protoc. 2016;5(4):e229.
Bousquet J, Chavannes NH, Guldemond N, Haahtela T, Hellings PW, Sheikh A. Realising the potential of mHealth to improve asthma and allergy care: how to shape the future. Eur Respir J. 2017;49(5):1700447.
Lau AY, Arguel A, Dennis S, Liaw ST, Coiera E. “Why Didn't it Work?” Lessons from a randomized controlled trial of a web-based personally controlled health management system for adults with asthma. J Med Internet Res. 2015;17(12):e283.
Simpson AJ, Honkoop PJ, Kennington E, Snoeck-Stroband JB, Smith I, East J, et al. Perspectives of patients and healthcare professionals on mHealth for asthma self-management. Eur Respir J. 2017. https://doi.org/10.1183/13993003.01966-2016.
Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5 Suppl):S147–334.
Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126(3):466–76.
Brozek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines—2016 revision. J Allergy Clin Immunol. 2017;140(4):950–8.
Bousquet J, Hellings PW, Agache I, Bedbrook A, Bachert C, Bergmann KC, et al. ARIA 2016: care pathways implementing emerging technologies for predictive medicine in rhinitis and asthma across the life cycle. Clin Transl Allergy. 2016;6:47.
Lombardi C, Musicco E, Rastrelli F, Bettoncelli G, Passalacqua G, Canonica GW. The patient with rhinitis in the pharmacy. A cross-sectional study in real life. Asthma Res Pract. 2015;1:4.
Bousquet J, Schunemann HJ, Hellings PW, Arnavielhe S, Bachert C, Bedbrook A, et al. MACVIA clinical decision algorithm in adolescents and adults with allergic rhinitis. J Allergy Clin Immunol. 2016;138(2):367–74 (e2).
Bourret R, Bousquet J, Mercier J, Camuzat T, Bedbrook A, Demoly P, et al. MASK rhinitis, a single tool for integrated care pathways in allergic rhinitis. World Hosp Health Serv. 2015;51(3):36–9.
Hellings PW, Muraro A, Fokkens W, Mullol J, Bachert C, Canonica GW, et al. A common language to assess allergic rhinitis control: results from a survey conducted during EAACI 2013 Congress. Clin Transl Allergy. 2015;5:36.
Klimek L, Bergmann KC, Biedermann T, Bousquet J, Hellings P, Jung K, et al. Visual analogue scales (VAS): measuring instruments for the documentation of symptoms and therapy monitoring in cases of allergic rhinitis in everyday health care: position Paper of the German Society of Allergology (AeDA) and the German Society of Allergy and Clinical Immunology (DGAKI), ENT Section, in collaboration with the working group on Clinical Immunology, Allergology and Environmental Medicine of the German Society of Otorhinolaryngology, Head and Neck Surgery (DGHNOKHC). Allergo J Int. 2017;26(1):16–24.
Bousquet J, Farrell J, Crooks G, Hellings P, Bel EH, Bewick M, et al. Scaling up strategies of the chronic respiratory disease programme of the European Innovation Partnership on Active and Healthy Ageing (Action Plan B3: Area 5). Clin Transl Allergy. 2016;6:29.
Bousquet J, Caimmi DP, Bedbrook A, Bewick M, Hellings PW, Devillier P, et al. Pilot study of mobile phone technology in allergic rhinitis in European countries: the MASK-rhinitis study. Allergy. 2017;72(6):857–65.
Bousquet J, Bewick M, Arnavielhe S, Mathieu-Dupas E, Murray R, Bedbrook A, et al. Work productivity in rhinitis using cell phones: the MASK pilot study. Allergy. 2017;72(10):1475–84.
Aristodimou A, Antoniades A, Pattichis CS. Privacy preserving data publishing of categorical data through k-anonymity and feature selection. Healthc Technol Lett. 2016;3(1):16–21.
Aldeen YA, Salleh M, Razzaque MA. A comprehensive review on privacy preserving data mining. Springerplus. 2015;4:694.
Protection of personal data. Article 29 data protection working party. Opinion 05/2014 on anonymisation techniques. European Commission Justice Data Protection. 2014;0829/14/EN WP216. http://ec.europa.eu/justice/data-protection/index_en.htm. Accessed 30 Sept 2018.
Regulation (EU) 2016/679 of the European Parliamant and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation). Official Organ of the European Union. 2016. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0679&from=EN. Accessed 30 Sept 2018.
Article 28 EU General Data Protection Regulation (EU-GDPR). 2018. https://www.eugdpr.org/. Accessed 30 Sept 2018.
Directive 2002/58/EC of the European Parliament and of the Council of 12 July 2002 concerning the processing of personal data and the protection of privacy in the electronic communications sector (Directive on privacy and electronic communications). Off J Eur Commun L 201, 37; 31 July 2002.
Directive 2009/136/EC of The European Parliament and of the Council of 25 November 2009 amending Directive 2002/22/EC on universal service and users’ rights relating to electronic communications networks and services, Directive 2002/58/EC concerning the processing of personal data and the protection of privacy in the electronic communications sector and Regulation (EC) No 2006/2004 on cooperation between national authorities responsible for the enforcement of consumer protection laws. Off J Eur Union, L 337, 11; 18 December 2009.
Sweeney L. k-anonymity: a model for protecting privacy. Int J Uncertain Fuz Knowl Syst. 2002;10:557–70.
El Emam K, Dankar FK, Issa R, et al. A globally optimal k-anonymity method for the de-identification of health data. J Am Med Inform Assoc. 2009;16:670–82.
Samreth D, Arnavielhe S, Ingenrieth F, Bedbrook A, Onorato GL, Murray R, et al. Geolocation with respect to personal privacy for the Allergy Diary app—a MASK study. World Allergy Organ J. 2018;11(1):15. https://doi.org/10.1186/s40413-018-0194-3.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10.
Bousquet J, Agache I, Aliberti MR, Angles R, Annesi-Maesano I, Anto JM, et al. Transfer of innovation on allergic rhinitis and asthma multimorbidity in the elderly (MACVIA-ARIA)—EIP on AHA Twinning Reference Site (GARD research demonstration project). Allergy. 2018;73(1):77–92.
Konig HH, Bernert S, Angermeyer MC, Matschinger H, Martinez M, Vilagut G, et al. Comparison of population health status in six european countries: results of a representative survey using the EQ-5D questionnaire. Med Care. 2009;47(2):255–61.
Smith AF, Pitt AD, Rodruiguez AE, Alio JL, Marti N, Teus M, et al. The economic and quality of life impact of seasonal allergic conjunctivitis in a Spanish setting. Ophthalmic Epidemiol. 2005;12(4):233–42.
Bousquet J, VandenPlas O, Bewick M, Arnavielhe S, Bedbrook A, Murray R, et al. The Work Productivity and Activity Impairment Allergic Specific (WPAI-AS) Questionnaire using mobile technology: the MASK study. J Investig Allergol Clin Immunol. 2018;28(1):42–4.
Azevedo P, Correia de Sousa J, Bousquet J, Bugalho-Almeida A, Del Giacco SR, Demoly P, et al. Control of Allergic Rhinitis and Asthma Test (CARAT): dissemination and applications in primary care. Prim Care Respir J. 2013;22(1):112–6.
Fonseca JA, Nogueira-Silva L, Morais-Almeida M, Azevedo L, Sa-Sousa A, Branco-Ferreira M, et al. Validation of a questionnaire (CARAT10) to assess rhinitis and asthma in patients with asthma. Allergy. 2010;65(8):1042–8.
Nogueira-Silva L, Martins SV, Cruz-Correia R, Azevedo LF, Morais-Almeida M, Bugalho-Almeida A, et al. Control of allergic rhinitis and asthma test—a formal approach to the development of a measuring tool. Respir Res. 2009;10:52.
van der Leeuw S, van der Molen T, Dekhuijzen PN, Fonseca JA, van Gemert FA, Gerth van Wijk R, et al. The minimal clinically important difference of the control of allergic rhinitis and asthma test (CARAT): cross-cultural validation and relation with pollen counts. NPJ Prim Care Respir Med. 2015;25:14107.
Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–81.
Leger D, Annesi-Maesano I, Carat F, Rugina M, Chanal I, Pribil C, et al. Allergic rhinitis and its consequences on quality of sleep: an unexplored area. Arch Intern Med. 2006;166(16):1744–8.
Kopp-Kubel S. International Nonproprietary Names (INN) for pharmaceutical substances. Bull World Health Organ. 1995;73(3):275–9.
Santo K, Richtering SS, Chalmers J, Thiagalingam A, Chow CK, Redfern J. Mobile phone apps to improve medication adherence: a systematic stepwise process to identify high-quality apps. JMIR Mhealth Uhealth. 2016;4(4):e132.
Thakkar J, Kurup R, Laba TL, Santo K, Thiagalingam A, Rodgers A, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med. 2016;176(3):340–9.
Bousquet J, Arnavielhe S, Bedbrook A, Alexis-Alexandre G, van Eerd M, Murray R, et al. Treatment of allergic rhinitis using mobile technology with real world data: the MASK observational pilot study. Allergy. 2018;73(9):1763–74.
Devillier P, Chassany O, Vicaut E, de Beaumont O, Robin B, Dreyfus JF, et al. The minimally important difference in the Rhinoconjunctivitis Total Symptom Score in grass-pollen-induced allergic rhinoconjunctivitis. Allergy. 2014;69(12):1689–95.
Onder G, Palmer K, Navickas R, Jureviciene E, Mammarella F, Strandzheva M, et al. Time to face the challenge of multimorbidity. A European perspective from the joint action on chronic diseases and promoting healthy ageing across the life cycle (JA-CHRODIS). Eur J Intern Med. 2015;26(3):157–9.
Bousquet J, Onorato GL, Bachert C, Barbolini M, Bedbrook A, Bjermer L, et al. CHRODIS criteria applied to the MASK (MACVIA-ARIA Sentinel NetworK) Good Practice in allergic rhinitis: a SUNFRAIL report. Clin Transl Allergy. 2017;7:37.
Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737–45.
Caimmi D, Baiz N, Tanno LK, Demoly P, Arnavielhe S, Murray R, et al. Validation of the MASK-rhinitis visual analogue scale on smartphone screens to assess allergic rhinitis control. Clin Exp Allergy. 2017;47(12):1526–33.
Bousquet J, Devillier P, Anto JM, Bewick M, Haahtela T, Arnavielhe S, et al. Daily allergic multimorbidity in rhinitis using mobile technology: a novel concept of the MASK study. Allergy. 2018;73(8):1622–31.
Tan R, Cvetkovski B, Kritikos V, Price D, Yan K, Smith P, et al. Identifying the hidden burden of allergic rhinitis (AR) in community pharmacy: a global phenomenon. Asthma Res Pract. 2017;3:8.
Carr WW, Yawn BP. Management of allergic rhinitis in the era of effective over-the-counter treatments. Postgrad Med. 2017;129(6):572–80.
Members of the Workshop. ARIA in the pharmacy: management of allergic rhinitis symptoms in the pharmacy. Allergic rhinitis and its impact on asthma. Allergy. 2004;59(4):373–87
Bousquet J, Cruz A, Robalo-Cordeiro C. Obstructive sleep apnoea syndrome is an under-recognized cause of uncontrolled asthma across the life cycle. Rev Port Pneumol. 2016;22(1):1–3
Bousquet J, Agache I, Anto JM, Bergmann KC, Bachert C, Annesi-Maesano I, et al. Google Trends terms reporting rhinitis and related topics differ in European countries. Allergy 2017;72(8):1261–6.
Bousquet J, O'Hehir RE, Anto JM, D'Amato G, Mösges R, Hellings PW, Van Eerd M, Sheikh A. Assessment of thunderstorm-induced asthma using Google Trends. J Allergy Clin Immunol. 2017;140(3):891–3.
Authors’ contributions
All authors are MAKS members and have contributed to the design of the project. Many authors also included users and disseminated the project in their own country. All authors read and approved the final manuscript.
Acknowledgements
None.
Mask Study Group
J Bousquet1–3, PW Hellings4, W Aberer5, I Agache6, CA Akdis7, M Akdis7, MR Alberti8, R Almeida9, F Amat10, R Angles11, I Annesi-Maesano12, IJ Ansotegui13, JM Anto14–17, S Arnavielle18, E Asayag19, A Asarnoj20, H Arshad21, F Avolio22, E Bacci23, C Bachert24, I Baiardini25, C Barbara26, M Barbagallo27, I Baroni28, BA Barreto29, X Basagana14, ED Bateman30, M Bedolla-Barajas31, A Bedbrook2, M Bewick32, B Beghé33, EH Bel34, KC Bergmann35, KS Bennoor36, M Benson37, L Bertorello23, AZ Białoszewski38, T Bieber39, S Bialek40, C Bindslev-Jensen41, L Bjermer42, H Blain43,44, F Blasi45, A Blua46, M Bochenska Marciniak47, I Bogus-Buczynska47, AL Boner48, M Bonini49, S Bonini50, CS Bosnic-Anticevich51, I Bosse52, J Bouchard53, LP Boulet54, R Bourret55, PJ Bousquet12, F Braido25, V Briedis56, CE Brightling57, J Brozek58, C Bucca59, R Buhl60, R Buonaiuto61, C Panaitescu62, MT Burguete Cabañas63, E Burte3, A Bush64, F Caballero-Fonseca65, D Caillot67, D Caimmi68, MA Calderon69, PAM Camargos70, T Camuzat71, G Canfora72, GW Canonica25, V Cardona73, KH Carlsen74, P Carreiro-Martins75, AM Carriazo76, W Carr77, C Cartier78, T Casale79, G Castellano80, L Cecchi81, AM Cepeda82, NH Chavannes83, Y Chen84, R Chiron68, T Chivato85, E Chkhartishvili86, AG Chuchalin87, KF Chung88, MM Ciaravolo89, A Ciceran90, C Cingi91, G Ciprandi92, AC Carvalho Coehlo93, L Colas94, E Colgan95, J Coll96, D Conforti97, J Correia de Sousa98, RM Cortés-Grimaldo99, F Corti100, E Costa101, MC Costa-Dominguez102, AL Courbis103, L Cox104, M Crescenzo105, AA Cruz106, A Custovic107, W Czarlewski108, SE Dahlen109, C Dario110, J da Silva111, Y Dauvilliers112, U Darsow113, F De Blay114, G De Carlo115, T Dedeu116, M de Fátima Emerson117, G De Feo118, G De Vries119, B De Martino120, N de Paula Motta Rubini121, D Deleanu122, P Demoly12,68, JA Denburg123, P Devillier124, S Di Capua Ercolano125, N Di Carluccio66, A Didier126, D Dokic127, MG Dominguez-Silva128, H Douagui129, G Dray103, R Dubakiene130, SR Durham131, G Du Toit132, MS Dykewicz133, Y El-Gamal134, P Eklund135, E Eller41, R Emuzyte136, J Farrell95, A Farsi81, J Ferreira de Mello Jr137, J Ferrero138, A Fink-Wagner139, A Fiocchi140, WJ Fokkens141, JA Fonseca142, JF Fontaine143, S Forti97, JM Fuentes-Perez144, JL Gálvez-Romero145, A Gamkrelidze146, J Garcia-Aymerich14, CY García-Cobas147, MH Garcia-Cruz148, B Gemicioğlu149, S Genova150, C George151, JE Gereda152, R Gerth van Wijk153, RM Gomez154, J Gómez-Vera155, S González Diaz156, M Gotua157, I Grisle158, M Guidacci159, NA Guldemond160, Z Gutter161, MA Guzmán162, T Haahtela163, J Hajjam164, L Hernández165, JO’B Hourihane166, YR Huerta-Villalobos167, M Humbert168, G Iaccarino169, M Illario170, JC Ivancevich171, EJ Jares172, E Jassem173, SL Johnston174, G Joos175, KS Jung176, M Jutel177, I Kaidashev178, O Kalayci179, AF Kalyoncu180, J Karjalainen181, P Kardas182, T Keil183, PK Keith184, M Khaitov185, N Khaltaev186, J Kleine-Tebbe187, L Klimek188, ML Kowalski189, M Kuitunen190, I Kull191, P Kuna47, M Kupczyk47, V Kvedariene192, E Krzych-Fałta193, P Lacwik47, D Larenas-Linnemann194, D Laune18, D Lauri195, J Lavrut196, LTT Le197, M Lessa198, G Levato199, J Li200, P Lieberman201, A Lipiec193, B Lipworth202, KC Lodrup Carlsen203, R Louis204, O Lourenço205, JA Luna-Pech206, K Maciej47, A Magnan94, B Mahboub207, D Maier208, A Mair209, I Majer210, J Malva211, E Mandajieva212, P Manning213, E De Manuel Keenoy214, GD Marshall215, MR Masjedi216, JF Maspero217, E Mathieu-Dupas18, JJ Matta Campos218, AL Matos219, M Maurer 220, S Mavale-Manuel221, O Mayora97, MA