Abstract
Introduction
Medication changes involving levothyroxine—either dose titrations or switching formulations—occur frequently in patients with erratic thyroid-stimulating hormone (TSH) levels and persistent hypothyroid symptoms. We investigated whether switching patients from levothyroxine tablets to a gel cap formulation of levothyroxine might reduce dose adjustments and improve tolerability and efficacy outcomes.
Objectives
Primary study objectives included quantifying the percentage of patients achieving TSH levels within a pre-specified range, median dose changes experienced, and the percentage of patients with improved hypothyroid symptom control after switching from levothyroxine tablets to levothyroxine gel caps.
Methods
A retrospective medical chart review was conducted among 99 randomly selected hypothyroid patients who were switched from a tablet to a gel cap formulation of levothyroxine. Patients were required to have been on levothyroxine monotherapy for ≥1 year prior to the medication switch. Data was collected for 6 months pre-switch and up to 6 months post-switch.
Results
Of the 99 patients studied, the majority (51.5%) experienced no documented change in TSH status after the switch (P < 0.0001). However, there was a decrease in the mean number of dose changes experienced (1.61 ± 0.96 vs. 0.73 ± 0.96; P < 0.0001). Improved hypothyroid symptom control was reported among 61.6% of patients (61 of 99; P < 0.0001).
Conclusion
The results of CONTROL Switch support a strategy of switching patients who may experience tolerability or efficacy problems with standard levothyroxine tablets to the levothyroxine gel cap formulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Changes in levothyroxine doses or formulations are common and can increase the use of healthcare resources and lead to poor clinical outcomes. |
Levothyroxine gel caps are a unique formulation that has been proven to be consistently absorbed in the presence of factors that limit the absorption of levothyroxine tablets. |
When patients were switched from levothyroxine tablets to gel caps, the majority experienced no improvement in TSH status but significant reductions in dose changes and significant improvement in hypothyroid symptom control. |
The results of the study were consistent regardless of the reason for medication switch (efficacy or adverse effects) or prior therapy (branded or generic levothyroxine tablets). |
1 Introduction
1.1 Background on Disease
Hypothyroidism is a common endocrine disorder resulting from deficiency of thyroid hormone. It is typically a primary process in which the thyroid gland is unable to produce sufficient amounts of thyroid hormone [1].
Third-generation thyroid-stimulating hormone (TSH) assays are readily available and are generally the most sensitive screening tool for primary hypothyroidism. The generally accepted reference range for normal serum TSH is the one contained in the most recent guidelines from the American Thyroid Association (ATA): 0.40–4.2 mIU/L [2].
1.2 Treatment
For hypothyroidism, thyroid hormone is administered to supplement or replace endogenous production—typically levothyroxine—the ‘gold standard’ of treatment for over 60 years. It is one of the most frequently used medications in the USA, with over 115 million prescriptions dispensed in 2013 [3].
In general, hypothyroidism can be adequately treated with a consistent daily dose of levothyroxine, which is determined after a period of initial dosing and titration. However, many patients continue to require dose changes during the course of therapy due to erratic biochemical control of hypothyroidism or persistent hypothyroid symptoms [4–9].
1.3 Levothyroxine Dose Changes
In the recently completed CONTROL Surveillance Project conducted in 925 patients taking levothyroxine monotherapy (94% for >2 years), 23.4% of respondents reported that they had experienced one levothyroxine dose change in the prior 12 months; 8% reported experiencing two or more dose changes in the past 12 months [10]. Thus, there appears to be a subset of patients whose hypothyroidism is difficult to control (biochemically and/or symptomatically) with traditional levothyroxine tablet therapy.
The common strategy of escalating the dose of levothyroxine with adjustments until targeted TSH status is achieved can increase overall healthcare costs and may lead to worsened outcomes. In the recently completed CONTROL HE (Health Economics) study, medical charts from 454 randomly selected patients were analyzed. Consumption of healthcare resources over a 24-month period (as measured by direct medical costs and the value of lost work productivity) was observed to increase significantly with each successive change in levothyroxine dose or formulation [11]. Changes to levothyroxine dosing have also been associated with the risk for prolonged exposure to supratherapeutic doses of levothyroxine [2, 12–14]. Data from five different studies have demonstrated that excessive exposure to levothyroxine is common, ranging from 14 to 22% of all patients [15–19]. Over-exposure to levothyroxine has been associated with adverse cardiovascular events and bone loss [12].
