Abstract
Background and Objective
Adult-onset Still’s disease (AOSD) is an idiopathic systemic inflammatory disease of unknown aetiology. Some patients exhibit resistance to conventional treatment during long-term therapy. Janus kinase inhibitors (JAKinibs) may contribute to the improvement in AOSD symptoms via the JAK–signal transducer and activator of transcription (STAT) pathway. We aimed to explore the efficacy and safety of baricitinib in patients with refractory AOSD.
Methods
Patients were enrolled if they fulfilled the Yamaguchi AOSD classification criteria in China between 2020 and 2022. All patients were recognized as having refractory AOSD and were treated with oral baricitinib at a dosage of 4 mg once daily. A systemic score and prednisone dosage were used to evaluate the efficacy of baricitinib at months 1, 3, and 6 and at the last follow-up visit. The safety profiles were recorded and analysed at every assessment.
Results
Seven female patients with refractory AOSD received baricitinib. The median age was 31 (IQR 10) years. Treatment was terminated in one patient due to progressive macrophage activation syndrome (MAS). Others continued baricitinib treatment until the last assessment. The systemic score decreased significantly at 3 months (p = 0.0216), 6 months (p = 0.0007), and the last follow-up visit (p = 0.0007) compared with baseline. One month after the initiation of baricitinib, the rates of improvement in fever, rash, sore throat, and myalgia symptoms were 71.4% (5/7), 40% (2/5), 80% (4/5), and 66.7% (2/3), respectively. Five patients remained symptom-free at the last follow-up visit. In most patients, their laboratory values had returned to normal by the last follow-up visit. A significant reduction in the levels of C-reactive protein (CRP) (p = 0.0165) and ferritin (p = 0.0047) was observed at the last visit compared with baseline. The daily prednisolone dosage significantly decreased from 35.7 ± 15.1 mg/day at baseline to 8.8 ± 4.4 mg/day by month 6 (p = 0.0256), and it was 5.8 ± 4.7 mg/day at the last assessment (p = 0.0030). Leukopenia due to MAS was noted in one patient. Except for mild abnormalities in lipid parameters, no other severe adverse events occurred during follow-up.
Conclusions
Our findings suggest that baricitinib therapy could provide rapid and durable clinical and laboratory improvement in patients with refractory AOSD. Treatment seemed to be well tolerated by these patients. The long-term efficacy and safety of baricitinib therapy for AOSD should be assessed further in prospective controlled clinical trials in the future.
Trial Registration
Trial registration number (TRN): ChiCTR2200061599. Date of registration: 29 June 2022 (retrospectively registered).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Inhibitors of the JAK-STAT pathway can control various proinflammatory cytokines. |
Baricitinib is increasingly being used to treat auto-inflammatory diseases. |
Additional clinical studies are needed to confirm the therapeutic effect of baricitinib in adult-onset Still’s disease. |
1 Introduction
Adult-onset Still’s disease (AOSD) is a rare autoinflammatory disease with multisystemic involvement characterized by spiking fever, salmon-pink rash, arthralgia or arthritis, neutrophilic leucocytosis and hyperferritinaemia [1, 2]. It was first described by George Still in 1897 [3]. The annual incidences of AOSD have been reported to be 0.16 and 0.62 per 100,000 persons worldwide [4,5,6], 3.9 per 100,000 individuals in Japan and 6.77 per 100,000 individuals in Turkey [4, 7].
A few AOSD patients have life-threatening complications, such as macrophage activation syndrome (MAS), fulminant hepatitis, disseminated intravascular coagulation (DIC), or thrombotic microangiopathy (TMA), which should be urgently considered and managed. The mortality rates of AOSD have been reported to be between 2.6% and 5.5% [8]. Glucocorticoids (GC), nonsteroidal anti-inflammatory drugs (NSAIDs), and conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) are regarded as conventional therapies for AOSD. However, the disease cannot be controlled by these traditional approaches in at least 30–40% of patients [9, 10].
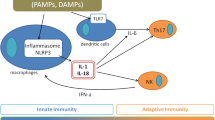
Research into the pathogenic mechanisms of AOSD has progressed over the past two decades. The proinflammatory cytokines released by innate immune cells, namely, interleukin (IL)-1, IL-6, IL-8, IL-17, IL-18, IL-23, tumour necrosis factor (TNF)-α, interferon (IFN)-γ, and macrophage inhibitory factors, can trigger a cytokine storm, thereby contributing to the initiation of AOSD [11,12,13]. Recently, the treatment efficacy of cytokine inhibitors targeting IL-1 and IL-6 has been determined in AOSD patients [10]. New evidence has been reported that Janus kinase (JAK) inhibitor (JAKinib) therapy is an option for patients with refractory AOSD, especially for those dependent on medium- to high-dose corticosteroids, as JAKinib therapy evidently reduced corticosteroid use [14].
