Abstract
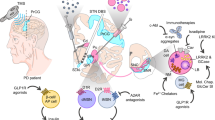
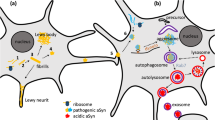
Parkinson’s disease (PD), the second most common neurodegenerative movement disorder, is characterized by progressive motor and non-motor symptoms [1]. Despite treatment with pharmacologic and surgical therapies, the disease will continue to relentlessly advance. Hence, there is a great deal of interest in potential disease-modifying therapies with the hope that the neurodegenerative process can be slowed or halted. The purpose of this review is to highlight the role toxic α-synuclein (α-syn) plays in PD pathogenesis and critically review the relevant literature about therapeutic modalities targeting α-syn. Toxic α-syn plays a key role in PD pathogenesis, disrupting important cellular functions, and, thus, targeting α-syn is a reasonable disease-modifying strategy. Current approaches under investigation include decreasing α-syn production with RNA interference (RNAi), inhibiting α-syn aggregation, promoting intracellular degradation of α-syn aggregates (via enhancing autophagy and enhancing lysosomal degradation), and promoting extracellular degradation of α-syn via active and passive immunization.
Similar content being viewed by others
References
GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–53.
Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, et al. Past, present, and future of Parkinson’s disease: a special essay on the 200th anniversary of the shaking palsy. Mov Disord. 2017;32:1264–310.
Fox SH, Katzenschlager R, Lim S-Y, Barton B, de Bie RMA, Seppi K, et al. International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33:1248–66.
Lotia M, Jankovic J. New and emerging medical therapies in Parkinson’s disease. Expert Opin Pharmacother. 2016;17:895–909.
Jankovic J. Pathogenesis-targeted therapeutic strategies in Parkinson’s disease. Mov Disord. 2019;34:41–4.
Spillantini GM, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40.
Polymeropoulos MH, Higgins JJ, Golbe LI, Johnson WG, Ide SE, Di Iorio G, et al. Mapping of a gene for Parkinson’s disease to chromosome 4q21-q23. Science. 1996;274:1197–9.
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–7.
Fanning S, Haque A, Imberdis T, Baru V, Barrasa MI, Nuber S, et al. Lipidomic analysis of α-synuclein neurotoxicity identifies stearoyl CoA desaturase as a target for Parkinson treatment. Mol Cell. 2019;73(5):1001–1014.e8.
Vincent BM, Tardiff DF, Piotrowski JS, Aron R, Lucas MC, Chung CY, et al. Inhibiting stearoyl-CoA desaturase ameliorates α-synuclein cytotoxicity. Cell Rep. 2018;25(2742–2754):e31.
Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis. 2008;5:55–9.
Adler CH, Dugger BN, Hentz JG, Hinni ML, Lott DG, Driver-Dunckley E, et al. Peripheral synucleinopathy in early Parkinson’s disease: submandibular gland needle biopsy findings. Mov Disord. 2016;31:250–6.
Ghiglieri V, Calabrese V, Calabresi P. Alpha-synuclein: from early synaptic dysfunction to neurodegeneration. Front Neurol. 2018;9:295.
Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9.
Bridi JC, Hirth F. Mechanisms of α-synuclein induced synaptopathy in Parkinson’s disease. Front Neurosci. 2018;12:80.
Wu K-P, Weinstock DS, Narayanan C, Levy RM, Baum J. Structural reorganization of alpha-synuclein at low pH observed by NMR and REMD simulations. J Mol Biol. 2009;391:784–96.
Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–4.
Peng C, Gathagan RJ, Covell DJ, Medellin C, Stieber A, Robinson JL, et al. Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature. 2018;557:558–63.
Mokretar K, Pease D, Taanman J-W, Soenmez A, Ejaz A, Lashley T, et al. Somatic copy number gains of α-synuclein (SNCA) in Parkinson’s disease and multiple system atrophy brains. Brain. 2018;141:2419–31.
Shachar T, Lo Bianco C, Recchia A, Wiessner C, Raas-Rothschild A, Futerman AH. Lysosomal storage disorders and Parkinson’s disease: Gaucher disease and beyond. Mov Disord. 2011;26:1593–604.
Blanz J, Saftig P. Parkinson’s disease: acid-glucocerebrosidase activity and alpha-synuclein clearance. J Neurochem. 2016;139(Suppl):198–215.
Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23:1–13.