1.4 Factors that Necessitate Levothyroxine Changes: Clinical Implications
Common factors that can necessitate levothyroxine dose adjustments include lack of medication persistence, changes in medication dosage, dosage errors, concomitant medical conditions/medications, switching to less bioavailable generic products, body mass changes, and diet. Because levothyroxine is a drug with a narrow therapeutic index, and its absorption is dependent on gastric pH, some co-morbidities may interfere with its absorption by altering gastric acidity [20–25].
Medication changes involving levothyroxine—either dose titrations or switching formulations—are frequent contributors to poor control of both TSH and hypothyroid symptoms [26]. In a recent large survey conducted by the American Association of Clinical Endocrinologists (AACE), ATA, and the Endocrine Society (ES), the majority of adverse outcomes associated with the generic substitution of levothyroxine products resulted in either mild symptoms of hypo- or hyperthyroidism and/or unexpected thyroid function tests that fell outside of normal limits, almost certainly resulting in lost productivity/wages, travel for further testing, and associated follow-up care [27].
1.5 Rationale for Study
Levothyroxine gel caps constitute a unique formulation that has been shown to produce consistent absorption even in the presence of factors that inhibit the absorption of standard tablet formulations of levothyroxine [28–31]. Given that the gel cap is free of many excipients known to cause tolerability problems (such as gluten), it seemed plausible that it may also be better tolerated. We hypothesized that switching difficult-to-control hypothyroid patients from tablet formulations of levothyroxine to the gel caps might reduce the need for dose adjustments, improve tolerability, and enhance efficacy outcomes in hypothyroid patients. In order to prove this, we undertook a retrospective chart review of patients previously on tablet formulations of levothyroxine who were switched to the gel cap. Such an evaluation had not previously been conducted.
2 Objectives of CONTROL Switch
The main objectives of our study were to quantify the percentage of patients who achieve TSH status within the ATA-recommended reference range of 0.40–4.2 mIU/L [2], the median number of dose changes experienced, and the percentage of patients with improved hypothyroid symptom control after switching from levothyroxine tablets to levothyroxine gel caps. Other objectives included describing patient characteristics, including any relevant co-morbidities, among the study cohort.
3 Methods
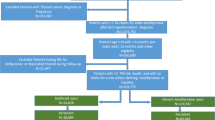
A retrospective medical chart review was conducted among randomly selected patients of providers in community healthcare practices across the USA. All patients who met study inclusion criteria had their medical charts abstracted by trained nurses. Each nurse abstractor was trained on and pre-certified to use the Institutional Review Board (IRB) approved data collection instrument. Study design and methods are shown in Appendix 1.
3.1 Study Populations
The target population consisted of 100 patients who had been treated with levothyroxine tablets for at least 1 year and whose medication had been changed from a tablet to the gel cap formulation. Patients were required to have been taking levothyroxine gel caps for 3–6 months post medication switch and to have medical charts that contained sufficient relevant data (see Appendix 2).
Table 1 provides a complete description of inclusion and exclusion criteria.
3.2 Study Period
Medical chart data were collected and reviewed for documented information on patients meeting inclusion criteria for the time period 6 months (minimum) pre-medication switch through to a period of 3–6 months post treatment switch.
Patients must have been switched to gel caps no later than 30 June 2015. Therefore, the chart abstraction range covered approximately 1 January 2007 (post-US Food and Drug Administration [FDA] approval of gel caps in late 2006) through to 31 December 2015.
3.3 Data Sources
Electronic Medical Records (EMRs) and paper medical records from participating research sites were utilized to identify relevant patients for data collection. Appendices 2 and 3 list the information that was collected, including a full listing of symptoms evaluated and medication adverse effects captured.
3.4 Statistical Analysis
3.4.1 Study Endpoints/Outcomes, Analysis Sets
Endpoints for this study included:
-
Achievement of target TSH status (defined as being within the ATA-recommended reference range of 0.40-4.2 mIU/L);
-
Improvement in hypothyroid symptom control (as documented by the treating clinician);
-
Number of dose changes after medication switch to achieve desired clinical results.
All patients meeting the entry criteria were analyzed in the full analysis.