Baricitinib, a JAK1/2 inhibitor, has been successfully used to treat severe inflammatory conditions. Although only sporadic cases have been reported thus far, as a crucial inhibitor of a variety of proinflammatory cytokines, baricitinib has shown excellent effectiveness in AOSD patients who have a low response to conventional and biological treatment [15]. Herein, we sought to report the efficacy and safety profile of baricitinib for patients with refractory AOSD. We further sought to evaluate the long-term clinical effects and therapeutic role of baricitinib, to address its applicability in clinical practice.
2 Methods
This was a prospective, single-centre, open-label, single-arm study of AOSD patients. The trial was a pilot study for a ‘proof-of-concept’ of the clinical efficacy and safety of baricitinib in refractory AOSD.
2.1 Patients Characteristics
Patients with AOSD were eligible for enrolment in the Department of Rheumatology and Immunology of Tianjin Medical University General Hospital if they fulfilled the Yamaguchi AOSD classification criteria [16] between 2020 and 2022. Before diagnosis or during a relapse episode, all patients underwent careful laboratory and radiologic screening to exclude some diseases mimicking AOSD, including infections, malignancy, autoimmune diseases, and other autoinflammatory disorders. All patients were diagnosed as having refractory AOSD as defined previously [17, 18].
Demographic and clinical information were obtained from all patients including sex, age, disease duration, and relevant investigations, as well as clinical AOSD-related characteristics. Enrolled patients were administered baricitinib 4 mg in tablet form once daily with background treatment. The follow-up duration was between 2 and 18 months.
2.2 Outcome Assessments
After starting baricitinib treatment, patients were closely monitored via clinical and laboratory assessments to evaluate the efficacy and safety of baricitinib at monthly visits.
The primary endpoints of the study were evaluated based on clinical and laboratory effects of baricitinib on refractory AOSD. Effective treatment was considered when all initial clinical manifestations and abnormal laboratory results had resolved, while ineffective treatment was considered when two or more clinical manifestations or abnormal laboratory tests persisted [19]. AOSD severity was measured by a modified Pouchot’s systemic score [20]. Patients were excluded if a major clinical event or a change in the therapeutic approach occurred within the first 2 consecutive weeks of baricitinib treatment. Secondary endpoints included corticosteroid-sparing effects during follow-up. The parameters used to verify the efficacy of baricitinib were the systemic score and prednisone dosage. These parameters were evaluated at baseline, at months 1, 3, and 6, and at the last follow-up visit.
2.3 Safety Assessments
Safety was determined by obtaining information on adverse events (AEs), serious adverse events (SAEs), and relevant laboratory changes, which were recorded at every visit, including white blood cell (WBC) count, neutrophil percentage, platelet count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), ferritin, plasma D-dimer, cholesterol, triglyceride, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C).
2.4 Statistical Analysis
All data analyses were performed using GraphPad Prism (version 9.0.0, GraphPad Software Inc., USA). Data such as demographic, clinical, and other disease-related variables are expressed as frequencies (percentages when appropriate) for categorical variables and the mean, standard deviation (SD), median, and interquartile range (IQR) for quantitative variables. After assessing normality with the Kolmogorov–Smirnov test, pairwise comparisons of qualitative data were performed using nonparametric ANOVA (Kruskal–Wallis test followed by Dunn’s multiple comparisons post-hoc test) by calculating the 95% confidence interval. Differences were considered to be significant at a level of p < 0.05 in all instances.
3 Results
3.1 Patient Characteristics
Seven female patients newly diagnosed with refractory AOSD who received baricitinib 4 mg once daily were included in the study. Patients were between 17 and 50 years old, with a median age of 31 (IQR 10) years. Every patient underwent baseline investigations to exclude infection, malignancy, other inflammatory diseases, connective tissue diseases, and pregnancy. Infectious aetiology testing included T-SPOT.TB (T-SPOT), procalcitonin (PCT), viral serology, and blood culture were performed. The median disease duration before baricitinib initiation was 11 (IQR 72) months. The clinical AOSD-related characteristics included fever (n = 7, 100%), abnormal liver function (n = 6, 85.7%), rash (n = 5, 71.4%), sore throat (n = 5, 71.4%), polyarthritis (n = 4, 57.1%), lymphadenopathy (n = 5, 71.4%), myalgia (n = 3, 42.9%), splenomegaly (n = 3, 42.9%), hepatomegaly (n = 2, 28.6%), arthralgia (n = 1, 14.3%), serositis (n = 1, 14.3%), abdominal pain (n = 1, 14.3%), and MAS (n = 1, 14.3%). The baseline demographic information, clinical characteristics, and differential diagnostic investigations are shown in Table 1.