Robak LA, Jansen IE, Van Rooij J, Uitterlinden AG, Kraaij R, Jankovic J, et al. Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain. 2017;140:3191–203.
Mor DE, Daniels MJ, Ischiropoulos H. The usual suspects, dopamine and alpha-synuclein, conspire to cause neurodegeneration. Mov Disord. 2019;34:167–79.
Rousseaux MWC, Shulman JM, Jankovic J. Progress toward an integrated understanding of Parkinson’s disease. F1000Research. 2017;6:1121.
Singleton AB, Farrer M, Johnson J, Singleton AB, Hague S, Kachergus J, et al. Alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841.
Chartier-Harlin M-C, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–9.
Brundin P, Dave KD, Kordower JH. Therapeutic approaches to target alpha-synuclein pathology. Exp Neurol. 2017;298:225–35.
Klein AD, Mazzulli JR. Is Parkinson’s disease a lysosomal disorder? Brain. 2018;141:2255–62.
Scrivo A, Bourdenx M, Pampliega O, Cuervo AM. Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol. 2018;17:802–15.
Schlatterer SD, Acker CM, Davies P. c-Abl in neurodegenerative disease. J Mol Neurosci. 2011;45:445–52.
Gaki GS, Papavassiliou AG. Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromolecular Med. 2014;16:217–30.
Ko HS, Lee Y, Shin J-H, Karuppagounder SS, Gadad BS, Koleske AJ, et al. Phosphorylation by the c-Abl protein tyrosine kinase inhibits parkin’s ubiquitination and protective function. Proc Natl Acad Sci USA. 2010;107:16691–6.
Imam SZ, Zhou Q, Yamamoto A, Valente AJ, Ali SF, Bains M, et al. Novel regulation of parkin function through c-Abl-mediated tyrosine phosphorylation: implications for Parkinson’s disease. J Neurosci. 2011;31:157–63.
Brahmachari S, Karuppagounder SS, Ge P, Lee S, Dawson VL, Dawson TM, et al. c-Abl and Parkinson’s disease: mechanisms and therapeutic potential. J Parkinsons Dis. 2017;7:589–601.
Hebron ML, Lonskaya I, Moussa CE-HE-H. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of α-synuclein in Parkinson’s disease models. Hum Mol Genet. 2013;22:3315–28.
Hebron ML, Lonskaya I, Moussa CE-H. Tyrosine kinase inhibition facilitates autophagic SNCA/α-synuclein clearance. Autophagy. 2013;9:1249–50.
Brahmachari S, Ge P, Lee SH, Kim D, Karuppagounder SS, Kumar M, et al. Activation of tyrosine kinase c-Abl contributes to α-synuclein-induced neurodegeneration. J Clin Invest. 2016;126:1–19.
Chang D, Nalls MA, Hallgrímsdóttir IB, Hunkapiller J, van der Brug M, Cai F, et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet. 2017;49:1511–6.
Blauwendraat C, Heilbron K, Vallerga C. Parkinson’s disease age at onset GWAS: defining heritability, genetic loci and α-synuclein mechanisms. Mov Disord. 2019;. https://doi.org/10.1101/424010.
Deng H, Wang P, Jankovic J. The genetics of Parkinson disease. Ageing Res Rev. 2018;42:72–85.
Sardi SP, Cedarbaum JM, Brundin P. Targeted therapies for Parkinson’s disease: from genetics to the clinic. Mov Disord. 2018;33:684–96.
Goedert M, Jakes R, Spillantini MG. The synucleinopathies: twenty years on. J Parkinsons Dis. 2017;7:S53–71.
Pihlstrøm L, Blauwendraat C, Cappelletti C, Berge-Seidl V, Langmyhr M, Henriksen SP, et al. A comprehensive analysis of SNCA-related genetic risk in sporadic Parkinson disease. Ann Neurol. 2018;84:117–29.
Petrucci S, Ginevrino M, Valente EM. Phenotypic spectrum of alpha-synuclein mutations: new insights from patients and cellular models. Parkinsonism Relat Disord. 2016;22(Suppl 1):S16–20.
Simón-Sánchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–12.
Sidransky E, Lopez G. The link between the GBA gene and parkinsonism. Lancet Neurol. 2012;11:986–98.
Inzelberg R, Hassin-Baer S, Jankovic J. Genetic movement disorders in patients of Jewish ancestry. JAMA Neurol. 2014;71:1567–72.
