Abstract
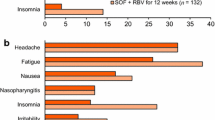
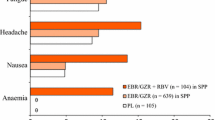
A fixed-dose tablet comprising ombitasvir (an NS5A replication complex inhibitor), paritaprevir (an NS3/4A protease inhibitor) and ritonavir (a cytochrome P450 inhibitor) taken in combination with dasabuvir (an NS5B polymerase inhibitor) is indicated for the treatment of chronic hepatitis C virus (HCV) genotype 1 infection in several countries, including the USA (copackaged as Viekira Pak™) and those of the EU (Viekirax® and Exviera®). In phase II and III trials, this interferon-free regimen, taken ± ribavirin, provided high rates of sustained virological response 12 weeks post-treatment in adults with chronic HCV genotype 1a or 1b infection, including those with compensated cirrhosis, liver transplants or HIV-1 co-infection. The regimen was generally well tolerated, with nausea, insomnia, asthenia, pruritus, other skin reactions and fatigue being among the most common tolerability issues. Thus, ombitasvir/paritaprevir/ritonavir plus dasabuvir is an effective interferon-free, direct-acting antiviral regimen for use ± ribavirin in a broad range of adults chronically infected with HCV genotype 1.

Similar content being viewed by others
References
Centers for Disease Control and Prevention. Infectious diseases related to travel: hepatitis C. 2014. http://wwwnc.cdc.gov. Accessed 9 Apr 2015.
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60(2):392–420.
World Health Organization. Hepatitis C: fact sheet No 164. 2014. http://www.who.int. Accessed 9 Apr 2015.
Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461(7265):798–801.
AASLD and IDSA. Recommendations for testing, managing, and treating hepatitis C. 2014. http://www.hcvguidelines.org/fullreport. Accessed 9 Apr 2015.
Stickel F, Helbling B, Heim M, et al. Critical review of the use of erythropoietin in the treatment of anaemia during therapy for chronic hepatitis C. J Viral Hepat. 2012;19(2):77–87.
Jensen DM. Advances in combination regimens in the management of HCV infection. Gastroenterol Hepatol. 2014;10(2):134–6.
Bichoupan K, Dieterich DT, Martel-Laferriere V. HIV-hepatitis C virus co-infection in the era of direct-acting antivirals. Curr HIV/AIDS Rep. 2014;11(3):241–9.
Asselah T, Marcellin P. Optimal IFN-free therapy in treatment-naïve patients with HCV genotype 1 infection. Liver Int. 2015;35:56–64.
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatology. doi:10.1016/j.jhep.2015.03.025.
Peter J, Nelson DR. Optimal interferon-free therapy in treatment-experienced chronic hepatitis C patients. Liver Int. 2015;35:65–70.
AbbVie Inc. VIEKIRA PAK (ombitasvir, paritaprevir, and ritonavir tablets; dasabuvir tablets): US prescribing information. 2015. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/. Accessed 9 Apr 2015.
AbbVie Limited. Viekirax 12.5 mg/75 mg/50 mg film-coated tablets: EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 8 Apr 2015.
AbbVie Limited. Exviera 250 mg film-coated tablets: EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 8 Apr 2015.
DeGoey DA, Randolph JT, Liu D, et al. Discovery of ABT-267, a pan-genotypic inhibitor of HCV NS5A. J Med Chem. 2014;57(5):2047–57.
Pilot-Matias T, Tripathi R, Cohen D, et al. In vitro and in vivo antiviral activity and resistance profile of the hepatitis C virus NS3/4A protease inhibitor ABT-450. Antimicrob Agents Chemother. 2015;59(2):988–97.
Kati W, Koev G, Irvin M, et al. In vitro activity and resistance profile of dasabuvir, a nonnucleoside hepatitis C virus polymerase inhibitor. Antimicrob Agents Chemother. 2014;59(3):1505–11.