3.4.2 Sample Size Considerations
Assuming the mean reduction in the number of dose changes following the switch to gel caps to be one, and a standard deviation (SD) of three dose changes, a sample size of 97 patients would provide 90% power at α = 0.05 (two-sided) to test the hypothesis.
We also estimated that somewhere between 50 and 80% of patients would achieve their target TSH status and hypothyroid symptom control with two or fewer dose changes after being switched to gel caps. A sample size of 100 patients would provide an estimate of this percentage within a 95% confidence interval of ± 10%.
3.4.3 Planned Analyses
Data outlined in Appendix 2 were extracted to allow six research questions to be answered through this study (Appendix 4).
3.4.4 Planned Method of Analysis
Descriptive and univariate analyses were conducted on abstracted variables before and after switch to gel caps, with inferential statistics including t tests and Chi-square tests used when appropriate. Statistical significance was evaluated at the two-sided 0.05 α level. A complete explanation of the analysis is available in Appendix 4.
4 Informed Consent
To ensure the research was in compliance with HIPAA (Health Insurance Portability and Accountability Act of 1996) legislation, an IRB waiver of authorization was obtained from Sterling IRB, Atlanta, GA, USA, according to 45 CFR 164.512 (i)(1)(i), which allowed the collection of protected health information without the authorization of study participants for research purposes.
5 Patient Confidentiality
Patients’ confidentiality was maintained for documents submitted to the study Sponsor. Patients were identified only by a unique identification number, and, where permitted, date of birth was documented and formatted in accordance with local laws and regulations.
6 Results
6.1 Demographics
Charts from 99 patients contained the information required for inclusion. The mean age for the entire cohort was 43.9 years, and 91% of patients were female; 40% were white, 4% Asian, 2% African American, and 53% did not specify race; 2% of patients were of Hispanic or Latino ethnicity. Most patients (75%) were taking branded levothyroxine tablet formulations pre-switch (Table 2). All patients were under the care of an endocrinologist.
Consistent with prior studies, there was a high prevalence of patients with co-morbid conditions that are known to complicate levothyroxine therapy. A total of 34.3% patients (34/99) had at least one reported co-morbidity. The majority (65.7%; 65/99) had no reported co-morbid condition. The following conditions were noted most frequently in medical records: gastroesophageal reflux disease (GERD) (11.1%), thyroid cancer (10.1%), celiac disease (6.1%), gastric bypass (3%), and irritable bowel syndrome (IBS) (3.0%) (see Table 3). Concomitant medications/dietary supplements that were noted in medical records for the entire cohort (n = 99) included calcium (15% of mentions), iron (17% of mentions), histamine H2 receptor antagonists (9% of mentions), and proton pump inhibitors (PPIs) (6% of mentions).
6.2 Reasons for Medication Changes
Among patients with documented reasons for switching to gel caps (n = 82), the most common reason for switching was adverse effects associated with prior therapy (n = 49, 59.8%). Reasons cited in medical chart notes include gastrointestinal-related adverse effects such as cramps and bloating and miscellaneous other adverse effects including insomnia, urticaria, and hair loss. Ineffectiveness of the previous medication was the next most common reason for switching to gel caps, documented in 39 (47.6%) patient records. In these cases, inadequate biochemical (TSH) and hypothyroid symptom control were the most cited. It should be noted that some patients were switched to gel caps for both reasons—adverse effects and suboptimal efficacy (n = 14).
6.3 Thyroid-Stimulating Hormone (TSH) Status
Among the full cohort of 99 patients switched to gel caps, 51.5% had no documented change in TSH status compared with prior to switch (P < 0.0001) (Fig. 1). More than 26% of patients had a documented improvement in their TSH status (26/99). Only a minority of patients (22.2%) experienced a worsening of their TSH status post-switch.
6.4 Dose Adjustments
Among the full cohort of 99 patients, 85.8% experienced one or fewer dose changes post-switch (33.3% experienced one dose change; 52.5% did not experience any dose changes). Prior to switching to gel caps, the study cohort experienced a mean of 1.61 ± 0.96 dose adjustments per patient; after switching to gel caps, the study cohort experienced a mean of 0.73 ± 0.96 dose adjustments per patient, a statistically significant change (P < 0.0001) (Fig. 2). The post-switch mean of 0.73 ± 0.96 dose adjustments per patient is a 54.7% decrease from the pre-switch mean number of dose adjustments.