Table 2 provides details about treatment history and baseline treatments at the initiation of baricitinib therapy. Patients had been previously administered GC (n = 7, 100%), cDMARDs (n = 6, 85.8%), NSAIDs (n = 2, 28.6%), and interleukin-6 (IL-6) receptor monoclonal antibody (tocilizumab, TCZ) (n = 1, 14.3%). Two severe patients received combination therapy with methylprednisolone pulse (500 mg once daily for 3 days [QD *3d]), plasma exchange (PLEX, once every other day *3 times), and intravenous immunoglobulin (IVIG, 20 g/d *3d) at baseline.
All patients received oral baricitinib at a dosage of 4 mg once daily. The use of concomitant medications (glucocorticoids, NSAIDs, and cDMARDs) was consistent from the initiation time. Six patients continued baricitinib treatment from initiation to the last assessment. Three patients decreased the dosage of baricitinib to 2 mg once daily after 1 year of treatment and maintained complete remission until the last visit. One patient terminated baricitinib after 2 months due to progressive MAS. The details regarding the above data are presented in Table 2.
3.2 Changes in Clinical Characteristics
At the 1-month follow-up, the symptoms of polyarthritis, arthralgia, serositis, and abdominal pain had quickly resolved, achieving complete control (100%). In addition, the rates of improvement in fever, abnormal liver function, rash, sore throat, lymphadenopathy, myalgia, and hepatomegaly were 71.4% (5/7), 50% (3/6), 40% (2/5), 80% (4/5), 60% (3/5), 66.7% (2/3), and 50% (1/2), respectively. No treatment response was observed regarding splenomegaly during the first month of therapy. At 3 months of follow-up, most symptoms had disappeared, except fever and lymphadenopathy. Five patients remained symptom free from 6 months to the last follow-up visit. One patient still had lymphadenopathy at the last visit (8 months). One patient relapsed when the dosage of prednisone was reduced to 10 mg/day at the 4- and 5-month visits. This patient recovered and maintained remission after the prednisone dosage was again increased to 15 mg/day at the 6-month assessment.
3.3 Changes in Inflammatory Markers
The median levels of inflammatory markers ESR, CRP, and serum ferritin were 45 mm/h (IQR 9), 56.9 mg/L (IQR 75.8), and 5666 ± 3803 mg/mL (IQR 6623), respectively, at treatment initiation (Fig. 1). At the 6-month assessment, serum ferritin decreased to 154 mg/mL (IQR 1061), indicating a significant decrease (p = 0.0253) from baseline (Fig. 2). A significant reduction in the levels of CRP (median 5.0, IQR 3, p = 0.0165) and ferritin (median 70, IQR 795, p = 0.0047) was observed at the last assessment (Fig. 2). In addition, serum IL-6 was assessed in patient 1 due to TCZ therapy, and the level of IL-6 was 25.6 pg/mL (normal ≤5.9 pg/mL) before TCZ was administered and 1000.0 pg/mL after one intravenous dose of TCZ. It should be emphasized that unusual changes in WBC count, neutrophil percentage, ESR, CRP, and lipid parameters were found in one patient due to MAS.
The statistical significance of the inflammatory parameters, systemic score, and Pred dosage. The statistical results for CRP (a), ferritin (b), modified Pouchot’s systemic score (c), and Pred dosage (d) during baricitinib treatment in the study are shown. Error bars represent the IQR for the median CRP value, ferritin level, modified Pouchot’s systemic score, and SD for the mean for Pred dosage. CRP C-reactive protein, IQR interquartile range, Pred prednisone, SD standard deviation
3.4 Changes in AOSD Severity Score
Measurements of efficacy in baricitinib treatment were performed at months 1, 3, and 6 and the last follow-up visit. The median systemic score was 7 points (IQR 3) at baseline, 2 points (IQR 3) at month 1, 0.5 points (IQR 2) at month 3, 0 points (IQR 0.25) at month 6, and 0 points (IQR 0.25) at the last visit. When compared with the initiation of baricitinib, the systemic score decreased significantly at 3 months (p = 0.0216), 6 months (p = 0.0007), and the last follow-up visit (p = 0.0007), with no significance between the 1-month follow-up and the baseline (Fig. 2). Complete remission was achieved in three out of six patients at the 3-month visit and five out of six patients at the 6-month visit. One patient achieved partial remission at the last visit.
3.5 Changes in Corticosteroid Sparing
The effect of sparing corticosteroids with baricitinib was also investigated in this study. At the beginning of baricitinib administration, the mean corticosteroid equivalent to prednisolone dosage was 35.7 ± 15.1 mg/day (10–50). The average dose of prednisone was 27.5 ± 12.8 mg/day (7.5–40) at the 1-month visit and 13.8 ± 5.9 mg/day (5–20) at the 3-month visit, while no significant difference was observed among the 1-month, 3-month and baseline assessments. The daily prednisolone dosage was 8.8 ± 4.4 mg/day (0.0–12.5; p = 0.0256) for the month-6 visit and 5.8 ± 4.7 mg/day (0–12.5; p = 0.0030) for the last visit, which was significantly reduced compared with the baseline (Fig. 2). One patient stopped prednisolone at a 12-month visit and maintained remission thereafter.