Medina-Avalos222, E Melén223, E Melo-Gomes26, EO Meltzer224, E Menditto225, J Mercier226, N Miculinic227, F Mihaltan228, B Milenkovic229, G Moda230, MD Mogica-Martinez231, Y Mohammad232, I Momas233,234, S Montefort235, R Monti236, D Mora Bogado237, M Morais-Almeida238, FF Morato-Castro239, R Mösges240, A Mota-Pinto241, P Moura Santo242, J Mullol243, L Münter244, A Muraro245, R Murray246, R Naclerio247, R Nadif3, M Nalin28, L Napoli248, L Namazova-Baranova249, H Neffen250, V Niedeberger251, K Nekam252, A Neou253, A Nieto254, L Nogueira-Silva255, M Nogues2,256, E Novellino257, TD Nyembue258, RE O’Hehir259, C Odzhakova260, K Ohta261, Y Okamoto262, K Okubo263, GL Onorato2, M Ortega Cisneros264, S Ouedraogo265, I Pali-Schöll266, S Palkonen115, P Panzner267, NG Papadopoulos268, HS Park269, A Papi270, G Passalacqua271, E Paulino272, R Pawankar273, S Pedersen274, JL Pépin275, AM Pereira276, M Persico277, O Pfaar278,279, J Phillips280, R Picard281, B Pigearias282, I Pin283, C Pitsios284, D Plavec285, W Pohl286, TA Popov287, F Portejoie2, P Potter288, AC Pozzi289, D Price290, EP Prokopakis291, R Puy259, B Pugin292, RE Pulido Ross293, M Przemecka47, KF Rabe294, F Raciborski193, R Rajabian-Soderlund295, S Reitsma141, I Ribeirinho296, J Rimmer297, D Rivero-Yeverino298, JA Rizzo299, MC Rizzo300, C Robalo-Cordeiro301, F Rodenas302, X Rodo14, M Rodriguez Gonzalez303, L Rodriguez-Mañas304, C Rolland305, S Rodrigues Valle306, M Roman Rodriguez307, A Romano308, E Rodriguez-Zagal309, G Rolla310, RE Roller-Wirnsberger311, M Romano28, J Rosado-Pinto312, N Rosario313, M Rottem314, D Ryan315, H Sagara316, J Salimäki317, B Samolinski193, M Sanchez-Borges318, J Sastre-Dominguez319, GK Scadding320, HJ Schunemann58, N Scichilone321, P Schmid-Grendelmeier322, FS Serpa323, S Shamai240, A Sheikh324, M Sierra96, FER Simons325, V Siroux326, JC Sisul327, I Skrindo378, D Solé328, D Somekh329, M Sondermann330, T Sooronbaev331, M Sova332, M Sorensen333, M Sorlini334, O Spranger139, C Stellato118, R Stelmach335, R Stukas336, J Sunyer14–17, J Strozek193, A Szylling193, JN Tebyriçá337, M Thibaudon338, T To339, A Todo-Bom340, PV Tomazic341, S Toppila-Salmi163, U Trama342, M Triggiani118, C Suppli Ulrik343, M Urrutia-Pereira344, R Valenta345, A Valero346, A Valiulis347, E Valovirta348, M van Eerd119, E van Ganse349, M van Hague350, O Vandenplas351, MT Ventura352, G Vezzani353, T Vasankari354, A Vatrella118, MT Verissimo211, F Viart78, G Viegi355, D Vicheva356, T Vontetsianos357, M Wagenmann358, S Walker359, D Wallace360, DY Wang361, S Waserman362, T Werfel363, M Westman364, M Wickman191, DM Williams365, S Williams366, N Wilson, J Wright367, P Wroczynski40, P Yakovliev368, BP Yawn369, PK Yiallouros370, A Yorgancioglu371, OM Yusuf372, HJ Zar373, L Zhang374, N Zhong200, ME Zernotti375, M Zidarn376, T Zuberbier35, C Zubrinich259, A Zurkuhlen377
1University Hospital, Montpellier, France. 2MACVIA-France, Fondation partenariale FMC VIA-LR, Montpellier, France. 3VIMA. INSERM U 1168, VIMA : Ageing and chronic diseases Epidemiological and public health approaches, Villejuif, Université Versailles St-Quentin-en-Yvelines, UMR-S 1168, Montigny le Bretonneux, France and Euforea, Brussels, Belgium. 4Laboratory of Clinical Immunology, Department of Microbiology and Immunology, KU Leuven, Leuven, Belgium. 5Department of Dermatology, Medical University of Graz, Graz, Austria. 6Transylvania University Brasov, Brasov, Romania. 7Swiss Institute of Allergy and Asthma Research (SIAF), University of Zurich, Davos, Switzerland. 8Project Manager, Chairman of the Council of Municipality of Salerno, Italy. 9Center for Health Technology and Services Research- CINTESIS, Faculdade de Medicina, Universidade do Porto; and Medida, Lda Porto, Portugal. 10Allergology department, Centre de l’Asthme et des Allergies Hôpital d’Enfants Armand-Trousseau (APHP); Sorbonne Université, UPMC Univ Paris 06, UMR_S 1136, Institut Pierre Louis d’Epidémiologie et de Santé Publique, Equipe EPAR, Paris, France. 11Innovación y nuevas tecnologías, Salud Sector sanitario de Barbastro, Barbastro, Spain. 12Epidemiology of Allergic and Respiratory Diseases, Department Institute Pierre Louis of Epidemiology and Public Health, INSERM and Sorbonne Université, Medical School Saint Antoine, Paris, France 13Department of Allergy and Immunology, Hospital Quirón Bizkaia, Erandio, Spain. 14ICREA and Climate and Health (CLIMA) Program, ISGlobal, Barcelona, Spain. 15IMIM (Hospital del Mar Research Institute), Barcelona, Spain. 16CIBER Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain. 17Universitat Pompeu Fabra (UPF), Barcelona, Spain. 18KYomed INNOV, Montpellier, France. 19Argentine Society of Allergy and Immunopathology, Buenos Aires, Argentina. 20Clinical Immunology and Allergy Unit, Department of Medicine Solna, Karolinska Institutet, Stockholm, and Astrid Lindgren Children’s Hospital, Department of Pediatric Pulmonology and Allergy, Karolinska University Hospital, Stockholm, Sweden. 21David Hide Asthma and Allergy Research Centre, Isle of Wight, United Kingdom. 22Regionie Puglia, Bari, Italy. 23Regione Liguria, Genoa, Italy. 24Upper Airways Research Laboratory, ENT Dept, Ghent University Hospital, Ghent, Belgium. 25Allergy and Respiratory Diseases, Ospedale Policlinico San Martino, University of Genoa, Italy. 26PNDR, Portuguese National Programme for Respiratory Diseases, Faculdade de Medicina de Lisboa, Lisbon, Portugal. 27Director of the Geriatric Unit, Department of Internal Medicine (DIBIMIS), University of Palermo, Italy. 28Telbios SRL, Milan, Italy. 29Universidade do Estado do Pará, Belem, Brazil. 30Department of Medicine, University of Cape Town, Cape Town, South Africa. 31Hospital Civil de Guadalajara Dr Juan I Menchaca, Guadalarara, Mexico. 32iQ4U Consultants Ltd, London, UK. 33Section of Respiratory Disease, Department of Oncology, Haematology and Respiratory Diseases, University of Modena and Reggio Emilia, Modena, Italy. 34Department of Respiratory Medicine, Academic Medical Center (AMC), University of Amsterdam, The Netherlands. 35Comprehensive Allergy Center Charité, Department of Dermatology and Allergy, Charité - Universitätsmedizin Berlin; Global Allergy and Asthma European Network (GA2LEN), Berlin, Germany. 36Deptt of Respiratory Medicine, National Institute of Diseases of the Chest and Hospital, Dhaka, Bangladesh. 37Centre for Individualized Medicine, Department of Pediatrics, Faculty of Medicine, Linköping, Sweden. 38Department of Prevention of Environmental Hazards and Allergology, Medical University of Warsaw, Poland. 39BIEBER. Department of Dermatology and Allergy, Rheinische Friedrich-Wilhelms-University Bonn, Bonn, Germany 40Dept of Biochemistry and Clinical Chemistry, Faculty of Pharmacy with the Division of Laboratory Medicine, Warsaw Medical University, Warsaw, Poland. 41Department of Dermatology and Allergy Centre, Odense University Hospital, Odense Research Center for Anaphylaxis (ORCA), Odense, Denmark. 42Department of Respiratory Medicine and Allergology, University Hospital, Lund, Sweden. 43Department of Geriatrics, Montpellier University Hospital, Montpellier, France. 