6.5 Symptom Control
Among the full cohort of 99 patients, 61.6% had physician-reported improvement of symptoms following switch to the gel caps formulation (P < 0.0001). Only 8.1% reported worsening of symptoms post-switch (Fig. 3).
6.6 Patients Switched for Efficacy Reasons Only
6.6.1 TSH Status
Among patients who were switched for efficacy reasons, almost one-third (32%; 8/25) experienced an improvement in TSH status post-switch. Most experienced no change (48%; 12/25). Only a minority of patients (5/25) experienced a worsening of TSH status after the switch. However, this result was not statistically significant (P = 0.2276).
6.6.2 Dose Adjustments
Among those patients who were switched for efficacy reasons, 88% (22/25) had one or fewer dose changes after switching to the gel cap (20% had one dose change; 68% experienced no dose adjustments). Prior to switching to gel caps, these patients experienced a mean of 1.60 ± 0.92 dose adjustments per patient. After switching to gel caps, this cohort experienced a mean of 0.44 ± 0.71 dose adjustments per patient, a statistically significant change of 72.5% from the pre-switch mean number of dose adjustments (P < 0.0001) (Fig. 4).
6.6.3 Symptom Control
Among patients switched for efficacy reasons, 64% (16/25) had physician-reported improvement in hypothyroid symptoms. Among those with no documented improvement in hypothyroid symptoms, the majority (7/9) experienced no change in symptoms. Only two patients (8%) experienced a worsening of their hypothyroid symptoms (Fig. 5).
7 Discussion
The results of CONTROL Switch showed improvements across a wide range of clinical variables when hypothyroid patients were switched from standard levothyroxine tablet formulations to levothyroxine gel caps. However, these results should be viewed within the context of the following:
-
The patient population studied;
-
Differences in results among patients switched for efficacy reasons versus non-efficacy reasons;
-
Changes in symptom control versus changes in TSH status;
-
Data collection period pre-/post-switch; and
-
Similarity or discordance with prior clinical studies.
7.1 Patient Population
The patient population in CONTROL Switch differed from those of prior studies. These differences include:
-
Patient age Patients in CONTROL Switch were younger (average age was 43.9 years). Current AACE/ATA guidelines list the prevalence of hypothyroidism among adult women in the USA to be as high as 12 per 1000 [2, 32]; the average age of patients is estimated at 58 years [2].
-
Co-morbidities The number of patients with gastrointestinal conditions (25.3%) is lower than that observed in other studies. In CONTROL Surveillance, a survey of 925 patients taking levothyroxine monotherapy, 47.0% of patients reported having one or more gastrointestinal condition that could affect levothyroxine therapy. Among these conditions, GERD (33.8%) and IBS (9.7%) were most prevalent [10].
-
Thyroid cancer surgery The prevalence of patients in the study who had experienced thyroid cancer surgery (10.1%) is also higher than that observed in the general population and may have influenced treatment decisions, especially the range of TSH values to which patients were managed.
-
Gastrointestinal surgery The percentage of patients with a history of gastric surgery in CONTROL Switch (3.0%) was higher than that observed in prior studies. Gastric surgery has been shown to limit the absorption of oral drug therapy, including levothyroxine [33].
Prior therapy Given the widely documented issues of consistency and potency noted among generic formulations of levothyroxine [27], it is reasonable to expect that patients taking generic levothyroxine formulations would see different results than patients taking branded levothyroxine formulations when switched to the gel cap. In CONTROL Switch, reductions in dose changes post-switch were similar among both groups. Prior to switching to gel caps, patients on branded levothyroxine tablets experienced a mean of 1.62 ± 0.72 dose adjustments per patient. After switching to gel caps, this cohort experienced a mean of 0.72 ± 0.93 dose adjustments per patient. The relative decrease of 55.6% in mean dose adjustments pre- and post-switch is comparable with that in patients switched from generic levothyroxine tablets (55.6 vs. 51.3%). There was no statistically significant difference in the mean number of dose adjustments between patients switched to gel caps from branded levothyroxine tablets and those switched from generic tablets (P = 0.8541) (Fig. 6). Both groups experienced similar improvements in hypothyroid symptom status: almost 61% (45/74) of patients on branded levothyroxine tablets pre-switch had documented physician- or patient-reported improvement of symptoms following the switch to gel caps, compared with 64.0% (16/25) of patients on generic levothyroxine tablets pre-switch. The result was not statistically significant, indicating there was no statistical difference in physician- or patient-reported documentation of symptom control between patients switched to gel caps from branded levothyroxine tablets and those switched from generic tablets (P = 0.9562) (Fig. 7).