3.6 Safety Assessments
None of the patients experienced severe adverse events during follow-up.
Before baricitinib treatment, the WBC count, neutrophil percentage, and platelet count were 11.6 ± 6.1*109/L (4.4–21.3), 85.6 ± 6.0% (77.4–92.8), and 277.9 ± 125.4 × 109/L (142–499) on average, respectively. The median plasma D-dimer level was 1886 ng/mL FEU (fibrinogen equivalent units) (IQR 2452). Despite temporary fluctuations, these indicators gradually returned to normal values and stabilized in six out of seven patients (Fig. 3). No significant difference was observed in the four parameters during the treatment visits compared with the baseline. Leukopenia was observed in one patient due to MAS. The neutrophil percentage mildly decreased and returned to normal in two patients.
Serological assays from the laboratory were monitored. The trends of WBC (a), Neu (b), D-dimer (c), and PLT (d) values during baricitinib treatment are shown. Leukopenia was found due to MAS in patient 7. FEU fibrinogen equivalent units, MAS macrophage activation syndrome, PLT platelet, WBC white blood cell
From initiation to the last visit, the levels of cholesterol, triglycerides, HDL-C, and LDL-C remained stable in four out of seven patients (Fig. 4). Increasing cholesterol and triglyceride levels were recorded at the 1-month visit for three patients independently. The cholesterol and triglyceride levels continuously increased in one patient due to MAS, while they showed only mild elevation in the other two patients at the last visit. HDL-C increased slightly in two patients at the 1-month and 3-month visits and decreased to normal at the last visit. In contrast, decreased HDL-C levels were found in one patient with MAS at the 1-month and 2-month visits. LDL-C was mildly elevated in two patients (Fig. 4). There was no significant variation in lipid parameters during the follow-up visits.
4 Discussion
AOSD is an idiopathic systemic inflammatory disease of unknown aetiology. With complicated and diverse clinical representations, AOSD more commonly affects young adults, with a higher prevalence observed in women [21, 22]. Corticosteroids are considered to be the first-choice treatment for the amelioration of symptoms in AOSD by nonspecifically suppressing inflammatory cytokines. However, corticosteroid dose dependence was common in patients with AOSD, which was induced by long-term treatment. Although a subset of patients will respond favourably to empirical treatment, long-term therapy with corticosteroids also brings about various side effects, such as infection and hypertension. Patients with refractory AOSD often have limited benefit and high rate of relapse, nevertheless, there is a relative paucity of literature focusing on therapy for refractory AOSD.
Treatment with cytokine-targeting biologics has been recommended in recent years for AOSD patients who are refractory to conventional corticosteroid and DMARD therapy [1, 6]. Both the IL-1 inhibitors anakinra and canakinumab are currently approved for the treatment of AOSD [23]. Notwithstanding, conventional therapy has remained the main choice for AOSD because anti-interleukin 1 (IL-1) agents are not currently available in mainland China. Based on the latest pathogenic and clinical studies, treatments that inhibit the JAK-STAT pathway may produce beneficial outcomes in patients with AOSD [14, 24,25,26]. Thus, we hypothesized that baricitinib could play a pivotal role in the treatment of refractory AOSD.
In this study, we administered baricitinib to seven female patients, all of whom were refractory to traditional therapy. Their clinical AOSD-related characteristics have been described previously. Of note, one patient in this study presented severe abdominal pain after TCZ treatment. A prominently increasing level of serum IL-6 was also observed at that time. Several studies have shown that the prevalence of abdominal pain, a serious complication of AOSD, is between 1.9 and 13% [2, 27]. Although the underlying pathological process is not fully clear, abdominal pain symptoms may be induced by vasculitis secondary to high levels of IL-6 in AOSD patients. Accurate evaluations should be performed for high-risk patients.
Once-daily oral baricitinib at a dosage of 4 mg obviously contributed to clinical improvement in most patients in this study. Systemic symptoms rapidly alleviated after the initiation of baricitinib, and symptoms of polyarthritis, arthralgia, serositis, and abdominal pain completely disappeared after 1 month and remained stable for a long time. Arthralgia and arthritis are very common in patients with AOSD, with the prevalence of articular involvement reported to be approximately 40–100% [25]. The control of joint symptoms in patients with AOSD is usually more difficult than the control of systemic symptoms. Recently, Kacar et al. [15] described significant efficacy for articular items in refractory AOSD patients. On the other hand, Smolen et al. [28] demonstrated the long-term efficacy of baricitinib in patients with early and refractory rheumatoid arthritis. Both findings indicated that a JAK 1/2 inhibitor with baricitinib may be beneficial for joint involvement in patients with refractory AOSD. The majority of manifestations gradually resolved after 3 months of treatment with baricitinib. Lymphadenopathy was observed in only one patient at the last assessment.