Krishnan P, Beyer J, Mistry N, et al. In vitro and in vivo antiviral activity and resistance profile of ombitasvir, an inhibitor of hepatitis C virus NS5A. Antimicrob Agents Chemother. 2014;59(2):979–87.
Krishnan P. Pooled analysis of resistance in patients treated with ombitasvir/ABT-450/r and dasabuvir with or without ribavirin in phase 2 and phase 3 clinical trials [abstract no. 1936]. Hepatology. 2014;40(4 Suppl 1):1134A–5A.
Krishnan P, Tripathi R, Schnell G, et al. Long-term follow-up of treatment-emergent resistance-associated variants in NS3, NS5A and NS5B with paritaprevir/r-, ombitasvir- and dasabuvir-based regimens [abstract no. O057]. J Hepatol. 2015;62(Suppl 2):S220.
Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594–603.
Zeuzem S, Jacobson IM, Baykal T, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1604–14.
Andreone P, Colombo MG, Enejosa JV, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147(2):359–65 e1.
Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983–92.
Dore GJ, Knysz B, Luo Y, et al. MALACHITE-II: phase 3b trial of ombitasvir/paritaprevir/r and dasabuvir + ribavirin or telaprevir + peginterferon/ribavirin in peginterferon/ribavirin treatment-experienced adults with HCV genotype 1 [abstract no. P0847 plus poster]. J Hepatol. 2015;62(Suppl 2):S656–7.
Conway B, Janczewska E, Luo Y, et al. MALACHITE-I: phase 3b trial of ombitasvir/paritaprevir/r and dasabuvir +/− ribavirin or telaprevir + peginterferon/ribavirin in treatment-naive adults with HCV genotype 1 [abstract no. P0842 plus poster]. J Hepatol. 2015;62(Suppl 2):S653–4.
AbbVie. An open-label, single arm, phase 2 study to evaluate ABT-450/r/ABT-267 and ABT-333 with ribavirin (RBV) in adults with genotype 1 HCV infection taking methadone or buprenorphine [clinicaltrials.gov identifier NCT01911845]. 2014. http://www.clinicaltrials.gov. Accessed 9 Apr 2015.
Bernstein DE, Luo Y, Lalezari JP, et al. PEARL-IV trial: subgroup analysis of genotype 1a-infected patients treated with ABT-450/r/ombitasvir with dasabuvir with or without ribavirin [abstract no. 1933]. Hepatology. 2014;60(4 Suppl 1):1132A–3A.
Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis (plus supplementary appendix). N Engl J Med. 2014;370(21):1973–82.
Kwo PY, Mantry PS, Coakley E, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014;371(25):2375–82.
Sulkowski MS, Eron JJ, Wyles D, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial (plus supplementary appendix). JAMA. 2015;313(12):1223–31.
Nelson DR, Reddy KR, Di Bisceglie AM, et al. ABT-450/r/ombitasvir + dasabuvir with or without ribavirin in HCV genotype 1-infected patients with history of depression or bipolar disorder: pooled analysis of efficacy and safety in phase 3 trials [abstract no. 1972]. Hepatology. 2014;60(4 Suppl):1159A–60A.
Wyles D, Eron J, Trinh R, et al. High SVR regardless of time to suppression with ABT-450/r/ombitasvir & dasabuvir + RBV [abstract no. 147]. In: Conference on Retroviruses and Opportunistic Infections; 2015.
Bernstein DE, Marinho RT, Cohen DE, et al. Adherence to prescribed doses of ABT-450/r/ombitasvir, dasabuvir, and ribavirin in the phase 3 PEARL-II, PEARL-III, and PEARL-IV trials [abstract no. 1953]. Hepatology. 2014;40(4 Suppl 1):1146A.
Hassanein T, Roberts S, Shafran SD, et al. Adherence to ombitasvir/paritaprevir/r, dasabuvir, and ribavirin is >98% in the SAPPHIRE-I and SAPPHIRE-II trials [abstract no. P0908]. J Hepatol. 2015;62(Suppl 2):S685.