Considering these points, it is not unreasonable to conclude that the patient population in CONTROL Switch was somewhat more difficult to manage than the general hypothyroid population. These make the improvements in TSH status and hypothyroid symptoms observed in the study more significant.
7.2 Results: Patients Switched for Efficacy Versus Adverse Effects
In CONTROL Switch, “side effects of prior medication” was listed as the primary reason for switching medication for most patients (59.8%). Assuming that many of these patients had TSH values within the recommended reference range pre-switch, one would expect little overall change in TSH status when switching to an alternate levothyroxine formulation. Results from our study bear this out. More than 78% of patients switched for “side effects” experienced no change in TSH status post-switch or an improvement in TSH status. Among patients switched primarily because of the “lack of efficacy of prior medication” (n = 25), the percentage of patients whose TSH status remained in range or improved was 72% (18/25). Only 28% of these patients (7/25) experienced a worsening of their TSH status. These results confirm our initial hypothesis that the clinician’s reason for the medication switch (lack of efficacy vs. adverse effects) would correlate to the results observed.
7.3 Changes in Symptom Control Versus Changes in TSH
In CONTROL Switch, the records of most patients indicate a notable improvement in hypothyroid symptom control post-medication switch. Patients switched for efficacy reasons experienced a greater improvement in hypothyroid symptom control (64.0%) than those switched primarily for non-efficacy reasons (62.8%; P > 0.05). The improvement in hypothyroid symptom control observed among patients switched for non-efficacy reasons may be attributable to improvements in medication tolerability. Briesacher et al. [34] and Kandukuri et al. [35] have demonstrated a strong correlation between drug tolerability or adverse effects and medication compliance.
Improvements in hypothyroid symptom control were observed even in the absence of laboratory test values that were within the recommended reference range. Among patients who experienced a worsening of their TSH status post-switch, 45.5% (10/22) experienced an improvement in hypothyroid symptom control post-switch. While seemingly paradoxical, this illustrates a phenomenon found frequently in clinical practice: the divergence between TSH values and patient symptoms. While the possibility of a ‘placebo effect’ cannot be ruled out, Hulisz [36] observed that some patients experience a “lack of well-being” in spite of reaching the euthyroid reference range of TSH. We view these data as hypothesis generating and advocate further study in a prospective and controlled trial.
7.4 Data Collection Period
In CONTROL Switch, there was a difference in the data collection period pre- and post-medication switch which could bias study results. Per protocol, data were collected for 6 months pre-switch and 3–6 months post–switch. When study results were examined, it was determined that almost 86% of study subjects (85/99) had their data analyzed for 6 months or more post-switch. Among these patients, there was no statistical difference in the number of dose changes experienced versus the entire study cohort (0.788 ± 0.989 vs. 0.727 ± 0.956; P > 0.05). Among patients whose records contained data for between 3 and 6 months post-switch (n = 14), there was also no statistical difference in the number of dose changes experienced versus the entire study cohort (0.429 ± 0.64 vs. 0.727 ± 0.956; P > 0.05). These data further support the robustness of the results observed in the study.
7.5 Similarity with Prior Studies
The results of CONTROL Switch are congruent with, and extend the results of, other studies involving levothyroxine gel caps. In a 2015 study, Santaguida et al. [31] reported that patients switched from tablet formulations of levothyroxine were able to be managed at a lower dose of levothyroxine gel caps. In CONTROL Switch most patients experienced no change in dose after initiation on the gel cap formulation. Among patients switched for efficacy reasons, a group among whom dose adjustments may be more likely, the majority who experienced dose changes post-switch had their medication titrated downward (62.5%; 5/8) rather than upward (37.5%; 3/8).