Regarding the laboratory assessments, serological test results showed significant normalization in almost all patients. Inflammatory markers such as CRP, ESR, and serum ferritin rapidly decreased from the initiation of baricitinib treatment. Transient leukopenia and abnormal variations in lipid parameters were found in one patient due to MAS. We also observed that the decrease in CRP did not occur in parallel to the clinical improvement in one patient with AOSD-related MAS. These phenomena should be thoroughly investigated in the future.
In the present study, we identified the positive efficacy of baricitinib with a modified Pouchot’s systemic score in patients with refractory AOSD. The response to baricitinib treatment was rapid and sustained in most of our patients. The systemic score significantly decreased after 3 months of treatment with baricitinib. Three out of six patients had achieved complete resolution at the 3-month visit, and five patients had achieved complete remission at the last visit. Relapse of the disease only occurred in one patient when the dosage of glucocorticoids was tapered to 10 mg/day at the 4-month visit. The patient recovered soon after the dosage was increased to 15 mg/day at the 6-month visit. Patient 7 withdrew from the study as a result of AOSD-related MAS exacerbation.
JAKinibs are currently the second-/third-line medication for the treatment of AOSD. However, successful treatment with baricitinib in AOSD patients who had failed conventional and biological agent therapies was recently reported by Kacar et al. [15] and Ladhari et al. [29]. In a previous study from China, Hu et al. [30] concluded that tofacitinib can contribute to disease remission/revolution in patients with refractory AOSD. Yoshida et al. [31] demonstrated that baricitinib was the strongest inhibitor of IFN-γ-mediated signalling pathways in innate immune cells. Both the literature and our study suggest that baricitinib could be used for the treatment of refractory AOSD.
Regarding secondary endpoints, the corticosteroid-sparing effect was carefully estimated during the entire follow-up. Encouragingly, important variations emerged in our study. The prednisone dosage was apparently reduced during baricitinib therapy. In terms of the data, statistical significance was observed at the 3-month visit for the first time. Corticosteroids were continuously reduced in six patients until the last assessment. One patient stopped glucocorticoids at the 12-month visit and maintained remission thereafter. Regarding the corticosteroid-sparing effect, a homologous result was observed with tofacitinib treatment for refractory AOSD [30]. Both of these studies illustrate the exceptional effectiveness of JAKinibs in terms of corticosteroid reduction. However, we noticed that some patients still needed long-term and low-dose corticosteroid treatment in these studies. Thus, further studies with a larger number of cases should be conducted to verify this activity.
Interestingly, patient 1 received TCZ therapy before baricitinib initiation in our study. This patient developed persistent fever, severe fatigue, abnormal liver function, and abdominal pain after one intravenous injection of TCZ. A methylprednisolone pulse dose, PLEX, and IVIG were administered immediately to address MAS-like manifestations. Subsequently, oral baricitinib was initiated in combination with CsA, resulting in a dramatic improvement in symptoms and complete remission 1 month later. Recently, MAS-like manifestations induced by biological agents have been reported in AOSD [32, 33]. In some cases of AOSD, MAS is aggravated during TCZ therapy [34, 35]. The transient enhancement in target cytokines after initial inhibition and aberrant cytokine levels observed during therapy were presumed to be the main mechanisms of MAS associated with TCZ [36, 37]. As a selective JAK1/JAK2 inhibitor, baricitinib may strongly control the cytokine storm likely induced by TCZ. Successful treatment of this patient illustrated that baricitinib can play a positive role in refractory AOSD, especially in patients who have a poor or no response to TCZ. Clinicians should carefully consider whether AOSD patients have existing MAS risk factors before adding biological agents. Additional studies are required in the future to confirm these observations.