Shihabuddin LS, Brundin P, Greenamyre JT, Stephenson D, Sardi SP. New frontiers in Parkinson’s disease: from genetics to the clinic. J Neurosci. 2018;38:9375–82.
Migdalska-Richards A, Schapira AHV. The relationship between glucocerebrosidase mutations and Parkinson disease. J Neurochem. 2016;139(Suppl):77–90.
Beavan MS, Schapira AHV. Glucocerebrosidase mutations and the pathogenesis of Parkinson disease. Ann Med. 2013;45:511–21.
Stirnemann J, Belmatoug N, Camou F, Serratrice C, Froissart R, Caillaud C, et al. A review of Gaucher disease pathophysiology, clinical presentation and treatments. Int J Mol Sci. 2017;18:441.
Cilia R, Tunesi S, Marotta G, Cereda E, Siri C, Tesei S, et al. Survival and dementia in GBA-associated Parkinson’s disease: the mutation matters. Ann Neurol. 2016;80:662–73.
Alessi DR, Sammler E. LRRK2 kinase in Parkinson’s disease. Science. 2018;360:36–7.
Yue Z, Yang XW. Dangerous duet: LRRK2 and α-synuclein jam at CMA. Nat Neurosci. 2013;16:375–7.
West AB. Achieving neuroprotection with LRRK2 kinase inhibitors in Parkinson disease. Exp Neurol. 2017;298:236–45.
Domingo A, Klein C. Genetics of Parkinson disease. Handb Clin Neurol. 2018;147:211–27.
Saunders-Pullman R, Mirelman A, Alcalay RN, Wang C, Ortega RA, Raymond D, et al. Progression in the LRRK2-associated Parkinson disease population. JAMA Neurol. 2018;75:312–9.
Marras C, Alcalay RN, Caspell-Garcia C, Coffey C, Chan P, Duda JE, et al. Motor and nonmotor heterogeneity of LRRK2-related and idiopathic Parkinson’s disease. Mov Disord. 2016;31:1192–202.
Volpicelli-Daley L, Brundin P. Prion-like propagation of pathology in Parkinson disease. Handb Clin Neurol. 2018;153:321–35.
Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211.
Rietdijk CD, Perez-Pardo P, Garssen J, van Wezel RJA, Kraneveld AD. Exploring Braak’s hypothesis of Parkinson’s disease. Front Neurol. 2017;8:37.
Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78:522–9.
Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, et al. Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology. 2017;88:1996–2002.
Killinger BA, Madaj Z, Sikora JW, Rey N, Haas AJ, Vepa Y, et al. The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci Transl Med. 2018;10:eaar5280. https://doi.org/10.1126/scitranslmed.aar5280.
Breen DP, Halliday GM, Lang AE. Gut-brain axis and the spread of α-synuclein pathology: vagal highway or dead end? Mov Disord. 2019;34:307–16.
Surmeier DJ, Obeso JA, Halliday GM. Parkinson’s disease is not simply a prion disorder. J Neurosci. 2017;37:9799–807.
Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–34.
Zhang Z, Nie S, Chen L. Targeting prion-like protein spreading in neurodegenerative diseases. Neural Regen Res. 2018;13:1875–8.
Brettschneider J, Del Tredici K, Lee VM-Y, Trojanowski JQ. Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci. 2015;16:109–20.
Goedert M. NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 2015;349:1255555.
Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol. 2016;79:940–9.
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, et al. Alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–4.
Recasens A, Carballo-Carbajal I, Parent A, Bové J, Gelpi E, Tolosa E, et al. Lack of pathogenic potential of peripheral α-synuclein aggregates from Parkinson’s disease patients. Acta Neuropathol Commun. 2018;6:8.
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167(1469–1480):e12.
Mittal S, Bjørnevik K, Im DS, Flierl A, Dong X, Locascio JJ, et al. Beta2-Adrenoreceptor is a regulator of the alpha-synuclein gene driving risk of Parkinson’s disease. Science. 2017;357:891–8.
Searles Nielsen S, Gross A, Camacho-Soto A, Willis AW, Racette BA. β2-adrenoreceptor medications and risk of Parkinson disease. Ann Neurol. 2018;84:683–93.
Benito-León J, Louis ED, Bermejo-Pareja F, Neurological Disorders in Central Spain Study Group. Risk of incident Parkinson’s disease and parkinsonism in essential tremor: a population based study. J Neurol Neurosurg Psychiatry. 2009;80:423–5.