44EA 2991, Euromov, University Montpellier, France. 45Department of Pathophysiology and Transplantation, University of Milan, IRCCS Fondazione Ca’Granda Ospedale Maggiore Policlinico, Milan, Italy. 46Argentine Association of Respiratory Medicine, Buenos Aires, Argentina. 47Division of Internal Medicine, Asthma and Allergy, Barlicki University Hospital, Medical University of Lodz, Poland. 48Pediatric Department, University of Verona Hospital, Verona, Italy. 49Department of Public Health and Infectious Diseases, Sapienza University of Rome, Italy. 50Second University of Naples and Institute of Translational Medicine, Italian National Research Council. 51Woolcock Institute of Medical Research, University of Sydney and Woolcock Emphysema Centre and and Sydney Local Health District, Glebe, NSW, Australia. 52Allergist, La Rochelle, France. 53Associate professor of clinical medecine, Laval’s University, Quebec city, Head of medecine department, Hôpital de la Malbaie, Quebec, Canada. 54Quebec Heart and Lung Institute, Laval University, Québec City, Quebec, Canada. 55Centre Hospitalier Valenciennes, France. 56Head of Department of Clinical Pharmacy of Lithuanian University of Health Sciences, Kaunas, Lithuania. 57Institute of Lung Health, Respiratory Biomedical Unit, University Hospitals of Leicester NHS Trust, Leicestershire, UK; Department of Infection, Immunity and Inflammation, University of Leicester, Leicester, UK. 58Department of Health Research Methods, Evidence and Impact, Division of Immunology and Allergy, Department of Medicine, McMaster University, Hamilton, ON, Canada. 59Chief of the University Pneumology Unit- AOU Molinette, Hospital City of Health and Science of Torino, Italy. 60Universitätsmedizin der Johannes Gutenberg-Universität Mainz, Mainz, Germany. 61Pharmacist, Municipality Pharmacy, Sarno, Italy. 62University of Medicine and Pharmacy Victor Babes, Timisoara, Romania. 63Instituto de Pediatria, Hospital Zambrano Hellion Tec de Monterrey, Monterrey, Mexico. 64Imperial College and Royal Brompton Hospital, London, UK. 65Centro Medico Docente La Trinidad, CaRacas, Venezuela. 66Regional Director Assofarm Campania and Vice President of the Board of Directors of Cofaser, Salerno, Italy 67Service de pneumologie, CHU et université d’Auvergne, Clermont-Ferrand, France. 68Department of Respiratory Diseases, Montpellier University Hospital, France. 69Imperial College London - National Heart and Lung Institute, Royal Brompton Hospital NHS, London, UK. 70Federal University of Minas Gerais, Medical School, Department of Pediatrics, Belo Horizonte, Brazil 71Assitant Director General, Montpellier, Région Occitanie, France. 72Mayor of Sarno and President of Salerno Province, Director, Anesthesiology Service, Sarno “Martiri del Villa Malta” Hospital, Italy. 73Allergy Section, Department of Internal Medicine, Hospital Vall d’Hebron & ARADyAL Spanish Research Network, Barcelona, Spain. 74Department of Paediatrics, Oslo University Hospital and University of Oslo, Oslo, Norway. 75CEDOC, Integrated Pathophysiological Mechanisms Research Group, Nova Medical School, Campo dos Martires da Patria, Lisbon, and Serviço de Imunoalergologia, Centro Hospitalar de Lisboa Central, EPE, Lisbon, Portugal. 76Regional Ministry of Health of Andalusia, Seville, Spain. 77Allergy and Asthma Associates of Southern California, Mission Viejo, CA, USA. 78ASA - Advanced Solutions Accelerator, Clapiers, France. 79Division of Allergy/Immunology, University of South Florida, Tampa, Fla, USA. 80Celentano pharmacy, Massa Lubrense, Italy. 81SOS Allergology and Clinical Immunology, USL Toscana Centro, Prato, Italy. 82Allergy and Immunology Laboratory, Metropolitan University Hospital, Branquilla, Columbia. 83Department of Public Health and Primary Care, Leiden University Medical Center, Leiden, The Netherlands 84Capital Institute of Pediatrics, Chaoyang district, Beijing, China. 85School of Medicine, University CEU San Pablo, Madrid, Spain. 86David Tvildiani Medical University - AIETI Highest Medical School, David Tatishvili Medical Center Tbilisi, Georgia. 87Pulmonolory Research Institute FMBA, Moscow, Russia and GARD Executive Committee, Moscow, Russia. 88National Heart & Lung Institute, Imperial College, London, UK. 89Specialist social worker, Sorrento, Italy. 90Argentine Federation of Otorhinolaryngology Societies, Buenos Aires, Argentina. 91Eskisehir Osmangazi University, Medical Faculty, ENT Department, Eskisehir,Turkey. 92Medicine Department, IRCCS-Azienda Ospedaliera Universitaria San Martino, Genoa, Italy. 93Universidade Federal da Bahia, Escola de Enfermagem, Brazil. 94Plateforme Transversale d’Allergologie, Institut du Thorax, CHU de Nantes, Nantes, France. 95LANUA International Healthcare Consultancy, Northern Ireland, UK. 96Innovación y nuevas tecnologías, Salud Sector sanitario de Barbastro, Barbastro, Spain. 97Innovation and Research Office, Department of Health and Social Solidarity, Autonomous Province of Trento, Italy. 98Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Braga, Portugal; ICVS/3B’s, PT Government Associate Laboratory, Braga/Guimarães, Portugal. 99Guadalarara, Mexico. 100FIMMG (Federazione Italiana Medici di Medicina Generale), Milan, Italy. 101UCIBIO, REQUINTE, Faculty of Pharmacy and Competence Center on Active and Healthy Ageing of University of Porto (Porto4Ageing), Porto, Portugal. 102Mexico City, Mexico. 103IMT Mines Alès, Unversité Montpellier, Alès, France. 104Department of Medicine, Nova Southeastern University, Davie, University of Miami Dept of Medicine, Miami, Florida, USA. 105Regional Director Assofarm Campania and Vice President of the Board of Directors of Cofaser, Salerno, Italy. 106ProAR – Nucleo de Excelencia em Asma, Federal University of Bahia, Brasil and WHO GARD Planning Group, Brazil. 107Centre for Respiratory Medicine and Allergy, Institute of Inflammation and Repair, University of Manchester and University Hospital of South Manchester, Manchester, UK. 108Medical Consulting Czarlewski, Levallois, France. 109The Centre for Allergy Research, The Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden. 110Azienda Provinciale per i Servizi Sanitari di Trento (APSS-Trento), Italy. 111Department of Internal Medicine, Federal University of Santa Catarina, Trindade, Florianópolis, Santa Catarina, Brazil. 112Sleep Unit, Department of Neurology, Hôpital Gui-de-Chauliac Montpellier, Inserm U1061, France. 113Department of Dermatology and Allergy, Technische Universität München, Munich, Germany; ZAUM-Center for Allergy and Environment, Helmholtz Center Munich, Technische Universität München, Munich, Germany. 114Allergy Division, Chest Disease Department, University Hospital of Strasbourg, Strasbourg, France. 115EFA European Federation of Allergy and Airways Diseases Patients’ Associations, Brussels, Belgium 116AQuAS, Barcelna, Spain & EUREGHA, European Regional and Local Health Association, Brussels, Belgium 117Policlínica Geral do Rio de Janeiro, Rio de Janeiro – Brasil 118Department of Medicine, Surgery and Dentistry “Scuola Medica Salernitana”, University of Salerno, Salerno, Italy. 