Notably, patient 7 exhibited MAS at the start of baricitinib treatment. Although the systemic score decreased from 8 to 4, the patient continued to have severe fatigue, lymphadenopathy, splenomegaly, abnormal liver function, hepatomegaly, and high-level serum ferritin at the 2-month assessment. VP16 combined with ruxolitinib was subsequently administered, and fortunately, the patient achieved complete remission 3 months later. MAS is one of the most serious and potentially life-threatening complications of AOSD, with a reported mortality rate ranging between 20% and 42% [38, 39]. MAS is also known as a hemophagocytic syndrome and is considered to be a form of secondary hemophagocytic lymphohistiocytosis (HLH). HLH-like manifestations are often referred to as MAS [26]. Ruxolitinib, a JAK1/JAK2 inhibitor, has already been approved for the treatment of myeloproliferative neoplasms (MPNs), steroid-refractory graft-vs-host disease (GVHD), and HLH [40, 41]. Recent studies have shown that ruxolitinib can reverse many HLH manifestations, including splenomegaly, cytopenia, hypercytokinaemia, peripheral organ effects, and CNS inflammation, and can significantly prolong survival [25, 42]. We speculate that baricitinib, as a structural analogue of ruxolitinib, may exert positive effects on AOSD-associated MAS. Despite the ineffective result for this patient, more basic and clinical studies are needed.
Adverse events were closely monitored in this study. JAKinibs block the downstream signalling of a variety of cytokines relevant for several physiological functions. Therefore, the various adverse effects that can arise from JAKinib treatment are concerning. Very common adverse effects of baricitinib are upper respiratory tract infections and hypercholesterolaemia. There is a growing concern that patients treated with JAKinibs may experience an increased risk for thromboembolic events (TEs) and cytopenia. During the follow-up, TEs due to platelet count elevation were not observed in patients; leukopenia and hypercholesterolaemia were observed in one patient due to MAS. The neutrophil percentage decreased but returned to normal in two patients after 6 months of treatment, but discontinuation of baricitinib was not needed. None of the patients developed anaemia. Although no severe adverse events were observed in the present study, the safety of baricitinib as a long-term therapy should be carefully assessed.
There are still some limitations to our study. There was a sex bias in this study, as all the enrolled patients were female. Furthermore, there was no patient-administered baricitinib monotherapy and no rigorously randomized controlled trial in the current study. We wonder whether baricitinib monotherapy would have created beneficial effects for AOSD patients, but this question awaits further research. In addition, further and broader studies with larger numbers of patients and longer duration of follow-up are needed to support the present results.
5 Conclusions
Our findings suggest that baricitinib therapy could provide prompt and persistent clinical and laboratory improvement in patients with AOSD, especially in those who have a poor response to conventional and biological therapy. Baricitinib appeared to be well tolerated. The long-term efficacy and safety of baricitinib in AOSD patients should be fully established in prospective controlled clinical trials in the future.
Abbreviations
- AOSD:
-
Adult-onset Still’s disease
- JAKinibs:
-
Janus kinase inhibitors
- STAT:
-
Signal transducer and activator of transcription
- IQR:
-
Interquartile range
- MAC:
-
Macrophage activation syndrome
- CRP:
-
C-reactive protein
- DIC:
-
Disseminated intravascular coagulation
- TMA:
-
Thrombotic microangiopathy
- GC:
-
Glucocorticoids
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- csDMARDs:
-
Conventional synthetic disease-modifying anti-rheumatic drugs
- IL:
-
Interleukin
- TNF:
-
Tumour necrosis factor
- IFN:
-
Interferon
- WBC:
-
White cell count
- PLT:
-
Platelet
- ESR:
-
Erythrocyte sedimentation rate
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- AEs:
-
Adverse events
- SAEs:
-
Serious adverse events
- SD:
-
Standard deviation
- T-SPOT:
-
T-SPOT.TB
- PCT:
-
Procalcitonin
- TCZ:
-
Tocilizumab
- PLEX:
-
Plasma exchange
- IVIG:
-
Intravenous immunoglobulin
- HLH:
-
Hemophagocytic lymphohistiocytosis
- MPNs:
-
Myeloproliferative neoplasms
- GVHD:
-
Graft-vs-host-disease (GVHD)
- TEs:
-
Thromboembolic events
- CT:
-
Computer tomography
- MRI:
-
Magnetic resonance imaging
- PET:
-
Positron emission tomography
- Infectious aetiology:
-
T-SPOT, PCT, viral serology, blood culture
- Diseases mimicking AOSD:
-
Infection, malignancy, other autoimmune diseases and inflammatory diseases
- DEX:
-
Dexamethasone
- MTX:
-
Methotrexate
- Pred:
-
Prednisone
- CsA:
-
Cyclosporine A
- CR:
-
Complete remission
- PR:
-
Partial remission
- HCQ:
-
Hydroxychloroquine
- AZA:
-
Azathioprine
- LEF:
-
Leflunomide
- VP16:
-
Etoposide
References
Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still’s disease. Nat Rev Rheumatol. 2018;14:603–18.
Sugiyama T, Furuta S, Hiraguri M, Ikeda K, Inaba Y, Kagami SI, et al. Latent class analysis of 216 patients with adult-onset Still’s disease. Arthritis Res Ther. 2022;24:7.
Still GF. On a form of chronic joint disease in children. Med Chir Trans. 1897;80:47-60.9.