CONTROL Switch further documents the utility of the gel cap formulation in treating patients with gastrointestinal disorders or other factors that may contribute to suboptimal and/or variable levothyroxine absorption. Among patients with gastrointestinal disorders in the study, there was a 36.3% reduction in the mean number of dose changes (1.83 ± 0.41 pre-switch reduced to 1.17 ± 1.83 post-switch). Improvements in hypothyroid symptom control were also observed. Among patients with gastrointestinal conditions (n = 25), 68% experienced an improvement in hypothyroid symptoms post-switch. The remainder (32%; 8/25) experienced no change. These results may be explained in part by the fact that the gel cap formulation is more fully absorbed when gastric pH is affected by certain gastrointestinal conditions, medications, or dietary supplements [28–31, 37]. In the case of patients with GERD, improved hypothyroid symptom control may be the result of the pharmacokinetics of the gel cap versus standard tablet formulations in the presence of PPIs. Seng Yue et al. [28] demonstrated the superior absorption of levothyroxine gel caps in individuals with altered gastric pH resulting from the coadministration of esomeprazole.
Given its lack of sensitizing excipients, it is not surprising that many patients were switched to the gel cap formulation after experiencing “side effects” with traditional levothyroxine therapies. McMillan et al. [10] reported that medical conditions that can be exacerbated by excipients found in tablet therapies may be present in as many as 15% of all levothyroxine-treated patients. In CONTROL Switch, 7.1% of patients had documentation in their medical charts indicating the presence of either celiac disease or lactose intolerance. However, these conditions are often undiagnosed. These patients experienced comparable reductions in dose changes relative to the overall study population. A majority of patients with celiac disease (83.3%; 5/6) experienced improvements in symptom control. The effect of the gel cap formulation in reducing dose changes and improving hypothyroid symptom control among patients with known medication tolerability concerns is further proof of its utility in treating these patients.
8 Study Limitations
Though adequately powered, our study involving 99 patients may not fully represent the broader hypothyroid patient population. There were a number of demographic differences found in this cohort of patients compared with the population at large, as reported in the medical literature. For example, a smaller percentage of African Americans (2%) was observed in our study than in the broader hypothyroid population (14%). The reason for this discrepancy is unclear.
Data were extracted if documented within patient charts. Certain datapoints, including demographic and co-morbidity information, were missing if they were not present in medical records. Missing data values were not assigned (imputed) based on inference from other known values.
For the analyses on dose changes, symptom control, and TSH status, there was no adjustment made to account for patient age, pregnancy status, etiology of hypothyroidism, or co-morbid conditions such as thyroid cancer. These factors are known to affect response to levothyroxine therapy and influence physician decisions regarding levothyroxine dosing [2, 38–42]. The symptom control analysis may have been improved by using a more detailed data collection instrument.
Finally, this was a retrospective study, not randomized, blinded, or placebo controlled. It should be noted that retrospective data analyses, by design, cannot establish cause and effect. Only a randomized, prospective study can draw such definitive conclusions.
9 Conclusions
Levothyroxine dose and medication changes are common in clinical practice and often result from lack of tolerability or intended efficacy. They may also contribute to unnecessary utilization of healthcare resources and patient dissatisfaction with care [26]. Minimizing levothyroxine dose and formulation changes may therefore be an appropriate strategy to both optimize healthcare resources and improve patient satisfaction. Our study suggests that for some patients, a strategy of switching from traditional levothyroxine tablet therapy to levothyroxine gel caps may eliminate or lower the need for such changes while improving hypothyroid symptom control.
The results of the CONTROL Switch study add to the growing body of evidence demonstrating the potential challenges of managing hypothyroid patients with levothyroxine. Suboptimally treated hypothyroidism, manifested by frequent levothyroxine medication or dose changes and/or poor symptom control, can produce undesirable clinical and societal outcomes. The utility of a strategy of switching from traditional levothyroxine tablet therapy to levothyroxine gel caps may offer an alternative that holds the possibility of improved quality of life for many patients.
References
Ward LS. The difficult patient: drug interaction and the influence of concomitant diseases on the treatment of hypothyroidism. Arq Bras Endocrinol Metabol. 2010;54:435–42.
Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670–751.
IMS Institute for Healthcare Informatics. Medicine use and shifting costs of healthcare. IMS Health. 2014;46. http://www.plannedparenthoodadvocate.org/2014/IIHI_US_Use_of_Meds_for_2013.pdf. Accessed 17 Nov 2016.