119Peercode BV, Geldermalsen,The Netherlands. 120Social workers oordinator, Sorrento, Italy. 121Federal University of the State of Rio de Janeiro, School of Medicine and Surgery, Rio de Janeiro, Brazil 122Allergology and Immunology Discipline, “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania. 123Department of Medicine, Division of Clinical Immunology and Allergy, McMaster University, Hamilton, Ontario, Canada. 124Laboratoire de Pharmacologie Respiratoire UPRES EA220, Hôpital Foch, Suresnes, Université Versailles Saint-Quentin, Université Paris Saclay, France. 125Farmacie Dei Golfi Group, Massa Lubrense, Italy. 126Rangueil-Larrey Hospital, Respiratory Diseases Department, Toulouse, France. 127University Clinic of Pulmology and Allergy, Medical Faculty Skopje, R Macedonia. 128Mexico City, Mexico. 129Service de Pneumo-Allergologie, Centre Hospitalo-Universitaire de Béni-Messous, Algiers, Algeria. 130Clinic of infectious, chest diseases, dermatology and allergology, Vilnius University, Vilnius, Lithuania. 131Allergy and Clinical Immunology National Heart and Lung Institute, Imperial College London, UK. 132Guy’s and st Thomas’ NHS Trust, Kings College London, UK. 133Section of Allergy and Immunology, Saint Louis University School of Medicine, Saint Louis, Missouri, USA. 134Pediatric Allergy and Immunology Unit, Children’s Hospital, Ain Shams University, Cairo, Egypt. 135Department of Computing Science, Umeå University, Sweden and Four Computing Oy, Finland. 136Clinic of Children’s Diseases, Faculty of Medicine, Vilnius University, Vilnius, Lithuania. 137University of São Paulo Medical School, Sao Paulo, Brazil 138Andalusian Agency for Healthcare Quality, Seville, Spain. 139Global Allergy and Asthma Platform GAAPP, Vienna, Austria. 140Division of Allergy, Department of Pediatric Medicine - The Bambino Gesù Children’s Research Hospital Holy see, Rome, Italy. 141Department of Otorhinolaryngology, Amsterdam, University Medical Centres, AMC, Amsterdam the Netherlands. 142CINTESIS, Center for Research in Health Technologies and Information Systems, Faculdade de Medicina da Universidade do Porto, Porto, Portugal and MEDIDA, Lda, Porto, Portugal 143Allergist, Reims, France. 144Hospital general regional 1 “Dr Carlos Mc Gregor Sanchez Navarro” IMSS, Mexico City, Mexico. 145Regional hospital of ISSSTE, Puebla, Mexico. 146National Center for Disease Control and Public Health of Georgia, Tbilisi, Georgia. 147Guadalarara, Mexico. 148Allergy Clinic, National Institute of Respiratory Diseases, Mexico City, Mexico. 149Department of Pulmonary Diseases, Istanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine, Istambul,Turkey. 150Allergology unit, UHATEM “NIPirogov”, Sofia, Bulgaria. 151Medical University, Faculty of Public Health, Sofia. 152Allergy and Immunology Division, Clinica Ricardo Palma, Lima, Peru. 153Department of Internal Medicine, section of Allergology, Erasmus MC, Rotterdam, The Netherlands. 154Allergy & Asthma Unit, Hospital San Bernardo Salta, Argentina. 155Allergy Clinic, Hospital Regional del ISSSTE ‘Lic. López Mateos’, Mexico City, Mexico. 156Head and Professor, Centro Regional de Excelencia CONACYT y WAO en Alergia, Asma e Inmunologia, Hospital Universitario , Universidad Autónoma de Nuevo León, Monterrey NL, Mexico. 157Center of Allergy and Immunology, Georgian Association of Allergology and Clinical Immunology, Tbilisi, Georgia. 158Latvian Association of Allergists, Center of Tuberculosis and Lung Diseases, Riga, Latvia. 159Federal District Base Hospital Institute, Brasília, Brazil. 160Institute of Health Policy and Management iBMG, Erasmus University, Rotterdam, The Netherlands 161University Hospital Olomouc – National eHealth Centre, Czech Republic. 162Immunology and Allergy Division, Clinical Hospital, University of Chile, Santiago, Chile. 163Skin and Allergy Hospital, Helsinki University Hospital, University of Helsinki, Helsinki, Finland. 164Centich : centre d’expertise national des technologies de l’information et de la communication pour l’autonomie, Gérontopôle autonomie longévité des Pays de la Loire, Conseil régional des Pays de la Loire, Centre d’expertise Partenariat Européen d’Innovation pour un vieillissement actif et en bonne santé, Nantes, France. 165Autonomous University of Baja California, Ensenada, Baja California, Mexico. 166Department of Paediatrics and Child Health, University College Cork, Cork, Ireland. 167Hospital General Regional 1 “Dr. Carlos MacGregor Sánchez Navarro” IMSS, Mexico City, Mexico. 168Université Paris-Sud; Service de Pneumologie, Hôpital Bicêtre; Inserm UMR_S999, Le Kremlin Bicêtre, France. 169Dipartimento di medicina, chirurgia e odontoiatria, università di Salerno, Italy. 170Division for Health Innovation, Campania Region and Federico II University Hospital Naples (R&D and DISMET) Naples, Italy. 171Servicio de Alergia e Immunologia, Clinica Santa Isabel, Buenos Aires, Argentina. 172President, Libra Foundation, Buenos Aires, Argentina. 173Medical University of Gdańsk, Department of Allergology, Gdansk, Poland. 174Airway Disease Infection Section, National Heart and Lung Institute, Imperial College; MRC & Asthma UK Centre in Allergic Mechanisms of Asthma, London, UK. 175Dept of Respiratory Medicine, Ghent University Hospital, Ghent, Belgium. 176Hallym University College of Medicine, Hallym University Sacred Heart Hospital, Gyeonggi-do, South Korea. 177Department of Clinical Immunology, Wrocław Medical University, Poland. 178Ukrainina Medical Stomatological Academy, Poltava, Ukraine. 179Pediatric Allergy and Asthma Unit, Hacettepe University School of Medicine, Ankara, Turkey. 180Hacettepe University, School of Medicine, Department of Chest Diseases, Immunology and Allergy Division, Ankara, Turkey. 181Allergy Centre, Tampere University Hospital, Tampere, Finland. 182First Department of Family Medicine, Medical University of Lodz, Poland. 183Institute of Social Medicine, Epidemiology and Health Economics, Charité - Universitätsmedizin Berlin, Berlin, and Institute for Clinical Epidemiology and Biometry, University of Wuerzburg, Germany. 184Department of Medicine, McMaster University, Health Sciences Centre 3V47, West, Hamilton, Ontario, Canada. 185National Research Center, Institute of Immunology, Federal Medicobiological Agency, Laboratory of Molecular immunology, Moscow, Russian Federation. 186GARD Chairman, Geneva, Switzerland. 187Allergy & Asthma Center Westend, Berlin, Germany. 188Center for Rhinology and Allergology, Wiesbaden, Germany. 189Department of Immunology and Allergy, Healthy Ageing Research Center, Medical University of Lodz, Lodz, Poland. 190Children’s Hospital and University of Helsinki, Finland. 191Centre for Clinical Research Sörmland, Uppsala University, Eskilstuna, Sweden. 192Faculty of Medicine, Vilnius University, Vilnius, Lithuania. 193Department of Prevention of Envinronmental Hazards and Allergology, Medical University of Warsaw, Poland. 194Center of Excellence in Asthma and Allergy, Médica Sur Clinical Foundation and Hospital, México City,, Mexico. 195Presidente CMMC, Milano, Italy. 196Head of the Allergy Department of Pedro de Elizalde Children’s Hospital, Buenos Aires, Argentina. 