Abstract
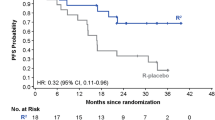
Lenalidomide (Revlimid®) is a targeted immunomodulatory drug with multiple mechanisms of action. In the USA and the EU, oral lenalidomide is indicated in combination with rituximab or a rituximab product for the treatment of patients with previously treated follicular lymphoma. In the pivotal, phase III AUGMENT trial, lenalidomide + rituximab significantly prolonged progression-free survival (PFS; primary endpoint) relative to placebo + rituximab in patients with relapsed or refractory indolent non-Hodgkin lymphoma, with the PFS benefit appearing to be specific to patients with follicular lymphoma and extending to elderly patients with this subtype. Lenalidomide + rituximab also demonstrated activity in an interim analysis of the phase III MAGNIFY trial in patients with relapsed or refractory indolent non-Hodgkin lymphoma, including those with rituximab-refractory disease. Lenalidomide had an acceptable tolerability profile. Although grade 3 or 4 neutropenia occurred more frequently with lenalidomide + rituximab than with placebo + rituximab, this was generally well managed with dosage adjustments and growth factor support. In conclusion, lenalidomide in combination with rituximab represents an important new treatment option for previously treated follicular lymphoma, including patients whose disease has become refractory to rituximab.
Similar content being viewed by others
References
Flowers C, Leonard JP, Fowler N. Lenalidomide in follicular lymphoma. Blood. 2020;135(24):2133–6.
Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol. 2020;95(3):316–27.
Feugier P, Filliatre-Clement L. Recent advances in the first-line treatment of follicular non-Hodgkin lymphoma. F1000Research. 2019. https://doi.org/10.12688/f1000research.16686.1.
Lymphoma Research Foundation. Follicular lymphoma: relapsed/refractory. 2018. http://www.lymphoma.org. Accessed 5 Aug 2020.
Dreyling M, Ghielmini M, Rule S, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl 5):v83–90.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines®): B-cell lymphomas. 2020. http://www.nccn.org. Accessed 5 Aug 2020.
Karmali R, Kimby E, Ghielmini M, et al. Rituximab: a benchmark in the development of chemotherapy-free treatment strategies for follicular lymphomas. Ann Oncol. 2018;29(2):332–40.
Celgene Corporation. REVLIMID (lenalidomide capsule): US prescribing information. 2019. http://dailymed.nlm.nih.gov. Accessed 5 Aug 2020.
National Institute for Health and Care Excellence. Treating follicular lymphoma. 2020. http://www.nice.org.uk. Accessed 5 Aug 2020.
European Medicines Agency. Revlimid (lenalidomide) hard capsules: summary of product characteristics. 2020. http://www.ema.europa.eu. Accessed 5 Aug 2020.
McCormack PL. Lenalidomide: a review of its continuous use in patients with newly diagnosed multiple myeloma not eligible for stem-cell transplantation. Drugs Aging. 2015;32(5):409–18.
Scott LJ, Lyseng-Williamson KA. Lenalidomide: a review of its use in the treatment of relapsed or refractory multiple myeloma. Drugs. 2011;71(5):625–49.
Syed YY. Lenalidomide: a review in newly diagnosed multiple myeloma as maintenance therapy after ASCT. Drugs. 2017;77(13):1473–80.
Syed YY, Scott LJ. Lenalidomide: a review of its use in patients with transfusion-dependent anaemia due to low- or intermediate-1-risk myelodysplastic syndrome associated with 5q chromosome deletion. Drugs. 2013;73(11):1183–96.
Gribben JG, Fowler N, Morschhauser F. Mechanisms of action of lenalidomide in B-cell non-Hodgkin lymphoma. J Clin Oncol. 2015;33(25):2803–11.
Chiu H, Trisal P, Bjorklund C, et al. Combination lenalidomide-rituximab immunotherapy activates anti-tumour immunity and induces tumour cell death by complementary mechanisms of action in follicular lymphoma. Br J Haematol. 2019;185(2):240–53.
Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol. 2019;37(14):1188–99.
Rummel MJ, Andorsky DJ, Coleman M, et al. MAGNIFY: phase IIIb interim analysis of induction lenalidomide + rituximab (R2) followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma [abstract no. EP1161]. Hemasphere. 2020;4(Suppl. 1):540.
Tuscano JM, Dutia M, Chee K, et al. Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. Br J Haematol. 2014;165(3):375–81.
Izutsu K, Minami Y, Fukuhara N, et al. Analysis of Japanese patients from the AUGMENT phase III study of lenalidomide + rituximab (R2) vs. rituximab + placebo in relapsed/refractory indolent non-Hodgkin lymphoma. Int J Hematol. 2019;111(3):409–16.
Scheliga A, Fogliatto L, Scheinberg P, et al. Analysis of Brazilian patients (pts) from AUGMENT: a phase III randomized study of lenalidomide plus rituximab vs rituximab plus placebo in pts with relapsed/refractory (R/R) indolent non-hodgkin lymphoma (INHL) [abstract no. 179 plus poster 348]. Hematol Transfus Cell Ther. 2019;41(Suppl 2):S67.
Trneny M, Leonard J, Zhang H, et al. Subgroup analyses of elderly patients aged ≥ 70 years in AUGMENT: a phase III randomized study of lenalidomide plus rituximab (R2) vs rituximab plus placebo in patients with relapsed/refractory indolent non-Hodgkin lymphoma [abstract no. 009 plus oral presentation]. In: Prague Hematological Days. 2019.
Leonard JP, Trneny M, Izutsu K, et al. Efficacy was improved with lenalidomide/rituximab (R2) vs rituximab/placebo in patients with follicular lymphoma irrespective of POD24 status in the phase III AUGMENT study [abstract no. PF483]. HemaSphere. 2019;3(Suppl 1):193–4.
Gribben J, Trneny M, Izutsu K, et al. AUGMENT: relapsed/refractory indolent NHL patients were more sensitive to next treatment following lenalidomide/rituximab (R2) than rituximab/placebo [abstract no 178]. Hematol Oncol. 2019;37(Suppl 2):227–9.
Leonard JP, Trneny M, Izutsu K, et al. Health-related quality of life (HRQoL) in relapsed/refractory (R/R) indolent NHL in the phase 3 AUGMENT trial of rituximab (R) plus lenalidomide (R2) versus R plus placebo [abstract no. 181]. Hematol Oncol. 2019;37(Suppl 2):232–4.
US National Institutes of Health. http://www.clinicaltrials.gov. Accessed 5 Aug 2020
Cheson BD, Morschhauser F, Martin P. Management of adverse events from the combination of rituximab and lenalidomide in the treatment of patients with follicular and low-grade non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2020. https://doi.org/10.1016/j.clml.2020.03.009.
National Institute for Health and Care Excellence. Lenalidomide with rituximab for previously treated follicular lymphoma. 2020. http://www.nice.org.uk. Accessed 5 Aug 2010.
Foster T, Miller JD, Boye ME, et al. Economic burden of follicular non-Hodgkin’s lymphoma. Pharmacoeconomics. 2009;27(8):657–79.
Acknowledgements
During the peer review process, the manufacturer of lenalidomide was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
H. Blair is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Additional information
Enhanced material
for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.12762095.
The manuscript was reviewed by: D. Dierickx, Department of Hematology, University Hospitals Leuven, Leuven, Belgium; S. Gonzalez, Hematology, University Hospital Marqués de Valdecilla, Santander, Spain; P. Scheinberg, Division of Hematology, Hospital A Beneficencia Portuguesa de São Paulo, São Paulo, Brazil.
Rights and permissions
About this article
Cite this article
Blair, H.A. Lenalidomide: A Review in Previously Treated Follicular Lymphoma. Drugs 80, 1337–1344 (2020). https://doi.org/10.1007/s40265-020-01381-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01381-1