Thenganatt MA, Jankovic J. The relationship between essential tremor and Parkinson’s disease. Parkinsonism Relat Disord. 2016;22(Suppl 1):S162–5.
Sapru MK, Yates JW, Hogan S, Jiang L, Halter J, Bohn MC. Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp Neurol. 2006;198:382–90.
Lewis J, Melrose H, Bumcrot D, Hope A, Zehr C, Lincoln S, et al. In vivo silencing of alpha-synuclein using naked siRNA. Mol Neurodegener. 2008;3:19.
Lambeth LS, Smith CA. Short hairpin RNA-mediated gene silencing. Methods Mol Biol. 2013;942:205–32.
Zharikov AD, Cannon JR, Tapias V, Bai Q, Horowitz MP, Shah V, et al. shRNA targeting α-synuclein prevents neurodegeneration in a Parkinson’s disease model. J Clin Invest. 2015;125:2721–35.
Xhima K, Nabbouh F, Hynynen K, Aubert I, Tandon A. Noninvasive delivery of an α-synuclein gene silencing vector with magnetic resonance-guided focused ultrasound. Mov Disord. 2018;33:1567–79.
McCormack AL, Mak SK, Henderson JM, Bumcrot D, Farrer MJ, Di Monte DA. Alpha-synuclein suppression by targeted small interfering RNA in the primate substantia nigra. PLoS One. 2010;5:e12122.
Kanaan NM, Manfredsson FP. Loss of functional alpha-synuclein: a toxic event in Parkinson’s disease? J Parkinsons Dis. 2012;2:249–67.
Collier TJ, Redmond DE, Steece-Collier K, Lipton JW, Manfredsson FP. Is alpha-synuclein loss-of-function a contributor to Parkinsonian pathology? Evidence from non-human primates. Front Neurosci. 2016;10:12.
Wild EJ, Tabrizi SJ. Therapies targeting DNA and RNA in Huntington’s disease. Lancet Neurol. 2017;16:837–47.
Wrasidlo W, Tsigelny IF, Price DL, Dutta G, Rockenstein E, Schwarz TC, et al. A de novo compound targeting α-synuclein improves deficits in models of Parkinson’s disease. Brain. 2016;139:3217–36.
Koike M, Price D, White B, Rockenstein E, Wrasidlo W, Tsigelny I, et al. The novel alpha-synuclein stabilizer NPT200-11 improves behavior, neuropathology and biochemistry in the murine thy1-ASYN transgenic model of Parkinson’s disease [poster]. Society for Neuroscience Congress, 15–19 Nov 2014. Washington, DC; 2014.
Szoke B, Wrasidlo W, Stocking E, Tsigelny I, Schwartz T, Konrat R, et al. Biophysical characterization of the interaction of NPT200-11 with alpha-synuclein [poster]. Society for Neuroscience Congress, 15–19 Nov 2014. Washington, DC; 2014.
Krishnan R, Tsubery H, Proschitsky MY, Asp E, Lulu M, Gilead S, et al. A bacteriophage capsid protein provides a general amyloid interaction motif (GAIM) that binds and remodels misfolded protein assemblies. J Mol Biol. 2014;426:2500–19.
Dehay B, Decressac M, Bourdenx M, Guadagnino I, Fernagut P-O, Tamburrino A, et al. Targeting α-synuclein: therapeutic options. Mov Disord. 2016;31:882–8.
Levin J, Schmidt F, Boehm C, Prix C, Bötzel K, Ryazanov S, et al. The oligomer modulator anle138b inhibits disease progression in a Parkinson mouse model even with treatment started after disease onset. Acta Neuropathol. 2014;127:779–80.
Wagner J, Ryazanov S, Leonov A, Levin J, Shi S, Schmidt F, et al. Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease. Acta Neuropathol. 2013;125:795–813.
Deeg AA, Reiner AM, Schmidt F, Schueder F, Ryazanov S, Ruf VC, et al. Anle138b and related compounds are aggregation specific fluorescence markers and reveal high affinity binding to α-synuclein aggregates. Biochim Biophys Acta. 2015;1850:1884–90.
Dexter DT, Wells FR, Agid F, Agid Y, Lees AJ, Jenner P, et al. Increased nigral iron content in postmortem parkinsonian brain. Lancet. 1987;2:1219–20.
Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem. 1989;52:1830–6.
Mandel S, Maor G, Youdim MBH. Iron and alpha-synuclein in the substantia nigra of MPTP-treated mice: effect of neuroprotective drugs R-apomorphine and green tea polyphenol −)-epigallocatechin-3-gallate. J Mol Neurosci. 2004;24:401–16.
Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid Redox Signal. 2014;21:195–210.
Finkelstein DI, Billings JL, Adlard PA, Ayton S, Sedjahtera A, Masters CL, et al. The novel compound PBT434 prevents iron mediated neurodegeneration and alpha-synuclein toxicity in multiple models of Parkinson’s disease. Acta Neuropathol Commun. 2017;5:53.
Hung S-Y, Fu W-M. Drug candidates in clinical trials for Alzheimer’s disease. J Biomed Sci. 2017;24:47.
Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Björklund A. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc Natl Acad Sci USA. 2013;110:E1817–26.
Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19:51–60.
Ghosh A, Tyson T, George S, Hildebrandt EN, Steiner JA, Madaj Z, et al. Mitochondrial pyruvate carrier regulates autophagy, inflammation, and neurodegeneration in experimental models of Parkinson’s disease. Sci Transl Med. 2016;8:368ra174.
Erlich S, Shohami E, Pinkas-Kramarski R. Neurodegeneration induces upregulation of Beclin 1. Autophagy. 2:49–51.
Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–9.
Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29:13578–88.
Hebron ML, Lonskaya I, Olopade P, Selby ST, Pagan F, Moussa CE-H. Tyrosine kinase inhibition regulates early systemic immune changes and modulates the neuroimmune response in α-synucleinopathy. J Clin Cell Immunol. 2014;5:259.
Pagan F, Hebron M, Valadez EH, Torres-Yaghi Y, Huang X, Mills RR, et al. Nilotinib effects in Parkinson’s disease and dementia with Lewy bodies. J Parkinsons Dis. 2016;6:503–17.
Wyse RK, Brundin P, Sherer TB. Nilotinib—differentiating the hope from the hype. J Parkinsons Dis. 2016;6:519–22.
Robledo I, Jankovic J. Media hype: patient and scientific perspectives on misleading medical news. Mov Disord. 2017;32:1319–23.
Sardi SP, Cheng SH, Shihabuddin LS. Gaucher-related synucleinopathies: the examination of sporadic neurodegeneration from a rare (disease) angle. Prog Neurobiol. 2015;125:47–62.
Migdalska-Richards A, Ko WKD, Li Q, Bezard E, Schapira AHV. Oral ambroxol increases brain glucocerebrosidase activity in a nonhuman primate. Synapse. 2017;71:e21967.
Pchelina SN, Nuzhnyi EP, Emelyanov AK, Boukina TM, Usenko TS, Nikolaev MA, et al. Increased plasma oligomeric alpha-synuclein in patients with lysosomal storage diseases. Neurosci Lett. 2014;583:188–93.
Zunke F, Moise AC, Belur NR, Gelyana E, Stojkovska I, Dzaferbegovic H, et al. Reversible conformational conversion of α-synuclein into toxic assemblies by glucosylceramide. Neuron. 2018;97(92–107):e10.
Deverman BE, Ravina BM, Bankiewicz KS, Paul SM, Sah DWY. Gene therapy for neurological disorders: progress and prospects. Nat Rev Drug Discov. 2018;17:641–59.
Fuji RN, Flagella M, Baca M, Baptista MAS, Brodbeck J, Chan BK, et al. Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci Transl Med. 2015;7:273ra15.
Zhao HT, John N, Delic V, Ikeda-Lee K, Kim A, Weihofen A, et al. LRRK2 antisense oligonucleotides ameliorate α-synuclein inclusion formation in a Parkinson’s disease mouse model. Mol Ther Nucleic Acids. 2017;8:508–19.
Lang AE, Espay AJ. Disease modification in Parkinson’s disease: current approaches, challenges, and future considerations. Mov Disord. 2018;33:660–77.
Denali Therapeutics announces positive clinical results from LRRK2 inhibitor program for Parkinson’s disease. 2018. http://investors.denalitherapeutics.com/news-releases/news-release-details/denali-therapeutics-announces-positive-clinical-results-lrrk2#ir-pages.
Witoelar A, Jansen IE, Wang Y, Desikan RS, Gibbs JR, Blauwendraat C, et al. Genome-wide pleiotropy between Parkinson disease and autoimmune diseases. JAMA Neurol. 2017;74:780–92.
Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 2017;546:656–61.