197University of Medicine and Pharmacy, Hochiminh City, Vietnam. 198Federal University of Bahia, Brazil. 199Sifmed, Milano, Italy. 200State Key Laboratory of Respiratory Diseases, Guangzhou Institute of Respiratory Disease, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China. 201Departments of Internal Medicine and Pediatrics (Divisions of Allergy and Immunology), University of Tennessee College of Medicine, Germantown, TN, USA. 202Scottish Centre for Respiratory Research, Cardiovascular & Diabetes Medicine, Medical Research Institute, Ninewells Hospital, University of Dundee, UK. 203Oslo University Hospital, Department of Paediatrics, Oslo, and University of Oslo, Faculty of Medicine, Institute of Clinical Medicine, Oslo, Norway. 204Department of Pulmonary Medicine, CHU Sart-Tilman, and GIGA I3 research group, Liege, Belgium. 205Faculty of Health Sciences and CICS – UBI, Health Sciences Research Centre, University of Beira Interior, Covilhã, Portugal. 206Department of Philosophical, Methodological and Instrumental Disciplines, CUCS, University of Guadalajara, Guadalajara, Mexico. 207Department of Pulmonary Medicine, Rashid Hospital, Dubai, UAE. 208Biomax Informatics AG, Munich, Germany. 209Director Gerneral for Health and Social Care, Scottish Government, Edinburgh, UK. 210Department of Respiratory Medicine, University of Bratislava, Bratislava, Slovakia. 211Coimbra Institute for Clinical and Biomedical Research (iCBR), Faculty of Medicine, University of Coimbra, Portugal; Ageing@Coimbra EIP-AHA Reference Site, Coimbra, Portugal. 212Medical center Iskar Ltd Sofia, Bulgaria. 213Department of Medicine (RCSI), Bon Secours Hospital, Glasnevin, Dublin, Ireland. 214Kronikgune, International Centre of Excellence in Chronicity Research Barakaldo, Bizkaia, Spain 215Division of Clinical Immunology and Allergy, Laboratory of Behavioral Immunology Research, The University of Mississippi Medical Center, Jackson, Mississippi, USA. 216Tobacco Control Research Centre;Iranian Anti Tobacco Association, Tehran, Iran. 217Argentine Association of Allergy and Clinical Immunology, Buenos Aires, Argentina. 218Mexico City, Mexico. 219University of Southeast Bahia, Brazil. 220Allergie-Centrum-Charité at the Department of Dermatology and Allergy, Charité - Universitätsmedizin Berlin, Germany 221Maputo Central Hospital--Department of Paediatrics, Mozambique. 222Veracruz, Mexico. 223Sachs’ Children and Youth Hospital, Södersjukhuset, Stockholm and Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden. 224Allergy and Asthma Medical Group and Research Center, San Diego, California, USA. 225CIRFF, Federico II University, Naples, Italy. 226Department of Physiology, CHRU, University Montpellier, Vice President for Research, PhyMedExp, INSERM U1046, CNRS UMR 9214, France. 227Croatian Pulmonary Society. 228National Institute of Pneumology M Nasta, Bucharest, Romania. 229Clinic for Pulmonary Diseases, Clinical Center of Serbia, Faculty of Medicine, University of Belgrade, Serbian Association for Asthma and COPD, Belgrade, Serbia. 230Regione Piemonte, Torino, Italy. 231Col Jardines de Sta Monica, Tlalnepantla, Mexico. 232National Center for Research in Chronic Respiratory Diseases, Tishreen University School of Medicine, Latakia, Syria. 233Department of Public health and health products, Paris Descartes University-Sorbonne Paris Cité, EA 4064 and Paris Municipal Department of social action, childhood, and health, Paris, France. 234Paris municipal Department of social action, childhood, and health, Paris, France. 235Lead Respiratory Physician Mater Dei Hospital Malta, Academic Head of Dept and Professor of Medicine University of Malta, Deputy Dean Faculty of Medicine and Surgery University of Medicine, La Valette, Malta. 236Department of Medical Sciences, Allergy and Clinical Immunology Unit, University of Torino & Mauriziano Hospital, Torino, Italy. 237Instituto de Prevision Social IPS HC, Socia de la SPAAI, Tesorera de la SLAAI, Asuncion, Paraguay. 238Allergy Center, CUF Descobertas Hospital, Lisbon, Portugal. 239Universidade de São Paulo, São Paulo, Brazil. 240Institute of Medical Statistics, and Computational Biology, Medical Faculty, University of Cologne, Germany and CRI-Clinical Research International-Ltd, Hamburg, Germany. 241General Pathology Institute, Faculty of Medicine, University of Coimbra, Portugal; Ageing@Coimbra EIP-AHA Reference Site, Coimbra, Portugal. 242Federal University of Bahia, Brazil. 243Rhinology Unit & Smell Clinic, ENT Department, Hospital Clínic; Clinical & Experimental Respiratory Immunoallergy, IDIBAPS, CIBERES, University of Barcelona, Spain. 244Danish Commitee for Health Education, Copenhagen East, Denmark. 245Food Allergy Referral Centre Veneto Region, Department of Women and Child Health, Padua General University Hospital, Padua, Italy. 246Director, Medical Communications Consultant, MedScript Ltd, Dundalk, Co Louth, Ireland and Honorary Research Fellow, OPC, Cambridge, UK Ireland. 247Johns Hopkins School of Medicine, Baltimore, Maryland, USA. 248General Manager of COFASER - Pharmacy Services Consortium, Salerno, Italy. 249Scientific Centre of Children’s Health under the MoH, Moscow, Russian National Research Medical University named Pirogov, Moscow, Russia. 250Director of Center of Allergy, Immunology and Respiratory Diseases, Santa Fe, Argentina Center for Allergy and Immunology, Santa Fe, Argentina. 251Dept of Otorhinolaryngology, Medical University of Vienna, AKH, Vienna, Austria. 252Hospital of the Hospitaller Brothers in Buda, Budapest, Hungary. 253Die Hautambulanz and Rothhaar study center, Berlin, Germany. 254Neumología y Alergología Infantil, Hospital La Fe, Valencia, Spain. 255Center for Health Technology and Services Research - CINTESIS and Department of Internal Medicine, Centro Hospitalar Sao Joao, Porto, Portugal. 256Caisse d’assurance retraite et de la santé au travail du Languedoc-Roussillon (CARSAT-LR), Montpellier, France. 257Director of Department of Pharmacy of University of Naples Federico II, Naples, Italy. 258ENT Department, University Hospital of Kinshasa, Kinshasa, Congo. 259Department of Allergy, Immunology and Respiratory Medicine, Alfred Hospital and Central Clinical School, Monash University, Melbourne, Victoria, Australia; Department of Immunology, Monash University, Melbourne, Victoria, Australia. 260Medical center “Research expert”, Varna, Bulgaria. 261National Hospital Organization, Tokyo National Hospital, Tokyo, Japan. 262Dept of Otorhinolaryngology, Chiba University Hospital, Chiba, Japan. 263Dept of Otolaryngology, Nippon Medical School, Tokyo, Japan. 264Jalisco, Guadalarara. 265Centre Hospitalier Universitaire Pédiatrique Charles de Gaulle, Ouagadougou, Burkina Faso. 266Dept of Comparative Medicine; Messerli Research Institute of the University of Veterinary Medicine and Medical University, Vienna, Austria. 267Department of Immunology and Allergology, Faculty of Medicine and Faculty Hospital in Pilsen, Charles University in Prague, Pilsen, Czech Republic. 