Balci MA, Pamuk ÖN, Pamuk GE, Uzundere FK, Donmez S. Epidemiology and outcome of adult-onset Still’s disease in Northwestern Thrace region in Turkey. Clin Exp Rheumatol. 2015;33:818–23.
Wakai K, Ohta A, Tamakoshi A, Ohno Y, Kawamura T, Aoki R, et al. Estimated prevalence and incidence of adult Still’s disease: findings by a nationwide epidemiological survey in Japan. J Epidemiol. 1997;7:221–5.
Gerfaud-Valentin M, Jamilloux Y, Iwaz J, Sève P. Adult-onset Still’s disease. Autoimmun Rev. 2014;13:708–22.
Asanuma YF, Mimura T, Tsuboi H, Noma H, Miyoshi F, Yamamoto K, et al. Nationwide epidemiological survey of 169 patients with adult Still’s disease in Japan. Mod Rheumatol. 2015;25:393–400.
Mehta BY, Ibrahim S, Briggs W, Efthimiou P. Racial/Ethnic variations in morbidity and mortality in adult onset still’s disease: an analysis of national dataset. Semin Arthritis Rheum. 2019;49:469–73.
Franchini S, Dagna L, Salvo F, Aiello P, Baldissera E, Sabbadini MG. Efficacy of traditional and biologic agents in different clinical phenotypes of adult-onset Still’s disease. Arthritis Rheum. 2010;62:2530–5.
Castañeda S, Blanco R, González-Gay MA. Adult-onset Still’s disease: advances in the treatment. Best Pract Res Clin Rheumatol. 2016;30:222–38.
Church LD, Cook GP, McDermott MF. Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat Clin Pract Rheumatol. 2008;4:34–42.
Jamilloux Y, Gerfaud-Valentin M, Martinon F, Belot A, Henry T, Sève P. Pathogenesis of adult-onset Still’s disease: new insights from the juvenile counterpart. Immunol Res. 2015;61:53–62.
Mitrovic S, Fautrel B. New markers for adult-onset still’s disease. Joint Bone Spine. 2018;85:285–93.
Gillard L, Pouchot J, Cohen-Aubart F, Koné-Paut I, Mouterde G, Michaud M, et al. JAK inhibitors in difficult-to-treat adult-onset Still’s disease and systemic-onset juvenile idiopathic arthritis. Rheumatology. 2022;00:1–12.
Kacar M, Fitton J, Gough AK, Buch MH, McGonagle DG, Savic S. Mixed results with baricitinib in biological-resistant adult-onset Still’s disease and undifferentiated systemic autoinflammatory disease. RMD Open. 2020;6: e001246.
Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424–30.
Fitzgerald AA, Leclercq SA, Yan A, Homik JE, Dinarello CA. Rapid responses to anakinra in patients with refractory adult-onset Still’s disease. Arthritis Rheum. 2005;52:1794–803.
Li T, Gu L, Wang X, Guo L, Shi H, Yang C, et al. A pilot study on tocilizumab for treating refractory adult-onset Still’s disease. Sci Rep. 2017;7:13477.
Vercruysse F, Barnetche T, Lazaro E, Shipley E, Lifermann F, Balageas A, et al. Adult-onset Still’s disease biological treatment strategy may depend on the phenotypic dichotomy. Arthritis Res Ther. 2019;21:53.
Rau M, Schiller M, Krienke S, Heyder P, Lorenz H, Blank N. Clinical manifestations but not cytokine profiles differentiate adult-onset Still’s disease and sepsis. J Rheumatol. 2010;37:2369–76.
Cagatay Y, Gul A, Cagatay A, Kamali S, Karadeniz A, Inanc M, et al. Adult-onset still’s disease. Int J Clin Pract. 2009;63:1050–5.
Sampalis JS, Esdaile JM, Medsger TA Jr, Partridge AJ, Yeadon C, Senécal JL, et al. A controlled study of the long-term prognosis of adult Still’s disease. Am J Med. 1995;98:384–8.
Efthimiou P, Kontzias A, Hur P, Rodha K, Ramakrishna GS, Nakasato P. Adult-onset still’s disease in focus: clinical manifestations, diagnosis, treatment, and unmet needs in the era of targeted therapies. Semin Arthritis Rheum. 2021;51:858–74.
Traves PG, Murray B, Campigotto F, Galien R, Meng A, Di Paolo JA. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann Rheum Dis. 2021;80:865–75.
Huarte E, Peel MT, Verbist K, Fay BL, Bassett R, Albeituni S, et al. Ruxolitinib, a JAK1/2 Inhibitor, Ameliorates cytokine storm in experimental models of hyperinflammation syndrome. Front Pharmacol. 2021;12: 650295.