Dhillon J-KS, Riffe C, Moore BD, Ran Y, Chakrabarty P, Golde TE, et al. A novel panel of α-synuclein antibodies reveal distinctive staining profiles in synucleinopathies. PLoS One. 2017;12:e0184731.
Lawand NB, Saadé NE, El-Agnaf OM, Safieh-Garabedian B. Targeting α-synuclein as a therapeutic strategy for Parkinson’s disease. Expert Opin Ther Targets. 2015;19:1351–60.
Dehay B, Bourdenx M, Gorry P, Przedborski S, Vila M, Hunot S, et al. Targeting α-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. Lancet Neurol. 2015;14:855–66.
Jankovic J. Immunologic treatment of Parkinson’s disease. Immunotherapy. 2018;10:81–4.
Schneeberger A, Tierney L, Mandler M. Active immunization therapies for Parkinson’s disease and multiple system atrophy. Mov Disord. 2016;31:214–24.
Mandler M, Valera E, Rockenstein E, Weninger H, Patrick C, Adame A, et al. Next-generation active immunization approach for synucleinopathies: implications for Parkinson’s disease clinical trials. Acta Neuropathol. 2014;127:861–79.
Kingwell K. Zeroing in on neurodegenerative α-synuclein. Nat Rev Drug Discov. 2017;16:371–3.
Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6:e19338.
Bergström A-L, Kallunki P, Fog K. Development of passive immunotherapies for synucleinopathies. Mov Disord. 2016;31:203–13.
Games D, Valera E, Spencer B, Rockenstein E, Mante M, Adame A, et al. Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson’s disease-like models. J Neurosci. 2014;34:9441–54.
Schenk DB, Koller M, Ness DK, Griffith SG, Grundman M, Zago W, et al. First-in-human assessment of PRX002, an anti-α-synuclein monoclonal antibody, in healthy volunteers. Mov Disord. 2017;32:211–8.
Jankovic J, Goodman I, Safirstein B, Marmon TK, Schenk DB, Koller M, et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti-α-synuclein monoclonal antibody, in patients with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2018;75:1206–14.
Brys M, Ellenbogen A, Fanning N, Penner N, Yang M, Welch M, et al. Randomized, double-blind, placebo-controlled, single ascending dose study of anti-alpha-synuclein antibody BIIB054 in patients with Parkinson’s disease. Neurology. 2018;90(15 Suppl):S26.001. http://n.neurology.org/content/90/15_Supplement/S26.001. Accessed 24 Mar 2019.
Weihofen A, Liu Y, Arndt JW, Huy C, Quan C, Smith BA, et al. Development of an aggregate-selective, human-derived α-synuclein antibody BIIB054 that ameliorates disease phenotypes in Parkinson’s disease models. Neurobiol Dis. 2018;124:276–88.
Martin-Bastida A, Ward RJ, Newbould R, Piccini P, Sharp D, Kabba C, et al. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci Rep. 2017;7:1398.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the publication of this review
Conflict of Interest
Dr. Daniel Savitt declares that there are no conflicts of interest relevant to this work. Dr. Joseph Jankovic declares the following: research/training funding—Allergan, Inc., CHDI Foundation, Civitas/Acorda Therapeutics, Dystonia Coalition, Dystonia Medical Research Foundation, F. Hoffmann-La Roche Ltd, Huntington Study Group, Medtronic Neuromodulation, Merz Pharmaceuticals, Michael J. Fox Foundation for Parkinson Research, National Institutes of Health, Neurocrine Biosciences, Parkinson’s Foundation, Nuvelution, Parkinson Study Group, Pfizer Inc., Prothena Biosciences Inc., Psyadon Pharmaceuticals, Inc., Revance Therapeutics, Inc., Teva Pharmaceutical Industries Ltd, and US WorldMeds; consultant/advisory board—Allergan, Inc., Merz Pharmaceuticals, Prothena Biosciences Inc., Retrophin, Inc.–Parexel, Revance Therapeutics, Inc., and Teva Pharmaceutical Industries Ltd; royalties—Cambridge, Elsevier, Future Science Group, Hodder Arnold, Medlink: Neurology, Lippincott Williams and Wilkins, and Wiley-Blackwell.
Rights and permissions
About this article
Cite this article
Savitt, D., Jankovic, J. Targeting α-Synuclein in Parkinson’s Disease: Progress Towards the Development of Disease-Modifying Therapeutics. Drugs 79, 797–810 (2019). https://doi.org/10.1007/s40265-019-01104-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01104-1