Liu Y, Larsen L, Zeuzem S, et al. Ombitasvir/paritaprevir/ritonavir and dasabuvir with ribavirin (RBV) has minimal impact in health-related quality of life (HRQOL) compared with placebo during 12-week treatment in treatment-naive adults with chronic hepatitis C (CHC) [abstract no. P0873]. J Hepatol. 2015;62(Suppl 2):S668.
Liu Y, Larsen L, Bourliere M, et al. Ombitasvir/paritaprevir/ritonavir and dasabuvir with ribavirin (RBV) has mild impact on health-related quality of life (HRQOL) compared with placebo during 12-week treatment in treatment-experienced adults with chronic hepatitis C (CHC) [abstract no. P0856]. J Hepatol. 2015;62(Suppl 2):S661.
Fried MW, Di Bisceglie AM, Vierling JM. Safety of ABT-450/r/ombitasvir + dasabuvir with or without ribavirin in HCV genotype 1-infected patients: results from phase 2 and phase 3 trials [abstract no. 1951]. Hepatology. 2014;40(4 Suppl 1):1145A.
Eron J, Wyles D, Sulkowski M, et al. TURQUOISE I: safety and efficacy of ABT-450/r/ombitasvir, dasabuvir and ribavirin in patients co-infected with HCV and HIV-1 [abstract no. V-673]. In: 54th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2014.
Flamm S. Safety of ABT-450/r/ombitasvir + dasabuvir with or without ribavirin in HCV genotype 1-infected patients ≥65 years of age: results from phase 2 and 3 trials [abstract no. 1969]. Hepatology. 2014;40(4 Suppl 1):1157A–8A.
Sulkowski M, Wyles D, Slim J, et al. Hematologic analysis of ABT-450/r/ombitasvir and dasabuvir + RBV in TURQUOISE-I [abstract no. 691]. In: Conference on Retroviruses and Opportunistic Infections; 2015.
Jacobson IM, Forns X, Zeusem S, et al. Characteristics of HCV-infected patients with cirrhosis requiring dose reduction during treatment with direct-acting antivirals [abstract no. 1973]. Hepatology. 2014;60(Suppl S1):1160A.
Pockros PJ, Reddy KR, Mantry PS, et al. Safety of ombitasvir/paritaprevir/ritonavir plus dasabuvir for treating HCV GT1 infection in patients with severe renal impairment or end-stage renal disease: the RUBY-I study [abstract no. L01 plus oral presentation]. J Hepatol. 2015;62(Suppl 2):S257.
Carrion AF, Gutierrez J, Martin P. New antiviral agents for the treatment of hepatitis C: ABT-450. Expert Opin Pharmacother. 2014;15(5):711–6.
Chayama K, Suzuki F, Ikeda K, et al. Ombitasvir/paritaprevir/ritonavir for treatment of HCV genotype 1b in Japanese patients with or without cirrhosis: results from GIFT-I [abstract no. G13]. J Hepatol. 2015;62(Suppl 2):S235.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit. Emma Deeks is a salaried employee of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: G. Bertino, Hepatology Unit, Regional Referral Center for HCV, HBV Treatment, Department of Clinical and Experimental Medicine, University of Catania, University Hospital G.Rodolico, Catania, Italy; P. Ferenci, Internal Medicine 3, Gastroenterology/Hepatology Division, Medical University of Vienna, Vienna, Austria; S. Karatapanis, First Department of Internal Medicine, General Hospital of Rhodes, Rhodes, Greece.
Rights and permissions
About this article
Cite this article
Deeks, E.D. Ombitasvir/Paritaprevir/Ritonavir Plus Dasabuvir: A Review in Chronic HCV Genotype 1 Infection. Drugs 75, 1027–1038 (2015). https://doi.org/10.1007/s40265-015-0412-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0412-z