268Division of Infection, Immunity & Respiratory Medicine, Royal Manchester Children’s Hospital, University of Manchester, Manchester, UK, and Allergy Department, 2nd Pediatric Clinic, Athens General Children’s Hospital “P&A Kyriakou,” University of Athens, Athens, Greece. 269Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, South Korea. 270Respiratory Medicine, Department of Medical Sciences, University of Ferrara, Ferrara, Italy. 271Allergy and Respiratory Diseases, Ospedale Policlino San Martino -University of Genoa, Italy. 272Farmacias Holon, Lisbon, Portugal. 273Department of Pediatrics, Nippon Medical School, Tokyo, Japan. 274University of Southern Denmark, Kolding, Denmark. 275Université Grenoble Alpes, Laboratoire HP2, Grenoble, INSERM, U1042 and CHU de Grenoble, France. 276Allergy Unit, CUF-Porto Hospital and Institute; Center for Research in Health Technologies and information systems CINTESIS, Universidade do Porto, Portugal. 277Sociologist, municipality area n33, Sorrento, Italy. 278Center for Rhinology and Allergology, Wiesbaden, Germany. 279Department of Otorhinolaryngology, Head and Neck Surgery, Universitätsmedizin Mannheim, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany. 280Centre for empowering people and communites, Dublin, UK. 281Conseil Général de l’Economie Ministère de l’Economie, de l’Industrie et du Numérique, Paris, France. 282Société de Pneumologie de Langue Française, Espace francophone de Pneumologie, Paris, France. 283Département de pédiatrie, CHU de Grenoble, Grenoble France. 284Medical School, University of Cyprus, Nicosia, Cyprus. 285Children’s Hospital Srebrnjak, Zagreb, School of Medicine, University J.J. Strossmayer, Osijek, Croatia. 286Karl Landsteiner Institute for Clinical and Experimental Pneumology, Hietzing Hospital, Vienna, Austria. 287University Hospital ‘Sv. Ivan Rilski’”, Sofia, Bulgaria. 288Allergy Diagnostic and Clinical Research Unit, University of Cape Town Lung Institute, Cape Town, South Africa. 289Vice-Presidente of IML, Milano, Italy. 290Centre of Academic Primary Care, Division of Applied Health Sciences, University of Aberdeen, Aberdeen, United Kingdom ; Observational and Pragmatic Research Institute, Singapore, Singapore. 291Department of Otorhinolaryngology University of Crete School of Medicine, Heraklion, Greece. 292European Forum for Research and Education in Allergy and Airway Diseases (EUFOREA), Brussels, Belgium. 293Cancun, Quintana Roo, Mexico. 294LungenClinic Grosshansdorf, Airway Research Center North, Member of the German Center for Lung Research (DZL), Grosshansdorf, Germany Department of Medicine, Christian Albrechts University, Airway Research Center North, Member of the German Center for Lung Research (DZL), Kiel, Germany. 295Department of Nephrology and Endocrinology, Karolinska University Hospital, Stockholm, Sweden. 296Farmácia São Paio, Vila Nova de Gaia, Porto, Portugal. 297St Vincent’s Hospital and University of Sydney, Sydney, New South Wales, Australia. 298Puebla, Mexico. 299Serviço de Pneumologia-Hosp das Clinicas UFPE-EBSERH, Recife, Brazil. 300Universidade Federal de São Paulo, São Paulo, Brazil. 301Centre of Pneumology, Coimbra University Hospital, Portugal. 302Polibienestar Research Institute, University of Valencia, Valencia, Spain. 303Pediatric Allergy and Clinical Immunology, Hospital Angeles Pedregal, Mexico City, Mexico. 304Getafe University Hospital Department of Geriatrics, Madrid, Spain. 305Association Asthme et Allergie, Paris, France. 306Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil. 307Primary Care Respiratory Research Unit Institutode Investigación Sanitaria de Palma IdisPa, Palma de Mallorca, Spain. 308Allergy Unit, Presidio Columbus, Rome, Catholic University of Sacred Heart, Rome and IRCCS Oasi Maria SS, Troina, Italy. 309Mexico City, Mexico. 310Regione Piemonte, Torino, Italy. 311Medical University of Graz, Department of Internal Medicine, Graz, Austria. 312Serviço de Imunoalergologia Hospital da Luz Lisboa Portugal. 313Hospital de Clinicas, University of Parana, Brazil. 314Division of Allergy Asthma and Clinical Immunology, Emek Medical Center, Afula, Israel. 315Honorary Clinical Research Fellow, Allergy and Respiratory Research Group, The University of Edinburgh, Edinburgh, UK. 316Showa University School of Medicine, Tokyo, Japan. 317Association of Finnish Pharmacies. 318Allergy and Clinical Immunology Department, Centro Médico-Docente la, Trinidad and Clínica El Avila, Caracas, Venezuela. 319Faculty of Medicine, Autnonous University of Madrid, Spain. 320The Royal National TNE Hospital, University College London, UK. 321DIBIMIS, University of Palermo, Italy. 322Allergy Unit, Department of Dermatology, University Hospital of Zurich, Zürich, Switzerland. 323Asthma Reference Center, Escola Superior de Ciencias da Santa Casa de Misericordia de Vitoria - Esperito Santo, Brazil. 324THe Usher Institute of Population Health Sciences and Informatics, The University of Edinburgh, Edinburgh, UK. 325Department of Pediatrics & Child Health, Department of Immunology, Faculty of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada. 326INSERM, Université Grenoble Alpes, IAB, U 1209, Team of Environmental Epidemiology applied to Reproduction and Respiratory Health, Université Joseph Fourier, Grenoble, France. 327Sociedad Paraguaya de Alergia Asma e Inmunologı´a, Paraguay. 328Division of Allergy, Clinical Immunology and Rheumatology, Department of Pediatrics, Federal University of São Paulo, São Paulo, Brazil. 329European Health Futures Forum (EHFF), Dromahair, Ireland. 330ENT, Aachen, Germany. 331Kyrgyzstan National Centre of Cardiology and Internal medicine, Euro-Asian respiratory Society, Bishkek, Kyrgyzstan. 332University Hospital Olomouc, Czech Republic. 333Department of Paediatric and Adolescent medicine, University Hospital of North Norway, Tromsø, Paediatric Research Group, Deptarment of Clinical Medicine, Faculty of Health Sciences, UiT The Arctic University of Norway, Tromsø, Norway. 334Presidente, IML (Lombardy Medical Initiative), Bergamo, Italy. 335Pulmonary Division, Heart Institute (InCor), Hospital da Clinicas da Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil. 336Public Health Institute of Vilnius University, Vilnius, Lithuania. 337Universidade Federal do Estado do Rio de Janeiro, Rio de Janeiro - Brazil 338RNSA (Réseau National de Surveillance Aérobiologique), Brussieu, France. 339The Hospital for Sick Children, Dalla Lana School of Public Health, University of Toronto, Canada. 340Imunoalergologia, Centro Hospitalar Universitário de Coimbra and Faculty of Medicine, University of Coimbra, Portugal. 341Department of ENT, Medical University of Graz, Austria. 342Campania Region, Division on Pharmacy and devices policy, Naples, Italy. 343Department of Respiratory Medicine, Hvidovre Hospital & University of Copenhagen, Denmark. 344Universidade Federal dos Pampas, Uruguaiana, Brazil. 345Division of Immunopathology, Department of Pathophysiology and Allergy Research, Center for Pathophysiology, Infectiology and Immunology, Medical University of Vienna, Vienna, Austria. 346Pneumology and Allergy Department CIBERES and Clinical & Experimental Respiratory Immunoallergy, IDIBAPS, University of Barcelona, Spain. 