Keenan C, Nichols KE, Albeituni S. Use of the JAK Inhibitor ruxolitinib in the treatment of hemophagocytic lymphohistiocytosis. Front Immunol. 2021;12: 614704.
Ohta A, Yamaguchi M, Kaneoka H, Nagayoshi T, Hiida M. Adult Still’s disease: review of 228 cases from the literature. J Rheumatol. 1987;14:1139–46.
Smolen JS, Xie L, Jia B, Taylor PC, Burmester G, Tanaka Y, et al. Efficacy of baricitinib in patients with moderate-to-severe rheumatoid arthritis with 3 years of treatment: results from a long-term study. Rheumatology (Oxford). 2021;60:2256–66.
Ladhari C, Jorgensen C, Pers YM. Treatment of refractory adult onset Still’s disease with combination anakinra and baricitinib therapy. Rheumatology (Oxford). 2019;58:736–7.
Hu Q, Wang M, Jia J, Teng J, Chi H, Liu T, et al. Tofacitinib in refractory adult-onset Still’s disease: 14 cases from a single centre in China. Ann Rheum Dis. 2020;79:842–4.
Yoshida S, Yamada S, Yokose K, Matsumoto H, Fujita Y, Asano T, et al. Interferon-γ induces interleukin-6 production by neutrophils via the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway. BMC Res Notes. 2021;14:447.
Gianella S, Schaer DJ, Schwarz U, Kurrer M, Heppner FL, Fehr J, et al. Retinal microangiopathy and rapidly fatal cerebral edema in a patient with adult-onset Still’s disease and concurrent macrophage activation syndrome. Am J Hematol. 2008;83:424–7.
Banse C, Vittecoq O, Benhamou Y, Gauthier-Prieur M, Lequerré T, Lévesque H. Reactive macrophage activation syndrome possibly triggered by canakinumab in a patient with adult-onset Still’s disease. Joint Bone Spine. 2013;80:653–5.
Yokota S, Imagawa T, Mori M, Miyamae T, Takei S, Iwata N, et al. Longterm safety and effectiveness of the anti-interleukin 6 receptor monoclonal antibody tocilizumab in patients with systemic juvenile idiopathic arthritis in Japan. J Rheumatol. 2014;41:759–67.
Puéchal X, DeBandt M, Berthelot JM, Breban M, Dubost JJ, Fain O, et al. Tocilizumab in refractory adult Still’s disease. Arthritis Care Res (Hoboken). 2011;63:155–9.
Virtanen AT, Haikarainen T, Raivola J, Silvennoinen O. Selective JAKinibs: prospects in inflammatory and autoimmune diseases. BioDrugs. 2019;33:15–32.
Kobayashi M, Takahashi Y, Yamashita H, Kaneko H, Mimori A. Benefit and a possible risk of tocilizumab therapy for adult-onset Still’s disease accompanied by macrophage-activation syndrome. Mod Rheumatol. 2011;21:92–6.
Wang R, Li T, Ye S, Tan W, Zhao C, Li Y, et al. Macrophage activation syndrome associated with adult-onset Still’s disease: a multicenter retrospective analysis. Clin Rheumatol. 2020;39:2379–86.
Yang XP, Wang M, Li TF, Li W, Zhang L, Liu SY. Predictive factors and prognosis of macrophage activation syndrome associated with adult-onset Still’s disease. Clin Exp Rheumatol. 2019;37(Suppl 121):83–8.
Verstovsek S, Mesa RA, Gotlib J, Gupta V, DiPersio JF, Catalano JV, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol. 2017;10:55.
Risitano AM, de Latour RP. Ruxolitinib for steroid-resistant acute GVHD. Blood. 2020;135:1721–2.
Maschalidi S, Sepulveda FE, Garrigue A, Fischer A, de Saint BG. Therapeutic effect of JAK1/2 blockade on the manifestations of hemophagocytic lymphohistiocytosis in mice. Blood. 2016;128:60–71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Science and Technology Project of Tianjin Health Commission (2020ZC20182).
Conflicts of interest/Competing interests
The authors declare that they have no competing interests.
Ethics approval
The study protocol was approved by the Ethical Committee of Tianjin Medical University General Hospital (IRB2022-WZ-034).
Consent to participate
All patients provided written informed consent for their clinical and molecular data to be used for research purposes.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Authors' contributions
ZS and XL designed the study, analysed the data, and wrote and revised the report. RL, YW, and FH recruited patients, collected data, advised on interpreting data and revised the report. FH, XL, and WW advised on revising the report, writing – reviewing, and editing. All authors read, reviewed, and approved the final version of the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sun, Z., Li, R., Wang, Y. et al. Efficacy of Baricitinib in Patients with Refractory Adult-Onset Still’s Disease. Drugs R D 23, 109–120 (2023). https://doi.org/10.1007/s40268-023-00417-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-023-00417-7