Dar RA, Chowdri NA, Parray FQ, et al. An unusual case of Hashimoto’s thyroiditis with four lobed thyroid gland. N Am J Med Sci. 2012;4:151–3.
Yaturu S, Fontinot J, Rowland T. Mixed medullary thyroid cancer and follicular cancer. Am J Case Rep. 2011;12:1–4.
Robertson HM, Narayanaswamy AK, Pereira O, et al. Factors contributing to high levothyroxine doses in primary hypothyroidism: an interventional audit of a large community database. Thyroid. 2014;24:1765–71.
Santini F, Pinchera A, Marsili A, et al. Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases. J Clin Endocrinol Metab. 2005;90:124–7.
Roos A, Linn-Rasker SP, van Domburg RT, et al. The starting dose of levothyroxine in primary hypothyroidism treatment. A prospective, randomized, double-blind trial. Arch Intern Med. 2005;165:1714–20.
Mandel SJ, Brent GA, Larscn PR. Levothyroxine therapy in patients with thyroid disease. Ann Intern Med. 1993;119:492–502.
McMillan M, Rotenberg KS, Vora K, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: results of the CONTROL Surveillance project. Drugs R D. 2016;16:53–68.
Ernst F, Barr P, Elmor R, et al. The economic impact of levothyroxine dose adjustments on healthcare utilization—the CONTROL HE study [abstract]. 86th Annual Meeting of the American Thyroid Association; 21–25 Sept 2016; Denver.
Flynn RW, Bonellie SR, Jung RT, et al. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab. 2010;9:186–93.
Shomon M. The thyroid treatment/osteoporosis controversy: does thyroid treatment contribute to loss of bone density? http://www.thyroid-info.com/articles/osteoporosis.htm. Accessed 31 Oct 2016.
Lindner H. Thyroid hormone: T3. http://www.hormonerestoration.com/Thyroid.html. Accessed 31 Oct 2016.
Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489–99.
Ross DS, Daniels GH, Gouveia D. The use and limitations of a chemiluminescent thyrotropin assay as a single thyroid function test in an out-patient endocrine clinic. J Clin Endocrinol Metab. 1990;71:764–9.
Parle JV, Franklyn JA, Cross KW, et al. Thyroxine prescription in the community: serum thyroid stimulating hormone level assays as an indicator of undertreatment or overtreatment. Br J Gen Pract. 1993;43:107–9.
Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–34.
Vaisman F, Coeli CM, Ward LS, et al. How good is the levothyroxine replacement in primary hypothyroidism patients in Brazil? Data of a multicentre study. J Endocrinol Invest. 2013;36:485–8.
Khandelwal D, Tandon N. Overt and subclinical hypothyroidism: who to treat and how. Drugs. 2012;72:17–33.
Liwanpo L, Hershman JM. Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab. 2009;23:781–92.
Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419–26.
Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244–51.
Cellini M, Santaguida MG, Gatto I, et al. Systematic appraisal of lactose intolerance as cause of increased need for oral thyroxine. J Clin Endocrinol Metab. 2014;99:E1454–8.
Ianiro G, Mangiola F, Di Rienzo TA, et al. Levothyroxine absorption in health and disease, and new therapeutic perspectives. Eur Rev Med Pharmacol Sci. 2014;18:451–6.
Ramadhan A, Tamilia M. Treatment-refractory hypothyroidism. CMAJ. 2012;184:205–9.
Hennessey JV, Malabanan AO, Haugen BR, et al. Adverse event reporting in patients treated with levothyroxine: results of the pharmacovigilance task force survey of the American Thyroid Association, American Association of Clinical Endocrinologists, and the Endocrine Society. Endocr Prac. 2010;16:357–70.
Seng Yue C, Benvenga S, Scarsi C, et al. When bioequivalence in healthy volunteers may not translate to bioequivalence in patients: differential effects of increased gastric pH on the pharmacokinetics of levothyroxine capsules and tablets. J Pharm Sci. 2015;18:844–55.
Vita R, Benvenga S. Tablet levothyroxine (L-T4) malabsorption induced by proton pump inhibitor; a problem that was solved by switching to L-T4 in gel cap capsule. Endocr Pract. 2014;20:e38–41.