347Vilnius University Institute of Clinical Medicine, Clinic of Children’s Diseases, and Institute of Health Sciences, Department of Public Health, Vilnius, Lithuania; European Academy of Paediatrics (EAP/UEMS-SP), Brussels, Belgium. 348Department of Lung Diseases and Clinical Immunology Allergology, University of Turku and Terveystalo allergy clinic, Turku, Finland. 349PELyon; HESPER 7425, Health Services and Performance Resarch - Université Claude Bernard Lyon, France. 350Immunology and Allergy Unit, Department of Medicine Solna, Karolinska Institutet and University Hospital, Stockholm. 351Department of Chest Medicine, Centre Hospitalier Universitaire UCL Namur, Université Catholique de Louvain, Yvoir, Belgium. 352University of Bari Medical School, Unit of Geriatric Immunoallergology, Bari, Italy. 353Pulmonary Unit, Department of Medical Specialties, Arcispedale SMaria Nuova/IRCCS, AUSL di Reggio Emilia, Italy. 354FILHA, Finnish Lung Association, Helsinki, Finland. 355Pulmonary Environmental Epidemiology Unit, CNR Institute of Clinical Physiology, Pisa, Italy ; and CNR Institute of Biomedicine and Molecular Immunology “A Monroy”, Palermo, Italy. 356Medical University, Plovdiv, Bulgaria, Department of Otorhinolaryngology, Plovdiv, Bulgaria. 357Sotiria Hospital, Athens, Greece. 358Dept of Otorhinolaryngology, Universitätsklinikum Düsseldorf, Germany. 359Asthma UK, Mansell street, London, UK. 360Nova Southeastern University, Fort Lauderdale, Florida, USA. 361Department of Otolaryngology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore. 362Department of Medicine, Clinical Immunology and Allergy, McMaster University, Hamilton, Ontario, Canada. 363Division of Immunodermatology and Allergy Research, Department of Dermatology and Allergy, Hannover Medical School, Hannover, Germany. 364Department of Medicine Solna, Immunology and Allergy Unit, Karolinska Institutet and Department of ENT diseases, Karolinska University Hospital, Stockholm, Sweden. 365Eshelman School of Pharmacy, University of North Carolina, Chapel Hill, NC, USA. 366International Primary Care Respiratory Group IPCRG, Aberdeen, Scotland. 367Bradford Institute for Health Research, Bradford Royal Infirmary, Bradford, UK. 368Allergologyst - Medical College of Medical Faculty, Thracian University, Stara Zagora, Bulgaria. 369Department of Research, Olmsted Medical Center, Rochester, Minnesota, USA. 370Cyprus International Institute for Environmental & Public Health in Association with Harvard School of Public Health, Cyprus University of Technology, Limassol, Cyprus; Department of Pediatrics, Hospital “Archbishop Makarios III”, Nicosia, Cyprus. 371Celal Bayar University Department of Pulmonology, Manisa, Turkey. 372The Allergy and Asthma Institute, Pakistan. 373Department of Paediatrics and Child Health, Red Cross Children’s Hospital, and MRC Unit on Child & Adolescent Health, University of Cape Town, Cape Town, South Africa. 374Department of Otolaryngology Head and Neck Surgery, Beijing TongRen Hospital and Beijing Institute of Otolaryngology, Beijing, China. 375Universidad Católica de Córdoba, Córdoba, Argentina. 376University Clinic of Respiratory and Allergic Diseases, Golnik, Slovenia. 377Gesundheitsregion KölnBonn - HRCB Projekt GmbH, Kohln, Germany. 378Akershus University Hospital, Department of Otorhinolaryngology, Akershus, Norway.
Competing interests
SBA reports personal fees from Boehringer Ingelheim, GSK, AstraZeneca, TEVA, grants from TEVA, MEDA outside the submitted work. JB reports personal fees and other from Chiesi, Cipla, Hikma, Menarini, Mundipharma, Mylan, Novartis, Sanofi-Aventis, Takeda, Teva, Uriach, other from Kyomed, outside the submitted work. AAC reports grants and personal fees from GlaxoSmithKline, personal fees from Boehrinher Ingelheim, personal fees from AstraZeneca, personal fees from Novartis, personal fees from Merk, Sharp & Dohma, personal fees from MEDA Pharma, personal fees from EUROFARMA, personal fees from Sanofi Aventis, outside the submitted work. MD reports other from Allergan, outside the submitted work. WF reports grants from Meda, outside the submitted work. TH reports personal fees from Mundipharma, Novartis, and Orion Pharma, outside the submitted work. JJ reports grants and personal fees from novartis, ALK abello, personal fees from thermofischer, astra zeneca outside the submitted work. PK reports personal fees from Adamed, Boehringer Ingelheim, AstraZeneca, Chiesi, FAES, Berlin Chemie, Novartis, Polpharma, Allergopharma, outside the submitted work. VK has received payment for consultancy from GSK and for lectures from Stallergens, Berlin-CHemie outside the submitted work. DLL reports personal fees from GSK, Astrazeneca, MEDA, Boehringer Ingelheim, Novartis, Grunenthal, UCB, Amstrong, Siegfried, DBV Technologies, MSD, Pfizer, grants from Sanofi, Astrazeneca, Novartis, UCB, GSK, TEVA, Chiesi, Boehringer Ingelheim, outside the submitted work. RM reports personal fees from ALK, grants from ASIT biotech, Leti, BitopAG, Hulka, Ursapharm, Optima; personal fees from allergopharma, Nuvo, Meda, Friulchem, Hexal, Servier, Bayer, Johnson&Johnson, Klosterfrau, GSK, MSD, FAES, Stada, UCB, Allergy Therapeutics; grants and personal fees from Bencard, Stallergenes; grants, personal fees and non-financial support from Lofarma; non-financial support from Roxall, Atmos, Bionorica, Otonomy, Ferrero; personal fees and non-financial support from Novartis. NP reports personal fees from Novartis, Faes Farma, BIOMAY, HAL, Nutricia Research, Menarini, Novartis, MEDA, Abbvie, MSD, Omega Pharma, Danone, grants from Menarini, outside the submitted work. JLP reports grants from Air Liquide Foundation, AGIR à dom, Astrazeneca, Fisher & Paykel, Mutualia, Philips, Resmed, Vitalaire, other from AGIR à dom, Astrazeneca, Boehringer Ingelheim, Jazz Pharmaceutical, Night Balance, Philips, Resmed, Sefam, outside the submitted work. OP reports grants and personal fees from ALK-Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, Biotech Tools S.A., Laboratorios LETI/LETI Pharma, Anergis S.A., grants from Biomay, Nuvo, Circassia, Glaxo Smith Kline, personal fees from Novartis Pharma, MEDA Pharma, Mobile Chamber Experts (a GA2LEN Partner), Pohl-Boskamp, Indoor Biotechnologies, grants from, outside the submitted work. AMTB reports grants and personal fees from Novartis, Boehringer Ingelheim, Mundipharma, GSK (GlaxoSmithKline), personal fees from Teva Pharma, AstraZeneca, grants from Leti, outside the submitted work. SW reports personnal fees from Merck, GSK, Novartis, Behring, Shire, Sanofi, Barid Aralez, Mylan Meda, Pediapharm outside the submitted work.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
FMC VIA LR.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bousquet, J., Arnavielhe, S., Bedbrook, A. et al. MASK 2017: ARIA digitally-enabled, integrated, person-centred care for rhinitis and asthma multimorbidity using real-world-evidence. Clin Transl Allergy 8, 45 (2018). https://doi.org/10.1186/s13601-018-0227-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13601-018-0227-6