Vita R, Saraceno G, Trimarchi F, et al. In patients with no interference on the intestinal absorption of L-T4 caused by gastro-intestinal disorders or drugs, a liquid formulation of L-T4 permits to reach target TSH levels that were missed by the conventional tablet formulation [abstract no. P73]. 36th Annual Meeting of the European Thyroid Association, 8-12 September 2012, Pisa. Eur Thyroid. 2012;J1:125.
Santaguida MG, Virili C, Del Duca SC, et al. Thyroxine softgel capsule in patients with gastric-related T4 malabsorption. Endocrine. 2015;49:51–7.
Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39–51.
Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11:41–50.
Briesacher BA, Andrade SE, Fouayzi H, et al. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437–43.
Kandukuri RC, Khan MA, Soltys SM. Nonadherence to medication in hypothyroidism: a case report. Prim Care Companion J Clin Psychiatry. 2010. doi:10.4088/PCC.09m00863gre.
Hulisz D. Current challenges in the management of hypothyroidism. US Pharmacist. 2012; Suppl:1–12. https://www.uspharmacist.com/courses/107997/107997.pdf. Accessed 31 Oct 2016.
Pabla D, Akhlaghi F, Zia H. A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur J Pharm Biopharm. 2009;72:105–10.
Ehrenkranz J, Bach PR, Snow GL, et al. Circadian and circannual rhythms in thyroid hormones: determining the TSH and Free T4 reference intervals based upon time of day, age, and sex. Thyroid. 2015;25:954–61.
Surks MI, Boucai L. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab. 2010;95:496–502.
Alexander EK, Marqusee E, Lawrence J, et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241–9.
Giusti M, Mortara L, Machello N, et al. Utility of a liquid formulation of levo-thyroxine in differentiated thyroid cancer patients. Drug Res (Stuttg). 2015;65:332–6.
Krhin B, Besic N. Effectiveness of L-thyroxine treatment on TSH suppression during pregnancy in patients with a history of thyroid carcinoma after total thyroidectomy and radioiodine ablation. Radiol Oncol. 2012;46:160–5.
Acknowledgements
The sponsor, Akrimax Pharmaceuticals, LLC, wishes to thank Indegene, Inc. (Kennesaw, GA, USA) for conducting and overseeing all aspects of this study, and Aesculapius Consulting, Inc. (East Brunswick, NJ, USA) for providing editorial assistance in the development of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All potential conflicts of interest and financial considerations are provided in the Disclosures section.
Author participation
Each of the authors participated in the data collection, organization, and/or writing of this manuscript.
Disclosures
This study was funded by Akrimax Pharmaceuticals, LLC, Cranford, NJ, USA. Frank Ernst, Jennifer Welstead, and Riad Elmor are employees of Indegene, Inc., which received a fee for services related to the development and execution of this study, and for the tabulation, analysis, and reporting of its results. Walter Sandulli and MaryKate Lavan are employees of Akrimax. Arnold Sterman has been a consultant for Akrimax, has contributed to research funded by Akrimax, and received an honorarium for his contributions to evaluating this study and to the development of this manuscript.
Appendices
Appendix 1
See Fig. 8.
Appendix 2
See Table 4.
Appendix 3
See Table 5.
Appendix 4
See Table 6.
4.1 Planned Method of Analysis
Both descriptive and univariate analyses were conducted to compare abstracted variables for each patient before and after switch to gel caps. Two-sided P values <0.05 denoting statistical significance were reported where applicable, and continuous variables were presented as data that included n, mean, standard deviation, median, interquartile range, minimum, and maximum. For categorical variables, presentation of data included frequency and percentages within each group.
Where applicable for each of the above, inferential statistics included t tests for important continuous variables (e.g., age) and Chi square tests for important categorical variables (e.g., sex, co-morbidities, and others as appropriate). Incidence rates for symptoms and control, calculated on the sample of patients included in the study, were provided with 95% confidence intervals, and cumulative incidences were analyzed as well (with confidence limits). Data were managed in Microsoft Excel® and all analyses were performed in SAS® version 9.2 or greater statistical software (SAS Institute, Cary, NC, USA).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ernst, F.R., Sandulli, W., Elmor, R. et al. Retrospective Study of Patients Switched from Tablet Formulations to a Gel Cap Formulation of Levothyroxine: Results of the CONTROL Switch Study. Drugs R D 17, 103–115 (2017). https://doi.org/10.1007/s40268-016-0150-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-016-0150-z